Xanthine oxidoreductase (XOR) is widely distributed in mammalian tissues and has long been known to be a major constituent of the milk fat globule membrane (MFGM), which surrounds fat globules in cow's milk (36, 50). This source has been exploited for isolation and characterization of the bovine enzyme for many decades (44). XOR is a complex enzyme comprising two identical 147,000-Mr subunits, each of which contains one molybdenum, one flavin adenine dinucleotide, and two nonidentical iron-sulfur redox centers (8, 32, 33). While the enzymology of XOR is well documented, its physiological role is unclear. The enzyme occurs in most mammalian tissues, and although it has a broad specificity for reducing substrates, its conventionally accepted role is in purine catabolism, catalyzing the oxidation of hypoxanthine to xanthine and the oxidation of xanthine to uric acid. Despite its wide tissue distribution, the enzyme is believed to be largely concentrated in endothelial and epithelial cells. Such a specific cellular location implies a physiological role apart from that of a simple housekeeping enzyme, and other functions have been sought. Indeed, the role of XOR in milk has long been a puzzle.
During the last 20 years, attention has been focused on the ability of the enzyme to generate reactive oxygen species (ROS). Mammalian XOR exists in two interconvertible forms, xanthine dehydrogenase (XDH) (EC 1.1.1.204), which predominates in vivo, and xanthine oxidase (XO) (EC 1.1.3.22). Both forms of the enzyme reduce molecular oxygen, although only XDH can reduce NAD+, which is its preferred electron acceptor.
Reduction of oxygen generates the ROS, superoxide anion, and hydrogen peroxide, and because of this, XOR has been implicated as a destructive agent, particularly in many forms of ischemia-reperfusion (IR) injury (27, 32, 46). Apart from a pathological context, ROS are increasingly recognized as key components in normal signal transduction pathways (20, 21, 29), and XOR is often a potential source of such species. The cytotoxic properties of ROS can also be beneficial, and an antimicrobial role for bovine milk XOR has been considered for many years. As early as 1943, two groups independently reported the antibacterial activity of purified milk enzyme and attributed this activity to XOR-catalyzed production of hydrogen peroxide (28, 42). Subsequently, the enzyme has been linked to infection in many diverse instances, and increased levels of expression have been demonstrated in both infected experimental animals (3, 16, 18, 62-64) and humans (32, 43, 60). Moreover, a protective role of XOR is indicated by observations that specific inhibition of XOR can increase microbial activity (14, 52, 58, 61, 64). Other than the general presumption of involvement of XOR-generated ROS (or urate), most publications have not considered the precise role of the enzyme in the various infected states. This question is addressed below.
XOR AND THE INFLAMMATORY RESPONSE
In recent years, a key role for XOR in the inflammatory response has been proposed (12, 13, 46). This concept follows from the hypothesis involving the enzyme in IR injury, which was first put forward by Granger et al. in 1981 (26). IR injury is cell death that commonly results from interruption of blood flow to specific tissue, such as that which occurs in myocardial infarction or stroke. On the basis of their studies of feline intestinal ischemia, Granger and colleagues proposed the following sequence of events. In the course of ischemia, the energy status of the cell falls, transmembrane ion gradients break down, and the levels of intracellular calcium increase. A consequence of this is activation of a calcium-dependent protease that irreversibly converts XDH, which is the predominant form in vivo, into the oxidase form, XO. Concurrently, cellular ATP is catabolized to hypoxanthine, which accumulates. On reperfusion, oxygen again becomes available and is reduced by the hypoxanthine-XO system, generating superoxide and hydrogen peroxide. These ROS then interact to generate hydroxyl radicals with consequent oxidation of biological molecules, including proteins, lipids, and nucleic acids, and tissue injury results. The publication of this hypothesis provoked many hundreds of further studies, in which workers investigated its relevance in a range of tissues and species. Over the years, following extensive discussions (32, 46), the proposed pathogenic mechanisms have been modified and extended, so that XO-generated ROS are envisaged not so much as primary destructive agents but as initiators of neutrophil infiltration. Neutrophils contain NADPH oxidase, another enzyme that catalyzes the reduction of molecular oxygen to superoxide anions. Superoxide rapidly dismutates to hydrogen peroxide, which combines with the former anion to generate the hydroxyl radical, as noted above. Neutrophils also contain the enzyme myeloperoxidase, which catalyzes the interaction of hydrogen peroxide and ambient chloride ions to give the potent oxidizing and chlorinating agent HOCl. The combination of these agents results in tissue damage (15).
Bulkley (12, 13) and Meneshian and Bulkley (46) made the point that clinically significant reperfusion is not a natural event and that IR injury most probably represents an aberrant response to a normal physiological process in the vascular endothelium, namely, inflammation. These workers proposed that in response to infection, cytokines or other proinflammatory mediators initiate XDH-to-XO conversion, generation of ROS, and neutrophil infiltration, as outlined above. The neutrophils then act to combat the infectious agents.
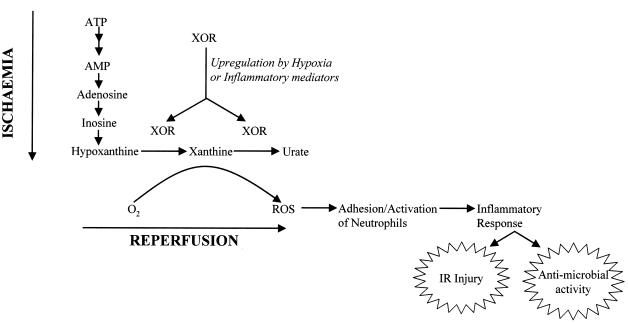
Although central to the original hypothesis of IR injury, the occurrence of significant XDH-to-XO conversion in relevant tissues has been disputed (32, 46). In fact, this conversion is not strictly essential either to this hypothesis or to the proposed mechanism of the inflammatory response. XOR as a whole is subject to upregulation both by hypoxia and by a range of proinflammatory agents (32). Moreover, generation of ROS is not confined to XO. XDH also reduces molecular oxygen in the presence of hypoxanthine, albeit not as efficiently as XO (55). Additionally, both forms of the enzyme show NADH oxidase activity, again with generation of ROS, and XDH is somewhat more effective in this respect (57). Accordingly, simple upregulation of XOR activity, irrespective of XDH/XO ratios, could well be triggered either by hypoxia or by proinflammatory agents and would serve to initiate neutrophil infiltration, as described above (Fig. 1).
FIG. 1.
Mechanisms by which XOR-derived ROS might initiate either the inflammatory response or IR injury. XOR (XDH or XO) is upregulated by either inflammatory mediators or hypoxia and, in the presence of molecular oxygen, reduces the oxygen to ROS. ROS, in turn, upregulate cell adhesion molecules on endothelial cells and neutrophils, leading to adhesion and activation of these cells. The ensuing inflammatory response constitutes a normal antimicrobial defense, but it can also result in IR injury. Adapted from reference 46 with permission of the publisher.
It may be that XOR is also involved in the antimicrobial activity of neutrophils themselves. Thus, in addition to NADPH oxidase, polymorphonuclear leukocytes and macrophages have been shown to contain XOR (52, 61, 62), which can be linked to the response of these cells to microbial infection.
XOR IN THE GASTROINTESTINAL TRACT
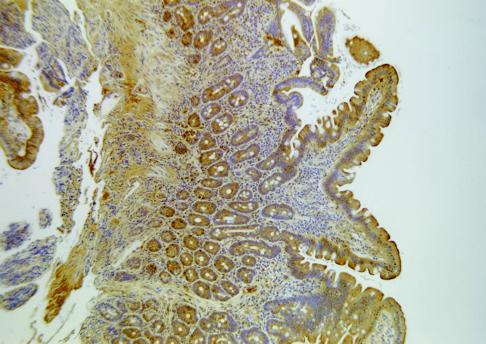
Expression of XOR is high in intestinal epithelial cells, where a barrier, microbicidal role has been proposed. The enzyme is particularly abundant in the first part of the digestive tract, and it is found in goblet cells and enterocytes of the small intestine, especially in the basal and apical layers (37, 38, 40, 41, 48) (Fig. 2). It is also present in epithelial Paneth cells, which are recognized as cells that play an antimicrobial defensive role (47). In ultrastructural studies of the rat digestive tract, Van den Munckhof et al. (65) detected XOR activity in enterocytes, in goblet cells, and in the mucus of the duodenum. The enzyme was also present in the apical cell layers of epithelia of the esophagus and tongue. Bacteria, apparently in the process of being destroyed, were clearly seen to be surrounded by XOR in the cornified layer. These authors proposed that XOR has an antimicrobial function in the gut resulting from the generation of ROS, superoxide, and hydrogen peroxide. Now, recent findings have added a new dimension to discussions of such a role.
FIG. 2.
Localization of XOR in the human small intestine. There is positive staining for anti-human milk XOR antibody in the epithelium of the villi, from midheight to the tips. Goblet cells are immunoreactive, and there is a concentration of staining at the brush border. There is also positive staining in the lamina propria and in cells within the crypts, such as Paneth cells and crypt base columnar cells. Reprinted from reference 43 with permission of the publisher.
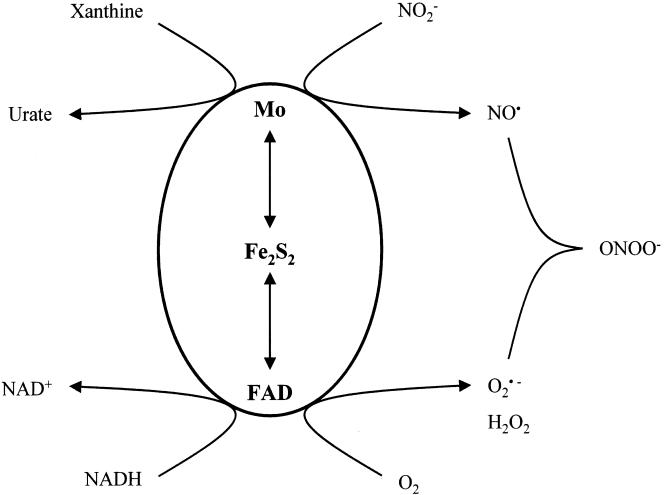
Under hypoxic conditions, XOR has been shown (24) to catalyze the reduction of inorganic nitrite to nitric oxide (NO), a much-studied signaling molecule with bactericidal properties. Moreover, if oxygen is present, it also is reduced, forming superoxide, which reacts rapidly with NO, yielding peroxynitrite (25), a still more potent bactericidal agent (11) (Fig. 3). While purified bovine milk XOR shows physiologically significant maximal rates of NO production in the presence of xanthine and nitrite, the Km values for nitrite are in the millimolar range. Such levels, which would need to be attained in order to approach maximal rates, are 2 orders of magnitude higher than the levels prevailing in the digestive tract. However, the nitrite levels are potentially much higher in the microenvironment of enteric bacteria. At least in anaerobic cultures, such bacteria can excrete millimolar levels of nitrite (19), which is derived from dissimilatory nitrate reductase (17). The concept that, by nitrite excretion, enteric bacteria might initiate their own destruction is attractive.
FIG. 3.
XOR-catalyzed generation of NO and peroxynitrite. Under hypoxic conditions and in the presence of nitrite and a reducing substrate, such as xanthine or NADH, NO is produced at the molybdenum site. Molecular oxygen is reduced at the flavin adenine dinucleotide (FAD) site, generating superoxide, which reacts with NO to give peroxynitrite. Reprinted from reference 32 with permission of the publisher.
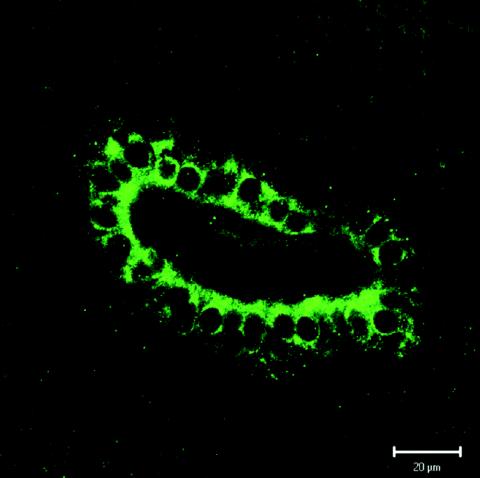
In forming part of the antimicrobial barrier of intestinal epithelia, XOR participates in the innate immune system. Bile has long been known to contribute to this system in the gut (54), and indeed, the enzyme has recently been detected at the luminal surface of bile duct epithelial cells (Fig. 4) and in bile itself (43).
FIG. 4.
Localization of XOR within human bile duct epithelial cells. Positive fluorescent staining for anti-human milk XOR antibody is diffusely distributed in the cytoplasm. The enzyme is apically localized, suggesting that there is secretion into the lumen of the bile duct. Reprinted from reference 43 with permission of the publisher.
Overall, therefore, XOR can be seen as potentially contributing to antimicrobial defense in the gastrointestinal tract by virtue of its presence in both the epithelial layer and bile.
ANTIMICROBIAL ACTIVITY OF XOR IN MILK
A third contribution to antimicrobial defense occurs in the special case of the neonatal gut. As noted above, the antibacterial activity of purified bovine milk XOR has long been recognized. Green and Pauli (28) and Lipmann and Owen (42) demonstrated that there was inhibition of the growth of Staphylococcus aureus by purified bovine milk XOR and explained their findings in terms of the cytotoxic properties of XOR-generated hydrogen peroxide. Similar antibacterial activity has been confirmed in the cases of Escherichia coli and Salmonella enteritidis (59), while Bjorck and Claesson (7) proposed that XOR-derived hydrogen peroxide exerts its antimicrobial effects by acting as a substrate for the lactoperoxidase system in milk.
In view of the recently discovered capacity of XOR to reduce nitrite to NO (see above), generation of reactive nitrogen species (RNS), particularly peroxynitrite, can be seen as a potential mechanism of antimicrobial activity of milk. As discussed above, nitrite concentrations are likely to be high in the immediate microenvironment of enteric bacteria, favoring XOR-catalyzed reduction to NO. Moreover, several factors promote the physical association of such bacteria with XOR, which is present on the MFGM. The MFGM is derived from the apical membrane of the mammary secretory cell and, as such, bears epithelial membrane antigens. Pathogenic bacteria target epithelial membranes of the digestive tract and may well bind to similar antigens on the MFGM (36). This not only diverts the bacteria from their primary target but also brings them into intimate contact with XOR. Such contact is further promoted by the known affinity of XOR for acidic polysaccharides, such as those that occur in many bacterial capsules (2).
In the generation of peroxynitrite, oxygen and nitrite compete for XOR-generated electrons, and there is an optimal oxygen tension for peroxynitrite production (25). In the case of the oxidase form of the enzyme, XO, this tension is low, at around 8 μM. With XDH, on the other hand, relatively high rates are maintained over the physiological range of oxygen tensions. Moreover, the maximal rate of peroxynitrite generation is approximately double the maximal rate for XO generation. It is noteworthy that it is the XDH form of the enzyme that predominates in freshly expressed milk (49).
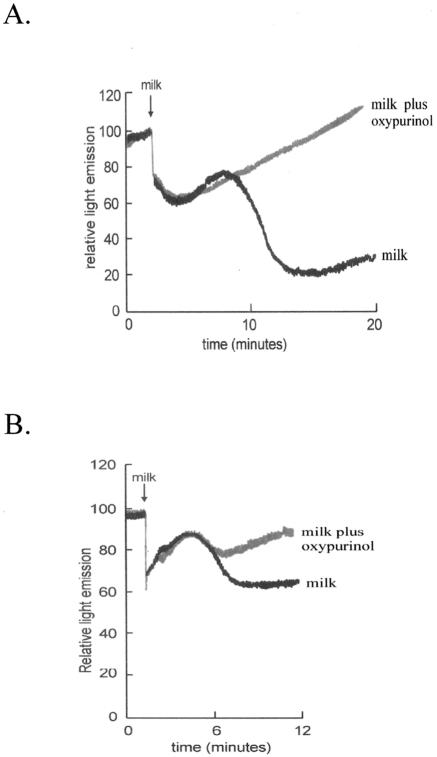
In support of the rationale described above, we recently obtained experimental evidence for an antibacterial activity of milk that appears to depend on XOR-generated RNS. Samples of fresh human milk and bovine milk were each shown to inhibit the metabolic activity of E. coli. We used a transformed E. coli construct that constitutively expresses the luxCDABE operon and, when metabolizing normally, emits luminescence (56). Impairment of the metabolic function results in reduced light emission, which can be quantitatively and continuously monitored. The milk-induced metabolic inhibition of E. coli was blocked by oxypurinol, a specific inhibitor of XOR (30) (Fig. 5). It was also found to be dependent on the presence of nitrite (30) and to be optimal at low oxygen tensions when XOR production of NO is maximal (25).
FIG. 5.
Antibacterial activity of bovine and human milk is mediated by XOR. Transformed E. coli, constitutively expressing the luxCDABE operon, was incubated under hypoxic conditions with a reducing substrate and inorganic nitrite. Light emission was continuously monitored as an indicator of bacterial growth. Addition of bovine milk (A) or human milk (B) led to initial artifactual variations in light emission that were succeeded by a sharp decrease (over 8 to 14 min [A] or 4 to 8 min [B]) corresponding to a loss of metabolic function. This decrease was, in turn, followed by a steady increase as the bacteria recovered and resumed growth. In the presence of the specific XOR inhibitor oxypurinol, the sharp decrease in light emission was largely abrogated. Reprinted from reference 30 with permission of the publisher.
Human milk is of particular interest. XOR purified from breast milk has low XO activity, which is only approximately 5% of the activity of purified bovine milk XOR (1, 57). This low activity has been shown to result from a low content of molybdenum (9, 23), and it is far from clear what advantage might be derived from a largely inactive molybdenum site (31). It may be significant that while XOR plays a key role in the process of milk secretion (45, 66), this process does not require active enzyme, depending rather on XOR protein (66). Moreover, the XO activity of XOR has been shown to peak dramatically in the first few weeks after birth (10), when the antibacterial activity of milk can be demonstrated (Fig. 5) (30) and when such activity is of most value to the infant.
After this, the specific activity rapidly falls to basal levels (10), and it is at this stage that breast milk is usually collected for XOR purification. It is conceivable that humans have evolved to spare the metabolically expensive process of molybdenum incorporation (22) in the later stages of milk secretion, when an antimicrobial function of milk is less critical.
CIRCULATING XOR
Healthy mammals have low levels of circulating XOR. In the case of humans, these levels can dramatically increase in response to a range of diseases, particularly those affecting the liver (32). Because such XOR is largely in the XO form, as a result of proteolysis (39), it tempting to see the circulating enzyme as a systemic source of antimicrobial ROS. Perhaps the most convincing example of such a role is found in Cape buffaloes, in which a natural resistance to trypanosome parasitemia has been attributed to hydrogen peroxide derived from serum XO (68). In general, however, the situation is far from clear. It is possible that the increased levels of serum XOR seen in liver disease simply reflect damage to liver cells, which are known to be rich in the enzyme. Moreover, circulating XOR, with its capacity to generate ROS, can be viewed as potentially pathogenic. It has the capacity to bind to glycosylaminoglycans on the surface of vascular endothelial cells (2, 53) and, thus concentrated, to initiate oxidative damage in distal organs (69). In this context, it is of interest that anti-XOR antibodies are also present in mammalian serum (68). In fact, the levels can be remarkably high, accounting for as much as 6% of the total immunoglobulin M antibodies in healthy human subjects (6). It is conceivable that such antibodies constitute a natural defense against excess levels of XOR, clearing them from the circulation in the form of immune complexes. A further complication is that XOR catalyzes production of uric acid, a powerful scavenger of ROS (4, 34), casting the enzyme once again in a protective role rather than a destructive role (37, 51).
CONCLUSIONS
Clearly, the physiological involvement of XOR is potentially complex. Apart from its generally accepted role in purine catabolism, the enzyme most probably participates in the metabolism of xenobiotics, particularly in the liver (5), a topic not addressed here. Moreover, the involvement of XOR in the process of milk secretion, which has been suspected for a long time (35), has recently been demonstrated (45, 66).
The capacity of XOR to generate ROS has excited research interest for many years, especially following the proposed key role of this reaction in IR injury. As Meneshian and Bulkley point out (46), it is likely that such injury represents an aberrant version of a normal physiological response, namely, inflammation, in which leukocytes are recruited, typically to combat infection. In this context, XOR acts as an agent of innate immunity, and other examples of this function are especially apparent in the gastrointestinal tract, where the enzyme can be seen as participating in antimicrobial defense by virtue of its localization in epithelial cells, in bile, and on the surface of maternal milk fat globules. In all of these instances, XOR is assumed to exert its antimicrobial action by means of ROS, and it seems increasingly possible that the RNS nitric oxide and peroxynitrite are also involved.
The role of circulating XOR is particularly ambivalent in that it is potentially a source of ROS and RNS, on the one hand, and a source of their scavenger, urate, on the other hand. Moreover, ROS and RNS have both the capacity to combat infection and the capacity to promulgate injury throughout the vascular system. It may be that, for these reasons, the levels of the enzyme are subject to rigorous control by the associated autoantibodies, a rare example of beneficial autoimmunity.
While much of the evidence for the antimicrobial role of XOR cited in this review is circumstantial, it at least makes a case for such a role, and the subject merits further investigation. In a broader context, it has been proposed that the enzyme is central to the evolution of the innate immune system in general (67), a concept supported by the above discussion.
Editor: J. B. Kaper
REFERENCES
- 1.Abadeh, S., J. Killacky, M. Benboubetra, and R. Harrison. 1992. Purification and partial characterization of xanthine oxidase from human milk. Biochim. Biophys. Acta 1117:25-32. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, T., T. Fukushima, Y. Usami, and K. Hirano. 1993. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial cell surface. Biochem. J. 289:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akaike, T., M. Ando, T. Oda, T. Doi, S. Ijiri, S. Amki, and H. Maeda, H. 1990. Dependence on O2-generation by xanthine oxidase of pathogenesis of influenza virus in mice. J. Clin. Investig. 85:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, B. F. 1993. Towards the physiological function of uric acid. Free Radic. Biol. Med. 14:615-631. [DOI] [PubMed] [Google Scholar]
- 5.Beedham, C. 2002. Molybdenum hydroxylases, p. 147-187. In C. Ionnides (ed.), Enzyme systems that metabolize drugs and other xenobiotics. John Wiley, New York, N.Y.
- 6.Benboubetra, M., A. Glesson, C. P. D. Harris, J. Khan, L. Arrar, D. Brennand, J. Reid, J. D. Reckless, and R. Harrison. 1997. Circulating anti-(xanthine oxidoreductase) antibodies in healthy human adults. Eur. J. Clin. Investig. 27:611-619. [DOI] [PubMed] [Google Scholar]
- 7.Bjorck, L., and O. Claesson. 1979. Xanthine oxidase as a source of hydrogen peroxide for the lactoperoxidase system in milk. J. Dairy Sci. 62:1211-1215. [Google Scholar]
- 8.Bray, R. C. 1975. Molybdenum iron-sulfur flavin hydroxylases and related enzymes, p. 299-419. In P. D. Boyer (ed.), The enzymes. Academic Press, New York, N.Y.
- 9.Bray, R. C., D. Lowe, B. Godber, R. Harrison, and R. Eisenthal. 1999. Properties of xanthine oxidase from human milk: the enzyme is grossly deficient in molybdenum and substantially deficient in iron-sulphur centres, p. 775-778. In S. Ghisla, P. M. Kroneck, P. Macheroux, and H. Sund (ed.), Flavins and flavoproteins. Agency for Scientific Publication, Berlin, Germany.
- 10.Brown, A.-M., M. Benboubetra, M. Ellison, D. Powell, J. D. Reckless, and R. Harrison. 1995. Molecular activation-deactivation of xanthine oxidase in human milk. Biochim. Biophys. Acta 1245:248-254. [DOI] [PubMed] [Google Scholar]
- 11.Brunelli, L., J. P. Crow, and J. S. Beckman. 1995. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch. Biochem. Biophys. 316:327-334. [DOI] [PubMed] [Google Scholar]
- 12.Bulkley, G. B. 1993. Endothelial xanthine oxidase: a radical transducer of inflammatory signals for reticuloendothelial activation. Br. J. Surg. 80:686. [DOI] [PubMed] [Google Scholar]
- 13.Bulkley, G. B. 1994. Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function. Lancet 344:934-936. [DOI] [PubMed] [Google Scholar]
- 14.Bungener, W. 1974. Influence of allopurinol on the multiplication of rodent malaria parasites. Tropenmed. Parasitol. 25:309-312. [PubMed] [Google Scholar]
- 15.Carden, D. L., and D. N. Granger. 2000. Pathology of ischemia-reperfusion injury. J. Pathol. 190:255-266. [DOI] [PubMed] [Google Scholar]
- 16.Christen, S., Y. D. Bifrare, C. Siegenthaler, S. L. Leib, and M. G. Tauber. 2001. Marked elevation in cortical urate and xanthine oxidoreductase activity in experimental bacterial meningitis. Brain Res. 900:244-251. [DOI] [PubMed] [Google Scholar]
- 17.Cole, J. 1996. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol. Lett. 136:1-11. [DOI] [PubMed] [Google Scholar]
- 18.Crosby, P. F., M. L. Matos, and E. Rivera-Collazo. 1969. Liver xanthine oxidase activity of mice infected with Schistosoma mansoni. J. Parasitol. 55:673. [PubMed] [Google Scholar]
- 19.DeMoss, J. A., and P.-Y. Hsu. 1991. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J. Bacteriol. 173:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel, T. 1999. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 65:337-340. [DOI] [PubMed] [Google Scholar]
- 21.Fuh, K. C., A. Meneshian, C. B. Patel, V. Takiar, and G. B. Bulkley. 2002. Signal transduction by reactive oxygen species: alternative paradigms for signaling specificity. Surgery 131:601-612. [DOI] [PubMed] [Google Scholar]
- 22.Garattini, E., R. Mendel, M. J. Romao, R. Wright, and M. Terao. 2003. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem. J. 372:15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godber, B., S. Sanders, R. Harrison, R. Eisenthal, and R. C. Bray. 1997. More than 95% of xanthine oxidase in human milk is present as the demolybdo form, lacking molybdopterin. Biochem. Soc. Trans. 25:519S. [DOI] [PubMed] [Google Scholar]
- 24.Godber, B. L. J., J. J. Doel, G. P. Sapkota, D. R. Blake, C. R. Stevens, R. Eisenthal, and R. Harrison. 2000. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 275:7757-7763. [DOI] [PubMed] [Google Scholar]
- 25.Godber, B. L. J., J. J. Doel, J. Durgan, R. Eisenthal, and R. Harrison. 2000. A new route to peroxynitrite: a role for xanthine oxidoreductase. FEBS Lett. 475:93-96. [DOI] [PubMed] [Google Scholar]
- 26.Granger, D. N., G. Rutili, and J. M. McCord. 1981. Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22-29. [PubMed] [Google Scholar]
- 27.Granger, D. N., M. E. Hollwarth, and D. A. Parks. 1986. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol. Scand. 548(Suppl.):47-63. [PubMed] [Google Scholar]
- 28.Green, D. E., and R. Pauli. 1943. The antibacterial action of the xanthine oxidase system. Proc. Soc. Exp. Biol. Med. 54:148-150. [Google Scholar]
- 29.Hancock, J. T., R. Desikan, and S. J. Neill. 2001. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 29:345-350. [DOI] [PubMed] [Google Scholar]
- 30.Hancock, J. T., V. Salisbury, M. C. Ovejero-Boglione, R. Cherry, C. Hoare, R. Eisenthal, and R. Harrison. 2002. Antimicrobial properties of milk: dependence on presence of xanthine oxidase and nitrite. Antimicrob. Agents Chemother. 46:3308-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison, R. 1997. Human xanthine oxidoreductase: in search of a function. Biochem. Soc. Trans. 25:786-791. [DOI] [PubMed] [Google Scholar]
- 32.Harrison, R. 2002. Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 33:774-797. [DOI] [PubMed] [Google Scholar]
- 33.Hille, R. 1996. The mononuclear molybdenum enzymes. Chem. Rev. 96:2757-2816. [DOI] [PubMed] [Google Scholar]
- 34.Hooper, D. C., G. S. Scott, A. Zborek, T. Mikheeva, R. B. Kean, H. Koprowski, and S. V. Spitsin. 2000. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 14:691-698. [DOI] [PubMed] [Google Scholar]
- 35.Jarasch, E.-D., C. Grund, G. Bruder, H. W. Heid, T. W. Keenan, and W. W. Franke. 1981. Localization of xanthine oxidase in mammary gland epithelium and capillary endothelium. Cell 25:67-82. [DOI] [PubMed] [Google Scholar]
- 36.Keenan, T. W., and S. Patton. 1995. The structure of milk: implications for sampling and storage. A. The milk lipid globule membrane, p. 5-50. In R. G. Jensen (ed.), Handbook of milk composition. Academic Press, New York, N.Y.
- 37.Kooij, A., K. S. Bosch, W. M. Frederiks, and C. J. F. Van Noorden. 1992. High levels of xanthine oxidoreductase in rat endothelial, epithelial and connective tissue cells. Virchows Arch. B Cell Pathol. 62:143-150. [DOI] [PubMed] [Google Scholar]
- 38.Kooij, A., M. Schijns, W. M. Frederiks, C. J. F. Van Noorden, and J. James. 1992. Distribution of xanthine oxidoreductase activity in human tissues—a histochemical and biochemical study. Virchows Arch. B Cell Pathol. 63:17-23. [DOI] [PubMed] [Google Scholar]
- 39.Kooij, A., H. J. Schiller, N. Schijns, C. J. F. Van Noorden, and W. M. Frederiks. 1994. Conversion of xanthine dehydrogenase to xanthine oxidase in rat liver and plasma at the onset of reperfusion after ischemia. Hepatology 19:1488-1495. [PubMed] [Google Scholar]
- 40.Kurosaki, M., M. Li Calzi, E. Scanziani, E. Garattini, and M. Terao. 1995. Tissue and cell-specific expression of mouse xanthine oxidoreductase gene in vivo: regulation by bacterial lipopolysaccharide. Biochem. J. 306:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linder, N., J. Rapola, and K. O. Raivio. 1999. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab. Investig. 79:967-974. [PubMed] [Google Scholar]
- 42.Lipmann, F., and C. R. Owen. 1943. The antibacterial effect of enzymatic xanthine oxidation. Science 98:246-248. [DOI] [PubMed] [Google Scholar]
- 43.Martin, H. M. 2003. Ph.D. thesis. University of Bath, Bath, United Kingdom.
- 44.Massey, V., and C. M. Harris. 1997. Milk xanthine dehydrogenase: the first one hundred years. Biochem. Soc. Trans. 25:750-755. [DOI] [PubMed] [Google Scholar]
- 45.McManaman, J. L., C. A. Palmer, R. M. Wright, and M. C. Neville. 2002. Functional regulation of xanthine oxidoreductase expression and localization in the mouse mammary gland: evidence of a role in lipid secretion. J. Physiol. 545:567-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meneshian, A., and G. B. Bulkley. 2002. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 9:162-175. [DOI] [PubMed] [Google Scholar]
- 47.Morita, Y. 2001. Identification of xanthine dehydrogenase/oxidase as a rat Paneth cell zinc-binding protein. Biochim. Biophys. Acta 1540:43-49. [DOI] [PubMed] [Google Scholar]
- 48.Moriwaki, Y., T. Yamamoto, K. Yamaguchi, S. Takahashi, and K. Higashino. 1999. Immunohistochemical localization of aldehyde and xanthine oxidase in rat tissues using polyclonal antibodies. Histochem. Cell Biol. 105:71-79. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura, M., and I. Yamazaki. 1982. Preparation of bovine milk xanthine oxidase as a dehydrogenase form. J. Biochem. 92:1279-1286. [DOI] [PubMed] [Google Scholar]
- 50.Patton., S., and T. W. Keenan. 1975. The milk fat globule membrane. Biochem. Biophys. Acta 415:273-309. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer, K. D., T. P. Huecksteadt, and J. R. Hoidal. 1994. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. J. Immunol. 153:1789-1797. [PubMed] [Google Scholar]
- 52.Potoka, D. A., S. Takao, T. Owaki, G. B. Bulkley, and A. S. Klein. 1998. Endothelial cells potentiate oxidant-mediated Kuppfer cell phagocytic killing. Free Radic. Biol. Med. 24:1217-1227. [DOI] [PubMed] [Google Scholar]
- 53.Radi, R., H. Rubbo, K. Bush, and B. A. Freeman. 1997. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilised xanthine oxidase-heparin complexes. Arch. Biochem. Biophys. 339:125-135. [DOI] [PubMed] [Google Scholar]
- 54.Reynoso-Paz, S., R. L. Coppel, I. R. Mackay, N. M. Bass, A. A. Ansari, and M. E. Gershwin. 1999. The immunobiology of bile and biliary epithelium. Hepatology 30:351-357. [DOI] [PubMed] [Google Scholar]
- 55.Saito, T., and T. Nishino. 1989. Differences in redox and kinetic properties between NAD-dependent and O2-dependent types of rat liver xanthine dehydrogenase. J. Biol. Chem. 264:10015-10022. [PubMed] [Google Scholar]
- 56.Salisbury, V., A. Pfoest, H. Wiesinger-Mayer, R. Lewis, K. E. Bowker, and A. P. MacGowan. 1999. Use of a clinical Escherichia coli isolate expressing lux genes to study the antimicrobial pharmacodynamics of moxiflaxacin. J. Antimicrob. Chemother. 43:829-832. [DOI] [PubMed] [Google Scholar]
- 57.Sanders, S. A., R. Eisenthal, and R. Harrison. 1997. NADH oxidase activity of human xanthine oxidoreductase. Generation of superoxide anion. Eur. J. Biochem. 245:541-548. [DOI] [PubMed] [Google Scholar]
- 58.Segal, B. H., N. Sakamoto, M. Patel, K. Maemura, A. S. Klein, S. M. Holland, and G. B. Bulkley. 2000. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47phox−/− mouse model of chronic granulomatous disease. Infect. Immun. 68:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens, C. R., T. M. Millar, J. G. Clinch, J. M. Kanczler, T. Bodamyali, and D. R. Blake. 2000. Antibacterial properties of xanthine oxidase in human milk. Lancet 356:829-830. [DOI] [PubMed] [Google Scholar]
- 60.Stirpe, F., M. Ravaioli, M. G. Batelli, M. G. S. Musiani, and G. L. Grazi. 2002. Xanthine oxidase activity in human liver disease., Am. J. Gastroenterol. 97:2079-2085. [DOI] [PubMed] [Google Scholar]
- 61.Takao, S., E. H. Smith, D. Wang, C. K. Chan, G. B. Bulkley, and A. S. Klein. 1996. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocyte killing. Am. J. Physiol. Cell Physiol. 271:C1278-C1284. [DOI] [PubMed] [Google Scholar]
- 62.Tubaro, E., B. Lotti, G. Cavallo, C. Groce, and G. Borelli. 1980. Liver xanthine oxidase increase in mice in three pathological models. A possible defense mechanism. Biochem. Pharmacol. 29:1939-1943. [DOI] [PubMed] [Google Scholar]
- 63.Tubaro, E., B. Lotti, C. Santiangeli, and G. Cavallo. 1980. Xanthine oxidase increase in polymorphonuclear leucocytes and macrophages in mice in three pathological situations. Biochem. Pharmacol. 29:1945-1948. [DOI] [PubMed] [Google Scholar]
- 64.Umezawa, K., T. Akaike, S. Fujii, M. Suga, K. Setoguchi, A. Ozawa, and H. Maeda. 1997. Induction of nitric oxide synthase and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect. Immun. 65:2932-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van den Munckhof, R. J. M., H. Vreeling-Sindelarova, J. P. M. Schellens, C. J. F. Van Noorden, and W. M. Frederiks. 1995. Ultrastructural localization of xanthine oxidase activity in the digestive tract of the rat. Histochem. J. 27:897-905. [PubMed] [Google Scholar]
- 66.Vorbach, C., A. Scriven, and M. R. Capecchi. 2002. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 16:3223-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vorbach, C., R. Harrison, and M. Capecchi. 2003. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 24:512-517. [DOI] [PubMed] [Google Scholar]
- 68.Wang, J., A. van Praagh, E. Hamilton, Q. Wang, B. Zou, M. Muranjan, N. B. Murphy, and S. J. Black. 2002. Serum xanthine oxidase: origin, regulation, and contribution to control of trypanosome parasitemia. Antioxid. Redox Signal. 4:161-178. [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama, Y., J. S. Beckman, T. K. Beckman, J. K. Wheat, T. G. Cash, B. A. Freeman, and D. A. Parks. 1990. Circulating xanthine oxidase: potential mediator of ischemic injury. Am. J. Physiol. 258:G565-G570. [DOI] [PubMed] [Google Scholar]