Abstract
A 67-year-old man was treated with cladribine for hairy cell leukaemia. A few weeks later, he presented with persistent headaches, intermittent hypoesthesia of the right upper limb and language impairment. Brain CT scan showed 3 contrast-enhancing lesions. MRI revealed infracentimetric nodular lesions with restricted diffusion. One of the lesions was surgically removed and tested positive for acid-fast bacilli. Moreover, Mycobacterium tuberculosis was confirmed by PCR. Antituberculous drugs were prescribed for 12 months, with complete resolution of neurological deficits. This case highlights the risk of mycobacterial infections associated with both hairy cell leukaemia and cladribine use, and the importance of screening and treatment of latent forms of tuberculosis in patients undergoing treatment with immunosuppressive drugs.
Background
Central nervous system tuberculosis (TB) can manifest itself in three distinct clinical forms: meningitis, intracranial tuberculoma and spinal tuberculous arachnoiditis. It occurs in ∼1% of all cases of TB and represents 4.5% of extrapulmonary disease.1 It is more common in paediatric patients and in adults with altered immunity.2 Intracranial tuberculomas are granulomatous inflammatory processes which develop in response to previously quiescent mycobacteria present for a variable time in brain tissue. Intracranial tuberculomas can occur in otherwise healthy individuals and should be considered as differential diagnosis in the discussion of intracranial mass lesions.3
Hairy cell leukaemia (HCL) is an uncommon chronic B-cell lymphoproliferative disorder, which has been associated with increased incidence of mycobacterial infections (4–9%).4 Cladribine is a purine analogue and one of the first-line drugs in the treatment of HCL, with a complete response rate of 76%.5 It induces a prolonged CD4+ lymphocytopaenia, with a median time to recover previous CD4+ count near 40 months.6
This case emphasises the elevated risk of TB reactivation in patients with HCL, in which treatment with cladribine may have an important contributing role.
Case presentation
We report the case of a 67-year-old Caucasian man with type 2 diabetes and a recent diagnosis of HCL, treated with a single course of cladribine. One week later this patient developed neutropaenia (480 neutrophils/μL), lymphopaenia (50 lymphocytes/μL) and cellulitis with methicillin-sensitive Staphylococcus aureus bacteraemia.
Ten weeks after cladribine infusion, the patient presented intermittent hypoesthesia in the right upper limb and language impairment. He had a persistent right frontal headache for a month. On admission, neurological examination was normal. A brain CT scan showed three contrast-enhancing lesions: in the left semioval centre, left anterior peri-insular cortex and in the right frontal lobe. He was haemodynamically stable and afebrile.
Investigations
The patient had a low lymphocyte count (950/μL) and a C reactive protein of 21.20 mg/L; the remaining routine blood tests were normal. Serology for HIV 1/2 and Toxoplasma and Cryptococcus antigen were negative.
The brain MRI revealed infracentimetric nodular lesions in the frontoinsular left cortex (figure 1A), medial and frontal right cortex (figure 1B), the left semioval centre (figure 1C) and in the right tegmental portion of the pons (figure 1D). The lesions were hypointense on T1 and T2, compared with the cortex, and showed a ring enhancement after gadolinium administration. The left frontoinsular lesion revealed restricted diffusion (figure 2). The white matter adjacent to the lesions was hyperintense on T2 and fluid-attenuated inversion recovery images and hypointense on T1, reflecting vasogenic oedema (figure 3).
Figure 1.
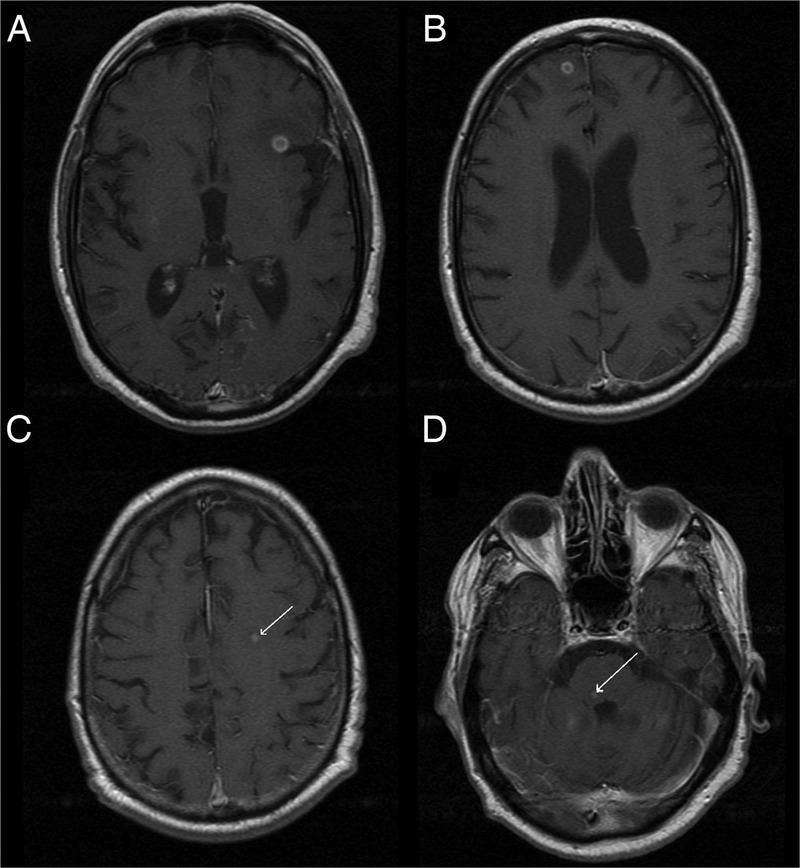
Cerebral MRI, T1 after gadolinium contrast: (A) left frontoinsular lesion, 5.6 mm; (B) medial and frontal right lesion, 4 mm; (C) left semioval centre lesion; (D) right pontine lesion (tegmental portion).
Figure 2.
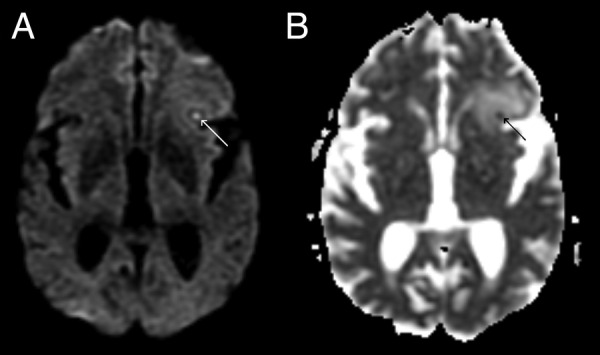
Left frontoinsular lesion: (A) diffusion-weighted imaging; (B) apparent diffusion coefficient.
Figure 3.
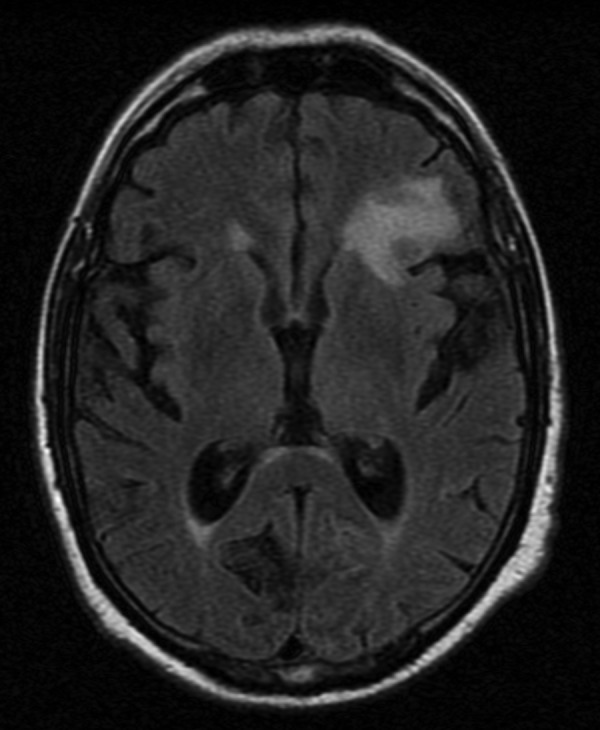
Left frontoinsular lesion, fluid-attenuated inversion recovery.
The frontal lesion was removed by craniotomy. The pathological study of the brain tissue revealed the presence of lymphocytes and reactive gliosis, and the Ziehl-Neelsen staining tested positive for acid-fast bacilli. Mycobacterium tuberculosis complex was confirmed by molecular detection from the brain tissue using real-time PCR (MTB Q—PCR Alert Kit, Nanogen). The liquid culture system (MGIT—Becton Dickinson) of the lesion was also positive and M. tuberculosis complex was identified by PCR and double-reverse hybridisation (Speed-Oligo Mycobacteria, Vircell). The strain was susceptible to all antituberculous drugs tested by MGIT (with the exception of streptomycin).
Differential diagnosis
Intracranial tuberculomas are cerebral mass lesions expressing ring enhancement on radiological evaluations. MRI techniques, using diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC), are useful to identify abscesses.7 It shows the restriction to the diffusion of water molecules of the viscous component in the core of the abscess, characterised by hyperintense signal on DWI and hypointense on ADC images,8 helping to differentiate abscesses from neoplasms.8 9 However, there are a few reported cases of solid tumours with hyperintense signal on DWI and low ADC.10 11
Some MRI studies of tuberculomas revealed different results in signal intensity of DWI and ADC.12–14 This may be related to a different degree of liquefaction of caseous necrosis, depending on the stage of the studied granuloma.
Lumbar puncture is contraindicated in patients with brain abscesses and focal signs or symptoms.15 Although there was no imaging evidence of intracranial hypertension, a lumbar puncture was not performed because cerebrospinal fluid culture in patients with tuberculomas is generally negative.16 In this perspective, a needle aspiration or surgical excision is mandatory for morphological and microbiological analysis.
Treatment
The patient began treatment with antituberculous drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) and prednisolone (50 mg/day), without recurrence of neurological deficits. A chest CT scan revealed a cavitary lesion in the right lung, with no signs of active infection (figure 4); there were no other signs of TB.
Figure 4.
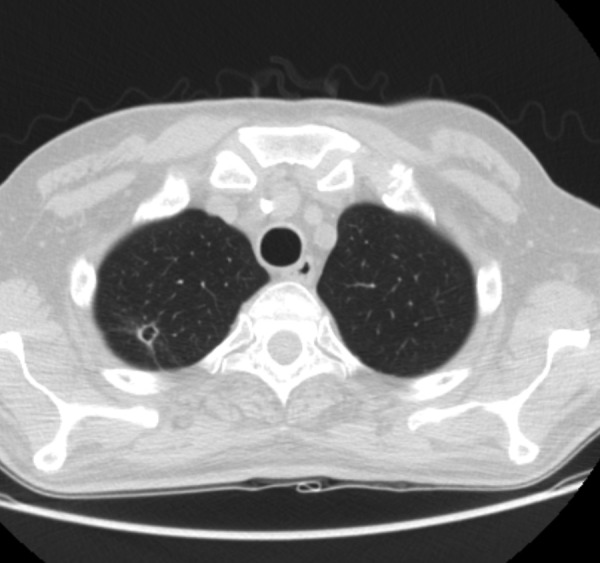
CT chest, cavitary lesion in the apical segment of the right upper lobe with 7.4 mm diameter.
Outcome and follow-up
The patient was discharged from the hospital on the 10th day of antituberculous therapy and began tapering of corticosteroids over the following 3 weeks. After 9 months of treatment, the follow-up brain CT scan did not show new parenchymal lesions. The patient completed 1 year of treatment. Moreover, after 4 years of follow-up, there is no recurrence of the disease. His peripheral blood immunophenotyping showed no recurrence of hairy cells.
Discussion
Increased incidence of mycobacterial infections, including disseminated TB, were reported in patients with HCL.4 17–22 However, only two cases of TB with central nervous system disease have been reported,23 24 one regarding a patient subjected to treatment with cladribine.24
Cladribine, by causing profound suppression in lymphocyte counts, has been a probable determinant factor in a less typical expression of active TB. It has been advocated that oncological patients should be screened for latent TB, and treated if positive, when submitted to chemotherapy. Furthermore, the importance of cladribine and HCL in promoting aggressive TB forms may have been under-recognised because of low prevalence of the disease. These data suggest that treatment of latent TB in cases of HCL treated with cladribine should be considered even in patients older than 65 years.
Learning points.
Hairy cell leukaemia (HCL) is a chronic B-cell lymphoproliferative disorder that has been associated with increased incidence of mycobacterial infections. Cladribine, one of the first-line drugs used in its treatment, leads to prolonged lymphocytopaenia and probably favours tuberculosis (TB) reactivation.
Intracranial tuberculomas are cerebral granulomatous inflammatory processes, which should be considered in the differential diagnosis of intracranial mass lesions especially in immunodeficiency states.
Diffusion MRI techniques are useful in differentiating abscesses from neoplasms.
The biopsy of an intracranial abscess is essential for the definitive diagnosis and identification of aetiological agent, since cerebrospinal fluid bacteriology is usually negative.
Screening and treatment of latent forms of TB in patients undergoing immunosuppressive therapies is important in countries of higher TB endemicity as illustrated by the present case.
Footnotes
Twitter: Follow Júlio de Oliveira at @Júlio R Oliveira
Contributors: SBF was involved in drafting the article. JRdO, CG and VL were involved in revising the article critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.CDC. Reported tuberculosis in the United States, 2009. Atlanta, GA: U.S. Department of Health and Human Services, CDC, October 2010. [Google Scholar]
- 2.Álvarez-Salgado JA, Ruiz-Ginés JA, Fuentes-Ventura CD et al. Intracranial tuberculoma simulating a malignant tumor: case report and literature review. Neurocirugia (Astur) 2011;22:600–4. [PubMed] [Google Scholar]
- 3.Suslu HT, Bozbuga M, Bayindir C. Cerebral tuberculoma mimicking high grade glial tumor. Turk Neurosurg 2011;21:427–9. 10.5137/1019-5149.JTN.2947-10.0 [DOI] [PubMed] [Google Scholar]
- 4.Thaker H, Neilly IJ, Saunders PG et al. Remember mycobacterial disease in hairy cell leukaemia (HCL). J Infect 2001;42:213–14. 10.1053/jinf.2001.0816 [DOI] [PubMed] [Google Scholar]
- 5.Else M, Dearden CE, Matutes E et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol 2009;145:733–40. 10.1111/j.1365-2141.2009.07668.x [DOI] [PubMed] [Google Scholar]
- 6.Seymour JF, Kurzrock R, Freireich EJ et al. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood 1994;83:2906–11. [PubMed] [Google Scholar]
- 7.Castillo M, Mukherji SK. Diffusion-weighted imaging in the evaluation of intracranial lesions. Semin Ultrasound CT MR 2000;21:405–16. 10.1016/S0887-2171(00)90033-7 [DOI] [PubMed] [Google Scholar]
- 8.Friedlander RM, Gonzalez RG, Afridi NA et al. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 16–2003. A 58-year-old woman with left-sided weakness and a right frontal brain mass. N Engl J Med 2003;348:2125–32. 10.1056/NEJMra022366 [DOI] [PubMed] [Google Scholar]
- 9.Leuthardt EC, Wippold FJ II, Oswood MC et al. Diffusion-weighted MR imaging in the preoperative assessment of brain abscesses. Surg Neurol 2002;58:395–402. 10.1016/S0090-3019(02)00929-1 [DOI] [PubMed] [Google Scholar]
- 10.Holtås S, Geijer B, Strömblad LG et al. A ring-enhancing metastasis with central high signal on diffusion-weighted imaging and low apparent diffusion coefficients. Neuroradiology 2000;42:824–7. 10.1007/s002340000431 [DOI] [PubMed] [Google Scholar]
- 11.Tung GA, Evangelista P, Rogg JM et al. Diffusion-weighted MR imaging of rim-enhancing brain masses: is markedly decreased water diffusion specific for brain abscess? AJR Am J Roentgenol 2001;177:709–12. 10.2214/ajr.177.3.1770709 [DOI] [PubMed] [Google Scholar]
- 12.Helmy A, Antoun N, Hutchinson P. Cerebral tuberculoma and magnetic resonance imaging. J R Soc Med 2011;104:299–301. 10.1258/jrsm.2011.100398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasudev MK, Jayakumar PN, Srikanth SG et al. Quantitative magnetic resonance techniques in the evaluation of intracranial tuberculomas. Acta Radiol 2007;48:200–6. 10.1080/02841850601067678 [DOI] [PubMed] [Google Scholar]
- 14.Sadeghi N, Rorive S, Lefranc F. Intracranial tuberculoma: is diffusion-weighted imaging useful in the diagnosis? Eur Radiol 2003;13:2049–50. 10.1007/s00330-002-1737-z [DOI] [PubMed] [Google Scholar]
- 15.Heilpern KL, Lorber B. Focal intracranial infections. Infect Dis Clin North Am 1996;10:879–98. 10.1016/S0891-5520(05)70331-7 [DOI] [PubMed] [Google Scholar]
- 16.Katti MK. Pathogenesis, diagnosis, treatment, and outcome aspects of cerebral tuberculosis. Med Sci Monit 2004;10:215–29. [PubMed] [Google Scholar]
- 17.Panchovska MS, Solakov PT, Anavi BL et al. Wrist tuberculosis in cladribine-induced remission of hairy cell leukosis. Folia Med (Plovdiv) 2000;42:37–40. [PubMed] [Google Scholar]
- 18.Au WY, Kwong YL, Ma SK et al. Hairy cell leukemia in Hong Kong Chinese: a 12-year retrospective survey. Hematol Oncol 2000;18:155–9. [DOI] [PubMed] [Google Scholar]
- 19.Broady R, Roberts S, Hawkins T. Mycobacterium-avium-intracellulare complex infection following 2-chlorodeoxyadenosine therapy for hairy cell leukaemia. Leuk Lymphoma 2000;36:639–42. 10.3109/10428190009148413 [DOI] [PubMed] [Google Scholar]
- 20.Rose C, Auxenfants E, Noel MP et al. Tuberculosis, mycobacterium infection and hairy cell leukemia. Presse Med 1997;26:110–14. [PubMed] [Google Scholar]
- 21.Bennett C, Vardiman J, Golomb H. Disseminated atypical mycobacterial infection in patients with hairy cell leukemia. Am J Med 1986;80:891–6. 10.1016/0002-9343(86)90634-0 [DOI] [PubMed] [Google Scholar]
- 22.Arslan F, Batırel A, Ozer S et al. A predisposing clinical condition for disseminated tuberculosis: hairy cell leukemia. Mikrobiyol Bul 2013;47:346–50. 10.5578/mb.4419 [DOI] [PubMed] [Google Scholar]
- 23.Hulin C, Clément L, Baty V et al. Hairy and febrile leukocytes. Rev Med Interne 1999;20(Suppl 2):313s–14s. 10.1016/S0248-8663(99)80479-2 [DOI] [PubMed] [Google Scholar]
- 24.Girardi K, Paviglianiti A, Cirillo M et al. Tuberculous meningoencephalitis in a patient with hairy cell leukemia in complete remission. J Clin Exp Hematop 2012;52:31–4. 10.3960/jslrt.52.31 [DOI] [PubMed] [Google Scholar]


