Abstract
Adherence of Mycoplasma fermentans to HeLa cells followed saturation kinetics, required a divalent cation, and was enhanced by preincubation of the organism at 37°C for 1 h in a low-osmolarity solution. Proteolytic digestion, choline phosphate, or anti-choline phosphate antibodies partially inhibited the adherence, supporting the notion that M. fermentans utilizes at least two surface components for adhesion, a protease-sensitive surface protein and a phosphocholine-containing glycolipid. Plasminogen binding to M. fermentans greatly increased the maximal adherence of the organism to HeLa cells. Anti-plasminogen antibodies and free plasminogen inhibited this increase. These observations suggest that in the presence of plasminogen the organism adheres to novel sites on the HeLa cell surface, which are apparently plasminogen receptors. Plasminogen-bound M. fermentans was detected exclusively on the cell surface of the infected HeLa cells. Nevertheless, plasminogen binding in the presence of the urokinase-type plasminogen activator (uPA) promoted the invasion of HeLa cells by M. fermentans. The latter finding indicates that the invasiveness of M. fermentans does not result from binding plasminogen but from activation of the bound plasminogen to plasmin. Cholesterol depletion and sequestration with β-cyclodextrin and filipin, respectively, did not affect the capacity of M. fermentans to adhere, but invasion of HeLa cells by uPA-activated plasminogen-bound M. fermentans was impaired, suggesting that lipid rafts are implicated in M. fermentans entry.
Mycoplasmas (class Mollicutes) are wall-less prokaryotes that are widely distributed in nature. Most mycoplasmas are parasites, exhibiting strict host and tissue specificities, and almost all of them are bound to the surface of the host cells. Almost all animal mycoplasmas depend on adhesion to host tissues for subsequent colonization and infection (26, 31). In these mycoplasmas, adherence is the major virulence factor, and adherence-deficient mutants are avirulent (3, 32). The best-studied adherence system is that of Mycoplasma pneumoniae, the causative agent of primary atypical pneumonia in humans. Adherence of this organism to cells in the respiratory tract is an initial and essential step in tissue colonization and the subsequent development of disease (20). A surface 169-kDa protein designated P1 (18) and a 30-kDa protein designated P30 (11) are densely clustered at the tip organelle of virulent M. pneumoniae and are associated with the adherence process.
The human pathogen Mycoplasma fermentans was isolated from the urogenital tract several decades ago (33). Interest in this organism has recently increased because of its possible role in the pathogenesis of rheumatoid arthritis and reports indicating that this organism may function as a cofactor accelerating the progression of human immunodeficiency virus disease (22, 28). Although M. fermentans is a typical extracellular microorganism able to adhere to human epithelial cells, ultrastructural studies performed with engulfed M. fermentans revealed mycoplasmas within membrane-bound vesicles (38, 39). However, the underlying mechanisms for the adherence to and invasion of host cells are only poorly understood.
Plasminogen (Pg) is a 92-kDa plasma glycoprotein. This protein is activated in vivo to the serine protease plasmin by the urokinase-type Pg activator (uPA) and the tissue-type Pg activator by cleavage of a single peptide bond (R561-V562), yielding two chains that remain connected by two disulfide bridges (34). The binding of Pg to mycoplasmas has been described previously (7, 40), and in M. fermentans two Pg binding proteins with molecular masses of about 32 and 55 kDa were identified (40). Pg binding to M. fermentans enhances the activation of Pg to plasmin by uPA, and it has been suggested that the ability of this organism to invade host cells stems from its potential to bind Pg and to activate it to plasmin (40). In the present study the adherence of M. fermentans to HeLa cells was characterized, and the roles of Pg binding and Pg activation by uPA in adherence and invasion were analyzed.
MATERIALS AND METHODS
Bacterial strains, cell lines, and culture conditions.
M. fermentans strain PG-18 (kindly provided by S.-C. Lo, Armed Forces Institute of Pathology, Washington, D.C.) was used throughout this study. In some experiments the respiratory isolate M. fermentans M-52 (kindly provided by P. C. T. Hannan, Mycoplasma Experience Ltd., Reigate, Surrey, United Kingdom) and M. pneumoniae strain M129 (obtained from the American Type Culture Collection, Rockville, Md.) were also utilized. M. fermentans strains were grown for 24 to 48 h at 37°C in a modified Chanock medium (13) supplemented with 5% heat-inactivated horse serum (Biological Industries, Beit Haemek, Israel). M. pneumoniae was grown for 72 h in the same medium supplemented with 20% horse serum. For metabolic labeling, the organisms were grown in a medium containing 0.5 μCi of [9,10(n)-3H]palmitic acid (53.0 Ci/mmol; New England Nuclear) per ml. The cells were harvested at the mid-exponential phase of growth (A640, 0.10 to 0.12; pH 6.6) by centrifugation for 20 min at 12,000 × g, washed once, and resuspended in a solution containing 0.25 M NaCl, 10 mM CaCl2, and 10 mM Tris adjusted to pH 7.5. The number of viable mycoplasmas was determined by plating and was expressed as the number of CFU per milliliter.
The epithelial cell line HeLa-229 (ATCC CCL2.1) was grown in T-25 flasks or in 24-well plates containing Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (Biological Industries), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The flasks were incubated at 37°C in a 5% CO2 atmosphere. For the adherence or internalization assays the cells were washed twice in phosphate-buffered saline (PBS) (pH 7.4).
Adherence assay.
Adherence of M. fermentans to HeLa cells was determined in a reaction mixture containing 106 HeLa cells and [3H]palmitate-labeled M. fermentans (100 μg of cell protein, 10,000 dpm) in 1 ml of PBS containing 10 mM CaCl2. The adherence mixtures (in duplicate) were incubated for up to 4 h in a 5% CO2 atmosphere. The nonadhering mycoplasmas were removed by washing the HeLa cells three times with 1 ml of PBS. The washed HeLa cells were trypsinized for 3 to 4 min and resuspended in PBS, aliquots were transferred to scintillation vials containing scintillation liquid, and radioactivity was counted. To determine the effects of Pg and uPA on the adherence, the native M. fermentans preparation in the adherence reaction mixture was replaced with M. fermentans that was preincubated for 1 h at 37°C with Pg (25 μg/ml) with or without uPA (100 U/ml). To test the effect of proteolysis on the adherence, intact M. fermentans cells (1 mg of cell protein/ml, 1011 CFU/ml) were treated for 15 to 60 min at 37°C with trypsin (25 μg/ml) or proteinase K (10 μg/ml). The proteolytic activity was stopped by adding trypsin inhibitor (50 μg/ml; Sigma) or by intensive washing with cold A buffer.
In vitro internalization assay.
Internalization of mycoplasmas was quantified by a standard antibiotic protection assay (2, 40) and by confocal laser scanning microscopy of immunofluorescent preparations. For immunofluorescence staining, M. fermentans-infected HeLa cells grown on coverslips were fixed at room temperature for 10 min with 4% formaldehyde in PBS. After three washes with PBS, the cells were permeabilized by incubation for 3 min with 0.2% Triton X-100 in PBS containing 1% bovine serum albumin (PBS-BSA), washed twice with PBS, and blocked by overnight incubation with 2% horse serum. The cultures were then overlaid for 60 min at room temperature with rabbit polyclonal anti-M. fermentans MfGL-II antiserum (4) diluted 1:100 in PBS-BSA. Nonbound antibody was removed by dipping the coverslips three times in PBS, and the cells were then incubated for 1 h at room temperature with goat anti-rabbit fluorescein isothiocyanate-conjugated immunoglobulin G serum (Jackson) diluted 1:150 in PBS-BSA. The coverslips were rinsed four times with PBS and mounted in a solution containing 90% glycerol, 7% PBS, 3% 1,4-diazabicyclo-(2,2)-octane as an antifading agent, and 0.1% sodium azide. The specificity of immunostaining was evaluated by omitting the antimycoplasma antibodies or by utilizing nonspecific antibodies (nonimmune rabbit serum). Immunofluorescent samples were analyzed by using a Zeiss 410 laser scanning confocal microscope (Zeiss, Oberkochen, Germany) equipped with an argon ion laser tuned at 488 nm.
Analytical methods.
The total protein content was determined as described by Bradford (8). Qualitative determination of Pg binding to mycoplasmas was performed by immunodot blotting (41). To assess the number and viability of HeLa cells, the cells were detached from the wells by EDTA-trypsin treatment, suspended in a 0.15% trypan blue solution, and analyzed microscopically. Pg was purified from human plasma as described by Deutsch and Mertz (12). Pg was activated as previously described (40) in a reaction mixture containing 1 mg of M. fermentans protein, 25 μg of Pg, and 100 U of uPA (American Diagnostic Inc.). For cholesterol depletion, HeLa cells were treated with methyl-β-cyclodextrine (MeCD) (Sigma) as previously described (17, 21). Lipids were extracted from HeLa cells by the method of Bligh and Dyer (6). Unesterified cholesterol and cholesterol esters were separated on Merck Silica Gel G glass plates and developed at room temperature with benzene-ethyl acetate (5:1, vol/vol). The unesterified cholesterol spot was extracted with chloroform at room temperature for 30 min, and the sterol content was determined by the phthaldialdehyde method (30). Radioactivity was determined with a scintillation spectrometer by using Opti-Flour scintillation liquor (Packard).
RESULTS AND DISCUSSION
Adherence of M. fermentans to HeLa cells.
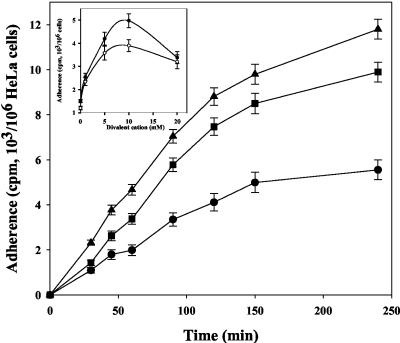
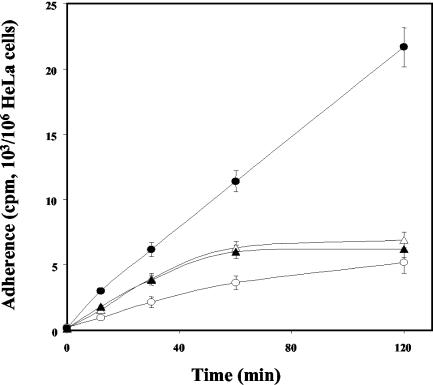
The adherence of M. fermentans to the HeLa cell line was measured as a function of time and temperature (Fig. 1). The extent of binding was calculated from the amount of radioactivity associated with the host cells after incubation for various periods of time. Binding was observed within 15 min of incubation and increased linearly during the next 120 min. Binding was significantly reduced at 4°C and reached about 50% of the binding measured at 37°C. Figure 1 shows that divalent cations had a marked influence on binding. Both Mg2+ and Ca2+ were effective, and the highest binding levels were observed at concentrations of 5 to 10 mM. Binding was almost the same with M. fermentans cells harvested at the early exponential (A640, 0.08; pH 7.2), late exponential (A640, 0.23; pH 6.4), and stationary (A640, 0.25; pH 6.0) phases of growth (data not shown). Pretreatment of M. fermentans with 4% paraformaldehyde for 15 min at 37°C reduced the binding to HeLa cells by only 15% ± 5%. However, the binding of native M. fermentans to paraformaldehyde-treated HeLa cells was 85% ± 5% lower than the binding to native HeLa cells. The ability to adhere was not influenced by pretreating M. fermentans for 15 min at 37°C with the uncoupler carbonyl cyanide m-chlorophenylhydrazone (5 μM), the ATPase inhibitor dicyclohexylcarbodiimide (20 μM), or the K+ ionophore valinomycin (5 μM) with or without 100 mM KCl, suggesting that adherence is not affected by the proton or electrochemical gradients across the M. fermentans membrane. Binding was not affected by pretreating HeLa cells for 30 min at 37°C with vinblastine, cytochalasine B, or taxol (5 μg of each per ml), suggesting that cytoskeletal components, including microtubules and microfilaments, do not play a role in the adherence process.
FIG. 1.
Adherence of M. fermentans strain PG-18 to HeLa cells as a function of time and temperature. Radiolabeled microorganisms were incubated with HeLa cells in the adherence assay mixture for up to 4 h at 37°C (▴), 22°C (▪), or 4°C (•), and binding was determined as described in Materials and Methods. The data are means ± standard deviations of three independent experiments. (Inset) Effect of divalent cations on binding. Symbols: (○), MgCl2; (•), CaCl2.
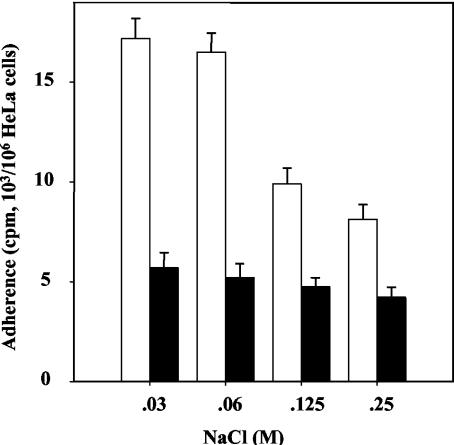
Preincubation of M. fermentans in an isosmotic NaCl solution (0.25 M NaCl) containing 10 mM CaCl2 at 37°C for 1 to 2 h resulted in increased competence for adherence (Fig. 2). The same level of competence was observed in the presence or absence of glucose (10 mM) and was not affected by adding chloramphenicol (100 μg/ml), carbonyl cyanide m-chlorophenylhydrazone (5 μM), dicyclohexylcarbodiimide (20 μM), or valinomycin (5 μM) to the incubation buffer (data not shown). These results suggest that neither the energetic state of M. fermentans nor the proton and electrochemical gradients across the cell membrane nor protein synthesis plays a role in increasing the competence of M. fermentans for adherence. The increased competence was much more pronounced when preincubation was performed in solutions having lower osmolarities (Fig. 2). As the wall-less mycoplasmas swell readily in solutions having low osmolarities (26), it can be proposed that stretching of the cell membrane upon cell swelling activates the adhesins either by improving the exposure of their active epitope(s) on the cell surface or by inducing conformational changes. The exposure to low osmolarities had no effect on the integrity and viability of M. fermentans, as indicated by plating experiments (data not shown).
FIG. 2.
Effect of preincubation of M. fermentans on binding to HeLa cells. Binding was performed as described in Materials and Methods by using microorganisms preincubated for 1 h at 37°C (open bars) in adherence reaction mixtures containing various concentrations of NaCl. Solid bars, control without preincubation. The data are means ± standards deviations of three to five independent experiments.
Pretreatment of intact M. fermentans cells with trypsin and proteinase K to remove surface proteins reduced the binding to HeLa cells by 60% ± 8% and 75% ± 10%, respectively. The proteolytic treatments did not affect the intracellular NADH dehydrogenase activity of M. fermentans; thus, proteolysis apparently did not affect cell intactness. These results suggest that although a protein is the major ligand on the cell surface of M. fermentans, mediating the binding to HeLa cells, a nonproteinaceous surface determinant is associated with the binding as well.
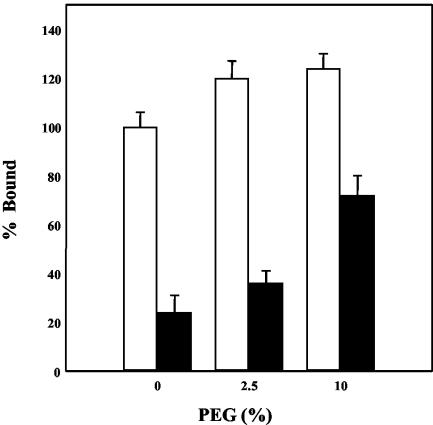
Figure 3 shows that in the presence of 10% polyethylene glycol 8000 (PEG), binding of native M. fermentans to HeLa cells was only slightly increased, whereas a two- to threefold increase in binding was observed with trypsin-treated M. fermentans preparations. It has been proposed that PEG functions as a dehydrating agent (23). This molecule alters the physical state of bulk water adjacent to the cell surface and the water of hydration of the polar groups of membrane phospho- and glycolipids, allowing more intimate contact between the two participating membranes (23). The more pronounced PEG effect on binding of the trypsin-treated mycoplasmas suggests that PEG is required for the binding of the protease-insensitive component of the adherence system. However, with native M. fermentans, binding to HeLa cells was only slightly affected by PEG (Fig. 3), suggesting that for adherence through the protease-sensitive component of the adherence system the proximity between the cell surface of HeLa cells and the cell membrane of M. fermentans is achieved even without PEG.
FIG. 3.
Stimulation of M. fermentans adherence by PEG. The binding of native (open bars) and trypsin-treated (solid bars) M. fermentans strain PG-18 to HeLa cells was determined in an adherence assay mixture containing various concentrations of PEG as described in Materials and Methods. The data are means ± standard deviations of five independent experiments.
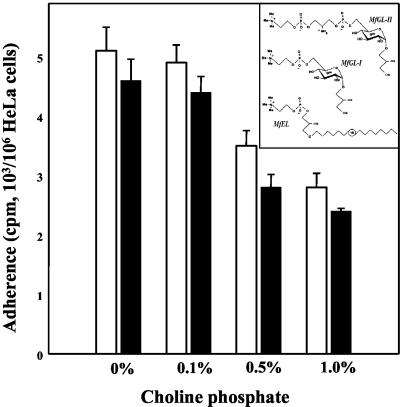
To identify possible receptor molecules on the cell surface of HeLa cells, simple carbohydrates were tested for adherence inhibition activity. The carbohydrates tested were d-glucose, α-methyl-d-glucoside, d-glucosamine, N-acetylglucosamine, d-mannose, d-mannitol, α-methyl-d-mannoside, d-galactose, α-methyl-d-galactoside, l-arabinose, d-galacturonic acid, xylose, rhamnose, maltose, raffinose, sucrose, lactose, inositol, ribitol, sorbitol, and dulcitol (all products of Sigma). All carbohydrates were tested in the adherence assay at a concentration of 1%. None of the carbohydrates tested had inhibitory activity. Nevertheless, adherence of M. fermentans to HeLa cells was significantly inhibited by 0.5 to 1.0% phosphocholine (Fig. 4), whereas free choline had no effect (data not shown). Phosphocholine-containing lipids (Fig. 4, inset) were detected in all M. fermentans strains tested (5). However, whereas type strain PG-18 contains almost exclusively MfGL-II (42), the respiratory strains (e.g., strain M-52) contain no MfGL-II but contain mainly MfGL-I and a phosphocholine-containing ether lipid (MfEL). Our data showing that the inhibitory effect of phosphocholine observed with strain PG-18 was almost identical to the inhibitory effect observed with the respiratory isolate (strain M-52) suggest that in addition to MfGL-II (4) other phosphocholine-containing lipids play a role in the M. fermentans-host cell interaction. The adherence inhibition induced by phosphocholine was much more pronounced when trypsin-treated M. fermentans was used in the adherence assay (90% ± 6% of inhibition obtained with 1% phosphocholine). Furthermore, antiserum against the phosphocholine-containing glycoglycerolipid MfGL-II inhibited the adherence of M. fermentans PG-18 to HeLa cells in vitro (data not shown), supporting preliminary data presented previously (4). As the anti MfGL-II repertoire is composed primarily of anti-phosphocholine antibodies (4), the data suggest that phosphocholine-containing glycolipids decorating the surface of M. fermentans play an important role in the adherence of M. fermentans to HeLa cells.
FIG. 4.
Inhibition of M. fermentans adherence by phosphocholine. Binding of M. fermentans strain PG-18 (open bars) or strain M52 (solid bars) to HeLa cells was determined in an adherence assay mixture containing various concentrations of phosphocholine as described in Materials and Methods. The data are means ± standard deviations of three independent experiments. (Inset) Major phosphocholine-containing lipids of M. fermentans.
The ability to adhere to eukaryotic cells is characteristic of all pathogenic Mycoplasma species, and in some of them expression of surface proteins that mediate the binding to host cells has been demonstrated (20, 37). The data reported here suggest that there is a second surface anchor mechanism in M. fermentans. By this mechanism M. fermentans is held at the host cell surface through the interaction of the phosphocholine-containing glycolipid of M. fermentans (5, 42) and a surface protein of the host cells. This conclusion is based on the following observations: (i) adherence was only partially inhibited by proteolysis; (ii) antibodies to the phosphocholine moiety of the surface glycolipid MfGL-II inhibited adherence; and (iii) adherence was inhibited by phosphocholine. It seems that M. fermentans utilizes at least two surface components for adhesion to HeLa cells, a protease-sensitive surface protein, apparently the lipoprotein recently described (37), and a phosphocholine-containing glycolipid. Involvement of choline-containing teichoic acid or lipoteichoic acid in the adhesion of streptococci has been described previously (29).
Pg binding mediates adherence and internalization.
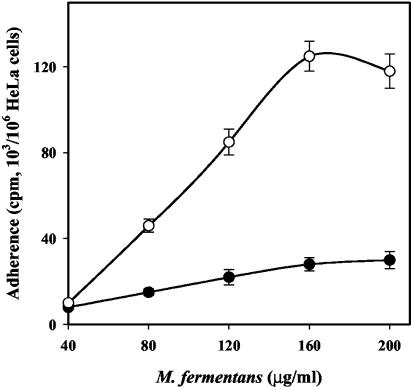
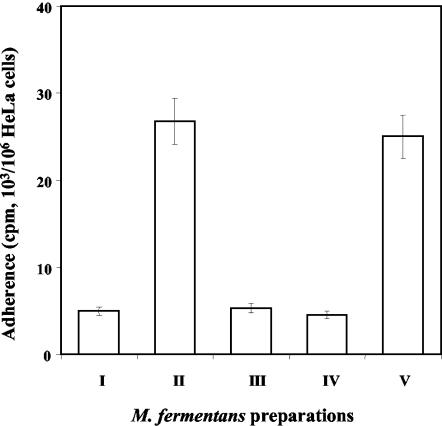
Figure 5 shows that Pg markedly increased the adherence of M. fermentans but had no effect on the adherence of M. pneumoniae to HeLa cells. Pg increased the maximal adherence of M. fermentans, which strongly suggests that in the presence of Pg, M. fermentans adheres to novel sites on the HeLa cells. In other words, the presence of Pg creates a novel epitope on the HeLa cells capable of binding M. fermentans. Figure 6 shows that in the presence of Pg, adherence of M. fermentans was dose dependent and saturable, two kinetic properties that argue for the specificity of the adherence in the presence of Pg. Furthermore, addition of exogenous Pg or anti-Pg antibodies caused substantial inhibition of the adherence (data not shown). Whereas native Pg had a pronounced effect on M. fermentans adherence, the Pg fragment containing the first three kringles (Pg site 1) or the fragment containing kringle 4 (Pg site 2) had no effect on the adherence process (Fig. 7), although these fragments were shown to bind to M. fermentans to about the same extent as native Pg (40). These findings suggest that the C-terminal domain of the Pg molecule, rather than the loop structures (kringles) located at the NH2 terminus, mediates the interaction of Pg-treated M. fermentans with HeLa cells.
FIG. 5.
Effect of Pg on adherence. Binding of Pg to M. fermentans or M. pneumoniae cells and adherence of these organisms to HeLa cells were evaluated as described in Materials and Methods. ○ and •, M. fermentans; ▵ and ▾, M. pneumoniae; open symbols, native mycoplasmas; solid symbols, Pg-bound mycoplasmas. The data are means ± standard deviations of three independent experiments.
FIG. 6.
Dose-dependent adherence of M. fermentans strain PG-18 to HeLa cells. HeLa cells (106 cells/well) were incubated with different amounts of native M. fermentans (•) or Pg-bound M. fermentans (○) for 2 h at 37°C, and adherence was assayed as described in Materials and Methods. The data are means ± standard deviations of five independent experiments.
FIG. 7.
Effect of Pg fragments on the adherence of M. fermentans to HeLa cells. Adherence to HeLa cells of native M. fermentans (I), Pg-bound M. fermentans (II), Pg site 1-bound M. fermentans (III), Pg site 2-bound M. fermentans (IV), and uPa-activated Pg-bound M. fermentans (V) was performed as described in Materials and Methods.
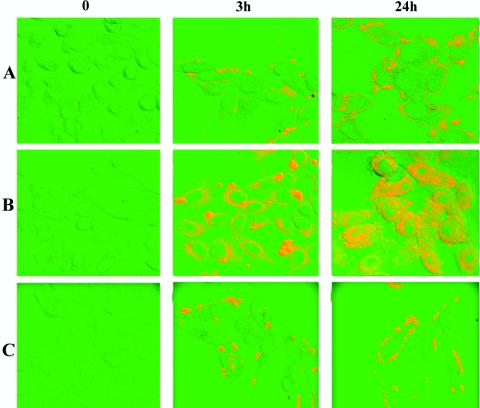
Although M. fermentans is a parasite associated with the cell surface of host cells (26), it was shown recently that under certain conditions this organism has also been detected within cells (40). The intracellular location of M. fermentans in HeLa cells was shown by the antibiotic protection assay (Table 1) by utilizing gentamicin to kill mycoplasmas that did not enter the host cells (1). When native M. fermentans was incubated with HeLa cells, no internalization was observed. However, when M. fermentans was preincubated with Pg and the Pg activator uPA, intracellularly located microorganisms were detected as early as 3 h postinfection. Up to 24 h postinfection the intracellular M. fermentans did not cause obvious damage to the host cells, as determined by the trypan blue viability assay, but as the infection proceeded, the number of intracellular organisms increased, and at a late stage of infection (>24 h) host cell integrity was disrupted and cell death followed (data not shown). Since Pg facilitates the adherence to HeLa cells and the formation of small clusters or microcolonies on the cell surface of the host cells (data not shown), it is possible that the escape from the killing effect of gentamicin in the antibiotic protection assay may have been due to aggregation and intimate adherence rather than internalization. To further establish the notion that M. fermentans does invade HeLa cells, immunofluorescence staining followed by analysis with a confocal laser scanning microscope was performed. HeLa cells were infected by uPA-treated Pg-bound M. fermentans, fixed with formaldehyde, permeabilized by treatment with Triton X-100, and incubated with anti-M. fermentans antibodies and then with a second fluorescein isothiocyanate-conjugated antibody. To obtain three-dimensional information on the cellular location of the fluorescence, a series of optical sections were made through infected HeLa cells. The results clearly demonstrated that in HeLa cells infected with the uPA-treated Pg-bound M. fermentans, there were both surface and intracellular foci of fluorescence corresponding to intracellular and extracellular mycoplasmas (Fig. 8B). Internalization was not detected in control HeLa cells infected with native M. fermentans, in which numerous foci of fluorescence corresponding to extracellular bound mycoplasmas were seen (Fig. 8A). Although M. pneumoniae binds Pg to the same extent as M. fermentans, as determined by the immunoblot assay (41), and the Pg-bound M. pneumoniae was activated to plasmin by uPA in a manner similar to the manner observed with Pg-bound M. fermentans (41), no internalization was detected with HeLa cells infected with uPA-treated Pg-bound M. pneumoniae (Fig. 8C). The amounts of intracellularly located M. fermentans increased during the first 24 h of infection, and internalization was temperature dependent. Thus, at 4°C fluorescence was predominantly associated with the surface of the host cells (data not shown). Our finding that Pg, which acts as an adherence factor, also mediates internalization by eukaryotic cells is not unique. The Inv, YadA, and Ail surface proteins of Yersinia enterocolitica (24) and AfaE of uropathogenic and diarrhea-associated Escherichia coli strains (19) have been shown to mediate both adhesion and internalization.
TABLE 1.
Effects of Pg and uPA on M. fermentans adherence to and invasion of HeLa cellsa
| M. fermentans prepn | Adherence to HeLa cells (cpm/106 HeLa cells) | Invasion of HeLa cells (CFU/106 cells) |
|---|---|---|
| Native | 2,450 ± 250 | 250 ± 150 |
| Pg treated | 9,800 ± 550 | 560 ± 250 |
| Pg and uPA treated | 9,200 ± 600 | 4,500 ± 1,100 |
M. fermentans was treated with 25 μg of Pg per ml or with Pg (25 μg/ml) and uPA (100 u/ml) for 30 min at 37°C. HeLa cells were then infected with the M. fermentans preparations at a multiplicity of infection of 100, and invasion was determined after 3 h of incubation at 37°C
FIG. 8.
Confocal micrographs demonstrating binding and internalization of M. fermentans and M. pneumoniae by HeLa cells. Following infection of HeLa cells with mycoplasmas for 0, 3, and 24 h, the cells were washed, fixed, and immunostained as described in Materials and Methods. Row A, HeLa cells infected with native M. fermentans; row B, HeLa cells infected with Pg- and uPA-treated M. fermentans; row C, HeLa cells infected with Pg- and uPA-treated M. pneumoniae.
Bacterial invasion of eukaryotic cells is a complex process that involves a variety of bacterial and host cell factors. It has been shown recently that bacterial invasion is based on the abilities of several bacteria to bind sulfated polysaccharides (14) or fibronectin (15). It was suggested that these compounds form a molecular bridge between the bacteria and eukaryotic surface proteins (9, 14) that enables invasion. The finding that in the presence of uPA internalization was apparent indicates that the ability of M. fermentans to invade host cell stems from its potential to bind Pg and to activate it to plasmin, a protease with broad substrate specificity. Pg and uPA are two proteins that play an important role in the invasion of several human malignant tumors (25); therefore, it is not surprising that the same system stimulates M. fermentans invasion. Our observation that M. pneumoniae cells pretreated with Pg and uPA are not internalized by HeLa cells (Fig. 8) (41), although this organism binds Pg to the same extent as M. fermentans (40), suggests that the role of Pg binding and activation is not solely to increase the proteolytic activity. It is possible that plasmin alters M. fermentans cell surface proteins and thereby enables internalization. The enhanced invasiveness of plasmin-coated Borrelia burgdorferi was described recently (10). Proteolytic modification of a bacterial and/or host cell surface protein(s) is an emerging theme among bacterial pathogens. Thus, the Pg activator of Yersinia pestis modifies specific bacterial outer membrane proteins and is associated with virulence (36), and a secreted protease has been shown to stimulate the fibronectin-dependent uptake of Streptococcus pyogenes into eukaryotic cells (9).
Membrane cholesterol regulates internalization.
To evaluate the importance of membrane cholesterol in the internalization process, we assessed the effects of MeCD and filipin on adherence and internalization of M. fermentans pretreated with Pg and uPA. MeCD has been reported to extract cholesterol from the membrane, whereas filipin, a polyene antibiotic from Saccharomyces filipinensis, sequesters cholesterol by forming multimeric globular complexes with it (16). Cholesterol depletion and sequestration by MeCD and filipin, respectively, result in the disruption of raft microdomains (21, 30). For cholesterol depletion cells were treated with 10 mM MeCD for 1 h at 37°C in Dulbecco modified Eagle medium without fetal calf serum. MeCD at this concentration did not cause cell death, as determined by trypan blue staining. Table 2 shows that after 1 h of treatment with MeCD the unesterified cholesterol content of HeLa cells dropped by over 70%. Cholesterol depletion had little effect on the binding of M. fermentans to HeLa cells but almost completely inhibited the internalization of M. fermentans pretreated with Pg and uPA. When filipin was used (1 μg/ml), the results were similar to the results obtained with MeCD (Table 2).
TABLE 2.
Effect of cholesterol depletion or sequestration on M. fermentans adherence to and invasion of HeLa cellsa
| HeLa cell treatment | Cholesterol concn (μg/107 HeLa cells)b | Adherence (cpm/106 HeLa cells) afterc
|
Invasion (CFU/106 HeLa cells) afterd
|
||
|---|---|---|---|---|---|
| 4 h | 24 h | 4 h | 24 h | ||
| Untreated | 22.4 | 5,180 ± 250 | 7,410 ± 550 | 5.6 × 103 | 8.0 × 104 |
| MeCD | 7.6 | 4,330 ± 180 | 6,028 ± 300 | 5.7 × 102 | 1 × 102 |
| Filipin | ND | 5,030 ± 250 | 7,170 ± 800 | NDe | 1 × 102 |
Hela cells were treated with 10 mM MeCD or 1 μg of fillipin per ml for 1 h at 37°C. M. fermentans preparations were treated with Pg and the Pg activator uPA.
Unesterified cholesterol was used.
The results are means ± standard deviations of three independent experiments.
Invasion was determined by the gentamicin protection assay.
ND, not determined.
Lipid rafts are specialized lipid microdomains enriched in glycosphingolipids, cholesterol, and glycosylphosphatidylinositol-anchored proteins. It is generally believed that cholesterol depletion or sequestration disrupts lipid rafts (35). These microdomains are most abundant at the plasma membrane, and they are involved in many important cellular processes, such as generation and maintenance of cellular polarity, chemotactic migration, and receptor signaling (19, 35). Our observation that internalization is reduced after cholesterol extraction but that binding is not impaired suggests that M. fermnetans binds to HeLa cells in a raft-independent manner but then triggers patching of raft domains and de novo caveole formation around the microorganism, a process required for internalization. The internalization of several viruses and bacteria has been shown to be cholesterol dependent (1, 16, 21, 27), and it has been suggested that interfering with raft assembly by cholesterol extraction may affect cell signaling or destroy the integrity of the actin network structure required for internalization (24, 27, 30).
Editor: V. J. DiRita
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreev, J., Z. Borovsky, I. Rosenshine, and S. Rottem. 1995. Invasion of HeLa cells by Mycoplasma penetrans and the induction of tyrosine phosphorylation of a 145-kDa host cell protein. FEMS Microbiol. Lett. 132:189-194. [DOI] [PubMed] [Google Scholar]
- 3.Baseman, J. B., M. Lange, N. L. Criscimagna, J. A. Giron, and C. A. Thomas. 1995. Interplay between mycoplasmas and host target cells. Microb. Pathog. 19:105-116. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Menachem, G., F. Wagner, U. Zähringer, E. T. Rietschel, and S. Rottem. 1997. Antibody response to MfGL-II, a phosphocholine containing major lipid of Mycoplasma fermentans membranes. FEMS Lett. 154:363-369. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Menachem, G., U. Zähringer, and S. Rottem. 2001. The phosphocholine motif in the cell membrane of M. fermentans strains. FEMS Microbiol. Lett. 199:137-141. [DOI] [PubMed] [Google Scholar]
- 6.Bligh, E. G., and W. J. Dyer. 1969. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 7.Bower, K., S. P. Djordjevic, N. M. Andronicos, and M. Ranson. 2003. Cell surface antigens of Mycoplasma species bovine group 7 bind to and activate plasminogen. Infect. Immun. 71:4823-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Chaussee, M. S., R. L. Cole, and J. P. M. van Putten. 2000. Streptococcal erythrogenic toxin B abrogates fibronectin-dependent internalization of Streptococcus pyogenes by cultured mammalian cells. Infect. Immun. 68:3226-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 67:3929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallo, S. F., A. Chavoya, and J. B. Baseman. 1990. Characterization of the gene for a 30-kDa adhesin-related protein of Mycoplasma pneumoniae. Infect. Immun. 58:4163-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutsch, D. G., and E. T. Mertz. 1970. Plasminogen purification from human plasma by affinity chromatography. Science 170:1095-1096. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch, J., M. Salman, and S. Rottem. 1995. An unusual polar lipid from the cell membrane of Mycoplasma fermentans. Eur. J. Biochem. 227:897-902. [DOI] [PubMed] [Google Scholar]
- 14.Duensing, T. D., J. S. Wing, and J. P. M. van Putten. 1999. Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect. Immun. 67:4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziewanowska, K., J. M. Platt, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fivaz, M., L. Abrami, and F. G. van der Goot. 1999. Pathogens, toxins and lipid rafts. Protoplasma 212:8-14. [DOI] [PubMed] [Google Scholar]
- 17.Ha, H., H. B. Kwak, S. K. Lee, D. S. Na, C. E. Rudd, Z. H. Lee, and H. H. Kim. 2003. Membrane rafts play a crucial role in receptor activator of nuclear factor kappa B signaling and osteoclast function. J. Biol. Chem. 278:18573-18580. [DOI] [PubMed] [Google Scholar]
- 18.Inamine, J. M., T. P. Denny, S. Loechel, U. Schaper, C. H. Huang, K. F. Bott, and P. C. Hu. 1988. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene 64:229. [DOI] [PubMed] [Google Scholar]
- 19.Jouve, M., M. I. Garcia, P. Courcoux, A. Labigne, P. Gounon, and C. Le Bouguénec. 1997. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the alpha-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 65:4082-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause, D. C. 1996. Mycoplasma pneumoniae cytadherence: unravelling the tie that binds. Mol. Microbiol. 20:247-253. [DOI] [PubMed] [Google Scholar]
- 21.Lafont, F., G. T. Van Nhieu, K. Hanada, P. Sansonetti, and F. G. van der Goot. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo, S. C. 1992. Mycoplasma and AIDS, p. 525-548. In J. Maniloff, R. N. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. ASM Press, Washington, D.C.
- 23.Lucy, J. A., and Q. F. Ahkong. 1989. Mermbrane fusion: fusogenic agents and osmotic forces, p. 189-228. In J. R. Harris and A. H. Etemedi (ed.), Subcellular biochemistry. Plenum Press, New York, N.Y. [PubMed]
- 24.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 56:1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao, J. S. 2003. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev. Cancer 3:489-501. [DOI] [PubMed] [Google Scholar]
- 26.Razin, S., and E. Jacobs. 1992. Mycoplasma adhesion. J. Gen. Microbiol. 138:407-422. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberger, C. M., J. H. Brumell, and B. B. Finlay. 2000. Microbial pathogenesis: lipid rafts as pathogen portals. Curr. Biol. 10:823-825. [DOI] [PubMed] [Google Scholar]
- 28.Rosengarten, R., C. Citti, M. Glew, A. Lischewski, M. Droesse, P. Much, F. Winner, M. Brank, and J. Spergser. 2000. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 290:15-25. [DOI] [PubMed] [Google Scholar]
- 29.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1977. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 30.Rottem, S. 1983. Characterization of membrane lipids, p. 269-275. In S. Razin and J. G., Tully (ed.), Methods in mycoplasmology. Academic Press, London, United Kingdom.
- 31.Rottem, S. 2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83:417-432. [DOI] [PubMed] [Google Scholar]
- 32.Rottem, S., and Y. Naot. 1998. Subversion and exploitation of host cells by mycoplasmas. Trends Microbiol. 6:436-440. [DOI] [PubMed] [Google Scholar]
- 33.Ruiter, M., and H. M. M. Wentholt. 1952. The occurrence of a pleuropneumonia-like organism in the fuso-spirillary infections of the human genital mucosa. J. Investig. Dermatol. 18:313-323. [DOI] [PubMed] [Google Scholar]
- 34.Saksela, O., and D. P. Rifkin. 1988. Cell-associated plasminogen activation: regulation and physiological functions. Annu. Rev. Cell Biol. 4:93-126. [DOI] [PubMed] [Google Scholar]
- 35.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 36.Sodeinde, O. A., Y. V. B. K. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 37.Spencer, A. L., and K. S. Wise. 2002. Identification and functional mapping of the Mycoplasma fermentans P29 adhesin. Infect. Immun. 70:4925-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtlander, C. T., H. L. Watson, J. W., Simecka, and G. H. Cassell. 1993. Cytopathogenicity of Mycoplasma fermentans (including strain incognitos). Clin. Infect. Dis. 17:289-301. [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Robinson, D., H. A. Davies, P. Sarathchandra, and P. M. Furr. 1991. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int. J. Exp. Pathol. 72:705-714. [PMC free article] [PubMed] [Google Scholar]
- 40.Yavlovich. A., A. R. Higazi, and S. Rottem. 2001. Binding and activation of plasminogen by M. fermentans. Infect. Immun. 69:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yavlovich, A., M. Tarshis, and S. Rottem. 2003. Internalization and intracellular survival of Mycoplasma pneumoniae by non-phagocytic cells. FEMS Microbiol. Lett. 233:241-246. [DOI] [PubMed] [Google Scholar]
- 42.Zähringer, U., F. Wagner, E. T. Rietschel, G. Ben-Menachem, J. Deutsch, and S. Rottem. 1997. Primary structure of a new phosphocholine-containing glycoglycerolipid of Mycoplasma fermentans. J. Biol. Chem. 272:26262-26270. [DOI] [PubMed] [Google Scholar]