Abstract
The mechanism of the apparent anti-inflammatory action of probiotic organisms is unclear. Lactobacillus reuteri is effective in inhibiting colitis in interleukin-10 (IL-10)-deficient mice. Nerve growth factor (NGF), in addition to its activity on neuronal cell growth, has significant anti-inflammatory effects in several experimental systems in vitro and in vivo, including a model of colitis. Our experiments were designed to explore the mechanism of effect of L. reuteri in the human epithelial cell lines T84 and HT29 on cytokine and NGF synthesis and IL-8 response to tumor necrosis factor alpha (TNF-α). Epithelial cells were cultured for various times with live and killed L. reuteri and examined by reverse transcription-PCR for NGF, IL-10, and TNF-α-induced IL-8 expression. An enzyme-linked immunosorbent assay was used to quantitate intracellular IL-8 and secreted product. Western blotting and confocal microscopy were used to determine the effects on IκB and NF-κB, respectively. Live but not heat-killed or gamma-irradiated L. reuteri upregulated NGF and dose dependently inhibited constitutive synthesis by T84 and HT29 cells of IL-8 and that induced by TNF-α in terms of mRNA and intracellular and secreted protein. Similarly, L. reuteri inhibited IL-8 synthesis induced by Salmonella enterica serovar Typhimurium. L. reuteri required preincubation and adherence for effect, inhibited translocation of NF-κB to the nuclei of HeLa cells, and prevented degradation of IκB. Neither cellular lysates nor media supernatants had any effect on TNF-α-induced IL-8. The conclusion is that L. reuteri has potent direct anti-inflammatory activity on human epithelial cells, which is likely to be related to the activity of ingested probiotics. L. reuteri also upregulates an unusual anti-inflammatory molecule, NGF, and inhibits NF-κB translocation to the nucleus.
Probiotics have been used in a large range of human and veterinary intestinal diseases (13, 21, 34). The mechanisms of action are obscure, but the epithelium may be a crucial player in the orchestration of the effects induced (17, 20, 37).
In the last decade, it has become clear that the epithelium is an active participant in gut physiology and can synthesize and secrete many mediators which play a role in homeostasis and innate immune responses to potentially pathogenic organisms (12, 35). These products include chemokines, cytokines, and arachidonic acid metabolites. Recently, it was shown that cholera toxin B subunit dose dependently increased both mRNA and interleukin-10 (IL-10) protein in three different human intestinal epithelial cell lines (18). There appeared to be an autocoid reciprocal stimulation pathway involved which included nerve growth factor (NGF), which has been shown to have potent immunoregulatory effects in a number of models including a hapten model of colitis (18).
Probiotic organisms have been used to treat a variety of human intestinal conditions including diarrhea (8, 23, 36) and aspects of inflammatory bowel disease such as pouchitis (7). In addition, Isolauri and associates have carried out randomized controlled studies of the use of a probiotic organism in atopic eczema in children and have shown improvement in clinical symptoms and severity of illness both upon cessation of treatment (13) and also in a 4-year follow-up study (14). In these studies, probiotic treatment resulted in a loss of increased intestinal permeability, reduced fecal eosinophil by-products, and an increase in serum IL-10.
An explanation for these effects is unclear, and while there is a hint from the work of Neish and colleagues (29) that nonpathogenic salmonella inhibited the synthesis of IL-8 induced by tumor necrosis factor alpha (TNF-α) in human intestinal epithelial cells (T84), this work until now has not been repeated with a probiotic organism. We have chosen to study Lactobacillus reuteri since this organism has been shown to be capable of inhibiting the onset of experimental colitis in transgenic IL-10-deficient mice (22) and has further been shown to reduce intestinal permeability to macromolecules (20).
In this paper, we show evidence that L. reuteri inhibited mRNA upregulation, cellular accumulation, and secretion of IL-8 induced by TNF-α in both T84 and HT-29 intestinal epithelial cells. This occurred only with live organisms in contact with the epithelial cells and inhibited translocation of NF-κB to the nucleus and also degradation of IκB. Furthermore, L. reuteri upregulated NGF mRNA in T84 and HT29 cells but had no effect on IL-10 mRNA. These results suggest some mechanisms of action of at least one clinically significant probiotic organism.
MATERIALS AND METHODS
Reagents.
The following reagents (with sources) were used: Dulbecco's modified Eagle's medium, F12 nutrient mixture (Ham) medium, Eagle's minimum essential medium with Earle's salts, McCoy's medium, fetal bovine serum (FBS), nonessential amino acids, penicillin-streptomycin (P-S), and l-glutamine (all from Gibco BRL Grand Island, N.Y.); brefeldin A, diethyl pyrocarbonate, mouse immunoglobulin G (IgG), peroxidase conjugated goat-antirabbit IgG, and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (all from Sigma Oakville, Ontario, Canada); recombinant human TNF-α, monoclonal mouse anti-human NF-κB p65, and polyclonal rabbit anti-IκB (all from BD PharMingen, Mississauga, Ontario, Canada); and Bacto lactobacilli MRS broth and MRS agar (Fisher Scientific, Nepean, Ontario, Canada). The primers used were synthesized by the Institute for Molecular Biology and Biotechnology, McMaster University.
Cells.
The human colon epithelial cell lines T84, HT-29, and CaCo-2 were used as described previously (18). The HeLa cell (human cervix epithelial-like cells) line was obtained from the American Type Culture Collection (Manassas, Va.), cultured in minimal essential medium with 0.1 mM nonessential amino acids, 1.5 mM l-glutamine, and 10% FBS and supplemented with 100 U of penicillin per ml and 100 μg of streptomycin per ml. Cells were maintained in 95% air, 5% CO2 at 37°C. Salmonella enterica serovar Typhimurium was a gift from Fergus Shanahan (National University of Ireland, Cork, Ireland).
Preparation of cell lysates.
Cell lysates were prepared by using CytoBuster Reagent (Novagen, Madison, Wis.), a proprietary formulation of detergents optimized for the extraction of soluble proteins from mammalian cells. Cells were washed twice with phosphate-buffered saline (PBS). CytoBuster reagent (300 μl) was added to each well in six-well plates containing attached cells and incubated for 5 min at room temperature. Attached cells were gently removed with a rubber policeman, pooled, and centrifuged at 16,000 × g for 5 min. The supernatants were collected and stored at −20°C.
Bacteria and related preparations. (i) L. reuteri (American Type Culture collection).
L. reuteri cells were suspended in MRS broth and plated in MRS agar, cultured anaerobically at 37°C for 24 h. The bacteria were checked by Gram stain and resuspended in 0.85% saline at different concentrations (see Results for details) based on the McFarland curve (measured by both turbidity and optical density). The bacteria were centrifuged at 890 × g for 15 min, resuspended at the designated concentration in T84, HT-29, or CaCo-2 medium without P-S, and used immediately.
(ii) Heat-killed (HK) L. reuteri.
HK L. reuteri was prepared by heating bacteria at 80°C for 10 min; bacteria were then centrifuged and resuspended as described above.
(iii) Gamma irradiated (IR) L. reuteri.
L. reuteri was exposed to gamma irradiation (cobalt-60 source) for 20 h at a dose of 8.05 Gy/min, centrifuged, and resuspended as described above.
(iv) L. reuteri broth culture medium (BCM) and soluble fraction (SF).
L. reuteri was cultured in MRS broth for 24 h. The BCM was separated by centrifugation at 890 × g for 15 min two times. BCM concentration was monitored by 1-ml BCM equivalent to 109 L. reuteri cells cultured. BCM was saved at −20°C until use. The precipitated bacteria were washed once and resuspended in 0.85% saline and centrifuged in an Eppendorf tube at 16,000 × g for 10 min at 4°C. The bacterial pellet was used for the preparation of SF. BugBuster reagent (Novagen), which is a formulation for the gentle disruption of bacteria for the liberation of soluble protein, was used per the manufacturer's instructions. Both BCM and SF were cultured and revealed no growth of bacteria in MRS agar. Serial dilutions of BCM and SF were used in the experiments.
(v) CM.
Conditioned medium (CM) was prepared by the incubation of L. reuteri with T84 media (without P-S) for 2 h according to the method for T84 cells. The culture medium was collected and centrifuged at 890 × g for 15 min. The supernatant was used as CM.
TNF-α induced IL-8 upregulation. (i) General sample preparation.
T84, HT-29, or CaCo-2 cells were cultured in six-well plates (Becton Dickinson Labware) at a concentration of 106 cells/ml for 3 days. The bacteria were added at the designated concentration and incubated in 95% air and 5% CO2 at 37°C for 2 h. Each well was either washed twice with PBS with new T84 medium added or left unwashed. TNF-α (10 ng/ml) or S. enterica serovar Typhimurium (107 cells/ml, which corresponded to 10 bacteria per T84 cell) was added to the designated wells and incubated for either 0.5, 1, 2, 4, 6, or 12 h. Cell viability was checked with trypan blue stain, and the viability was always higher than 95%. Supernatants were saved for enzyme-linked immunosorbent assays (ELISAs). Each well was then washed twice with PBS, followed by RNA isolation.
(ii) Intracellular IL-8 sample preparation.
Optimal concentrations of L. reuteri were added to the epithelial cell culture (without P-S). After 2 h of incubation, TNF-α (10 ng/ml) and brefeldin A (5 μg/ml; for blocking IL-8 release from the cells) were added to each culture, and the incubation continued for 6 h. The supernatants were collected for an IL-8 ELISA. The attached cells were washed twice with PBS, lysed, and used for the assay of intracellular IL-8.
RNA extraction and RT PCR analysis for IL-8, IL-10, and NGF.
RNA extraction was carried out by using TRIzol reagent as per the vendor's instructions (GIBCO BRL). Total RNA (1 μg) was reverse transcribed at 37°C for 1 h by using 500 μM random hexamers [pd(N)6, Amersham Pharmacia Biotech], 500 μM deoxynucleotide-5′-triphosphate (Amersham Pharmacia Biotech), 50 mM Tris · HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT), and 400 U of Moloney murine leukemia virus reverse transcriptase (RT; GIBCO BRL) in a final volume of 40 μl. For each PCR, 2 μl of RT product was amplified. PCR was performed with primers described previously (18) in 1.5 mmol/l MgCl2, 10 mM Tris · HCl (pH 8.3), 50 mM KCl, a 500 μmol concentration of each deoxynucleoside triphosphate, a 50 pmol concentration of each primer, and 1 U of AmpliTag DNA polymerase (Roche Molecular Systems, Branchburg, N.J.) in a final volume of 50 μl. A 5-min denaturation step at 94°C was followed by amplification cycles (30 cycles for IL-8 and 35 cycles for NGF and IL-10). Each cycle consisted of 94°C for 1 min, 61°C for 1 min, 72°C for 1.5 min, and a final extension at 72°C for 8 min in an automated thermal cycler (Perkin Elmer Cetus, Norwalk, Conn.). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control, with a GAPDH/cytokine ratio of 1:20. Fifteen microliters of each reaction was run onto a 2% agarose gel, stained with ethidium bromide, and visualized by UV illumination. Lambda DNA-HindIII/ϕX-174 DNA-HincII digest (Amersham Pharmacia Biotech) was run as molecular weight markers. Semiquantitative analysis of the DNA bands was performed by a Kodak Digital Science ID Electrophoresis Documentation and Analysis System. Data were expressed as the ratio of each specific expression to GAPDH calculated from the respective densities.
ELISA for IL-8.
IL-8 was quantified in supernatants and cell lysates with the BD OptEIA human IL-8 ELISA kit, performed as per the vendor's instructions (BD Biosciences).
Immunohistochemical staining for intracellular NF-κB translocation.
Immunohistochemistry was used to view intracellular NF-κB localization. HeLa cells were grown on coverslips in six-well plates for 3 days at a starting concentration of 106 cells/ml. Cells were incubated with L. reuteri (107 cells/ml) for 2 h, followed by TNF-α (10 ng/ml) incubation for 30 min. Coverslips were washed twice with PBS, fixed with 98% ethanol for 5 min, and then washed twice with PBS containing 0.2% Tween 20 (PBST). Cells were incubated with PBST containing 5% mouse serum for 15 min and then incubated with either 6 μg of mouse anti-NF-κB antibody or mouse IgG per ml for 30 min. Cells were washed three times in PBST, blocked with 5% goat serum in PBST for 15 min, incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:64) for 30 min, washed three times, and mounted in glycerol-PBS. Slides were viewed in an LSM510 (Carl Zeiss, Jena, Germany) confocal microscope.
Western blotting for IκB.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on a 12% precast gel (Bio-Rad, Hercules, Calif.) by using 10 μg per lane of whole-cell lysate. The protein was then transferred onto a BioTrace nitrocellulose membrane (VWR Scientific, Mississauga, Ontario, Canada) by using Tris-buffered saline with Tween 20 (TBST) and blocked with 10% FBS-TBST for 1 h. The membrane was washed with TBST three times. A 1:1,000 dilution of primary rabbit anti-IκB antibody was incubated with the membrane for 1 h and washed with TBST three times. A 1:25,000 dilution of goat anti-rabbit IgG conjugated with peroxidase was incubated with the membrane for 30 min, followed by washing six times with TBST. The results were revealed with an ECL system (Amersham Pharmacia Biotech, Piscataway, N.J.), and bands were semiquantified from scanned images by using Kodak Digital Science analysis software.
Statistics.
Experimental results are expressed as means ± the standard errors of the means. Statistical analyses were performed with unpaired two-tailed Student's t tests or one-way analysis of variance, followed by Newman-Keuls test for comparing all pairs of groups (GraphPad PRISMTM version 2.0). A P value of <0.05 was considered statistically significant, and n represents the number of experiments performed.
RESULTS
TNF-α induced IL-8 production. (i) Time course of response.
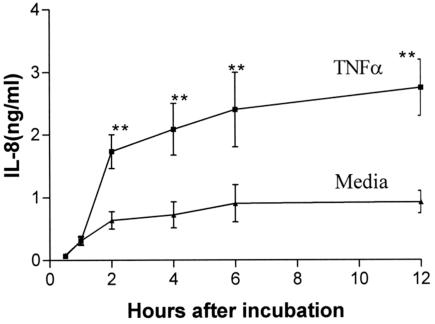
To obtain a time course for optimal IL-8 production, T84 cells were incubated with TNF-α (at a concentration of 10 ng/ml for 0.5, 1, 2, 4, 6, and 12 h). Significant increases in IL-8 production caused by TNF-α could be observed first at 2 h (Fig. 1). The 2-h incubation time was therefore chosen in all experiments on IL-8 induction of synthesis and secretion.
FIG. 1.
Time course of TNF-α-induced IL-8 response. T84 cells were incubated with TNF-α (10 ng/ml) for 0.5, 1, 2, 4, 6, and 12 h. T84 cells constitutively secreted IL-8. TNF-α significantly increased IL-8 secretion starting from 2 h of incubation with T84 cells. **, P < 0.01, compared with media control (n = 3).
(ii) Dose response.
To determine the optimal dose of TNF-α in this model, TNF-α (5 and 10 ng/ml) was incubated with T84 cells for 2 h. At a concentration of 10 ng/ml, TNF-α significantly (P < 0.01) induced IL-8 production (data not shown). This concentration was then used in all subsequent experiments.
L. reuteri inhibited TNF-α-induced upregulation of IL-8. (i) Dose-response effect.
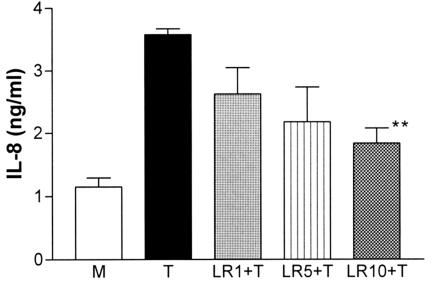
Different concentrations of L. reuteri (0.1 × 107, 0.5 × 107, and 1 × 107 cells/ml, which were relatively expressed as 1, 5, or 10 bacteria per T84 cell) were used to see the dose response of L. reuteri on TNF-α-induced IL-8 production. At a concentration of 107 cells/ml, live L. reuteri significantly (P < 0.01) inhibited (Fig. 2) but did not reduce the IL-8 secretion to baseline (media control). This concentration of L. reuteri was subsequently used in all experiments. The same effect of L. reuteri was also achieved in HT-29 cells (Fig. 3). CaCo-2 cells showed a significant constitutive secretion of IL-8 and less IL-8 secretion in response to TNF-α (data not shown), so this cell line was not used further in these experiments.
FIG. 2.
Dose response of L. reuteri on TNF-α-induced IL-8 production. L. reuteri at a concentration of 0.1 × 107 (LR1, 1 bacterium/cell), 0.5 × 107 (LR5, 5/cell), or 1 × 107 (LR10, 10/cell) cells/ml was used. Significant inhibition by L. reuteri of TNF-α-induced IL-8 secretion occurred at 1 × 107 cells/ml. M, media control; **, P < 0.01, compared to TNF-α control (n = 3). LR, L. reuteri; T, TNF-α.
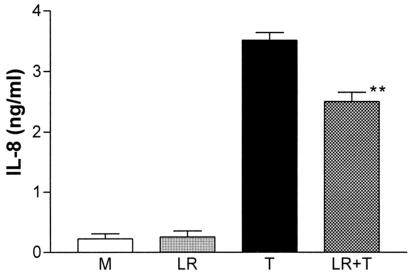
FIG. 3.
L. reuteri effect on TNF-α-induced IL-8 secretion by HT-29 cells. HT-29 cells constitutively secreted low levels of IL-8. L. reuteri significantly inhibited TNF-α-induced IL-8 secretion but not to the media (M) baseline. **, P < 0.01, compared with TNF-α (n = 3). LR, L. reuteri; T, TNF-α.
(ii) Intracellular IL-8.
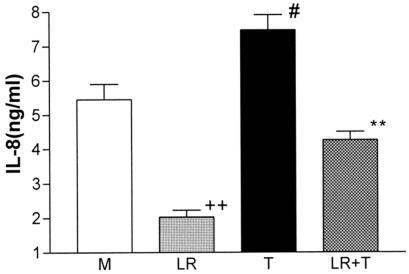
L. reuteri was more effective in the inhibition of TNF-α-induced intracellular IL-8 accumulation in brefeldin A-treated T84 cells (Fig. 4) than seen in the secretion studies. TNF-α effects were reduced to the baseline levels of medium alone. Despite relatively high normal constitutive levels of accumulated IL-8, L. reuteri preincubation significantly (P < 0.01) reduced this below baseline.
FIG. 4.
L. reuteri effect on TNF-α-induced intracellular IL-8 accumulation in brefeldin A-treated T84 cells. The cell lysates were tested for IL-8. L. reuteri inhibited TNF-α-induced IL-8 intracellular accumulation to baseline and constitutive levels to below the baseline. **, P < 0.01, compared with TNF-α-treated cells; #, P < 0.05, compared to media control; ++, P < 0.01, compared to media control (n = 3). LR, L. reuteri; T, TNF-α; M, media control.
(iii) IL-8, IL-10, and NGF mRNA.
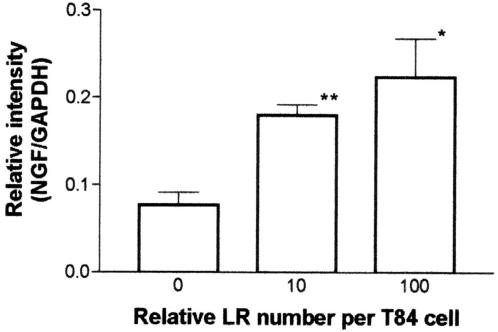
Preincubation with L. reuteri (10 organisms/cell plated) significantly (P < 0.01) reduced increases in TNF-induced mRNA for IL-8 both at 30 min (data not shown) and 2 h (Fig. 5). These treatments did not affect IL-10 mRNA in T84 and HT-29 cells (data not shown); however, NGF mRNA was significantly upregulated (Fig. 6) in both these cell lines at both time points. In contrast, the pathogenic bacterium S. enterica serovar Typhimurium did not affect NGF mRNA when plated at 10 organisms/cell for 2 h (data not shown).
FIG. 5.
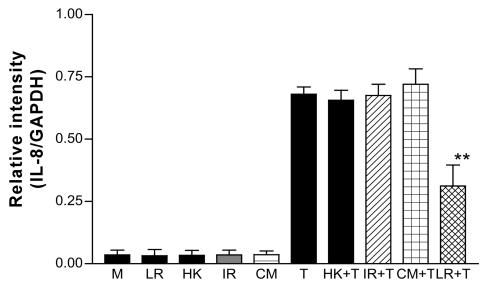
Effects of L. reuteri and related products on TNF-α-induced IL-8 mRNA increase in T84. Live L. reuteri, heat-killed (HK) L. reuteri, gamma-irradiated (IR) L. reuteri, and CM did not change constitutive expression. Live L. reuteri, but not heat-killed or gamma-irradiated L. reuteri or CM, significantly inhibited TNF-α (T)-induced IL-8 mRNA increase. **, P < 0.01, compared with TNF-α-treated cells (n = 3). LR, L. reuteri; T, TNF-α.
FIG. 6.
Effect of two concentrations of L. reuteri on NGF mRNA expression in T84 cells. L. reuteri was tested at zero, 1 × 107 cells/ml (10 bacteria/cell) or 10 × 107 cells/ml (100 bacteria/cell). L. reuteri significantly upregulated NGF mRNA in a dose-dependent manner. LR, L. reuteri; *, P < 0.05; **, P < 0.01 (P values are based on a comparison with media control [0]; n = 3).
L. reuteri inhibits IL-8 release induced by S. enterica serovar Typhimurium.
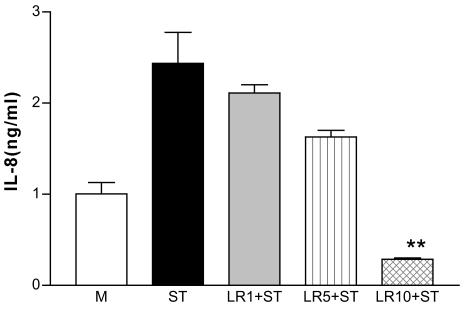
As previously reported, S. enterica serovar Typhimurium induced synthesis of IL-8 by T84 cells (25) to a degree similar to that observed following exposure to TNF-α. L. reuteri (0.1 × 107, 0.5 × 107, and 1 × 107 cells/ml, which were relatively expressed as 1, 5, or 10 bacteria per T84 cell) was used to see the dose response of L. reuteri on serovar Typhimurium-induced IL-8 production. With a 2-h preincubation with 107 cells/ml, live L. reuteri significantly (P < 0.01) inhibited (Fig. 7) the serovar Typhimurium-induced IL-8 secretion.
FIG. 7.
Dose response of L. reuteri on S. enterica serovar Typhimurium-induced IL-8 production. L. reuteri at a concentration of 0.1 × 107 (LR1, 1 bacterium/cell), 0.5 × 107 (LR5, 5/cell), or 1 × 107 (LR10, 10/cell) cells/ml was used. Significant inhibition by L. reuteri of S. enterica serovar Typhimurium (ST)-induced IL-8 secretion occurred at 1 × 107 cells/ml. M, media control; **, P < 0.01, compared to serovar Typhimurium control (n = 3). LR, L. reuteri.
Live L. reuteri is essential for the effect on the TNF-α- and serovar Typhimurium-induced IL-8 elevation.
L. reuteri was preincubated with T84 cells for 2 h, and the culture was then washed extensively (three times) in PBS. Control wells were Gram stained and examined. Very few bacteria were seen compared with unwashed cells cultured with bacteria for the same period. After washing there were no differences in IL-8 mRNA levels between L. reuteri pretreated and nontreated cells after the addition of TNF-α to cultures for 30 min or 2 h (data not shown). When killed L. reuteri was preincubated with T84 cells for 2 h, no effect on TNF-α-induced IL-8 mRNA upregulation was observed (Fig. 5). Similarly, the CM alone was without effect. The secretion of IL-8 revealed by ELISA showed the same patterns. Neither CM nor BCM nor killed L. reuteri had any inhibitory effect on the TNF-α-induced IL-8 increase in both T84 and HT-29 cells (data not shown). Only when L. reuteri organisms were alive, adherent to the cells, and present in coculture was the inhibitory effect on TNF-α-induced IL-8 mRNA upregulation and protein secretion observed. Similarly, IL-8 secretion induced by serovar Typhimurium was not altered by preincubation with either heat-killed or gamma-irradiated L. reuteri (data not shown).
Loss of effect of L. reuteri when simultaneously incubated with TNF-α.
When L. reuteri and TNF-α were added simultaneously to the T84 cell culture, the bacteria had no effect on TNF-α-induced IL-8 secretion or increases in mRNA (data not shown). This indicates that preincubation with L. reuteri was essential for the effect, which was not due to interference with the binding of TNF-α to its receptor.
Preincubation of L. reuteri with TNF-α did not abolish the subsequent effect of TNF-α on IL-8 production.
To further exclude any degradative effect of L. reuteri on TNF-α itself, TNF-α (10 ng/ml) was incubated with L. reuteri (107 cells/ml in the culture medium without P-S). The media were collected after a 2-h incubation and centrifuged at 890 × g for 15 min. Supernatants were then used as a source of TNF-α. The preincubated TNF-α performed as it did in the native form prior to incubation with bacteria, causing elevation of IL-8 secretion. This and the previous experiment show that the bacteria did not, at least at the concentrations of bacteria and TNF-α used in the experiments, inactivate or degrade TNF-α activity.
L. reuteri prevented TNF-α-induced NF-κB translocation.
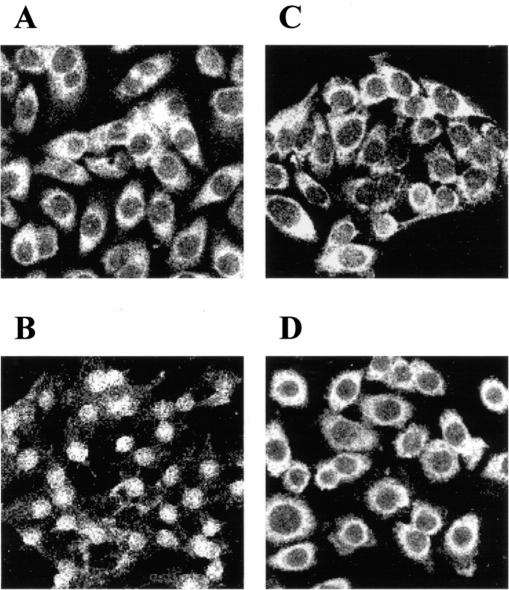
It is known that NF-κB plays a key role in the regulation of several gene groups, and its translocation from cytoplasm to nucleus activates the process, including IL-8 gene expression and protein synthesis (4). HeLa cells were used as a model to assess NF-κB activation since they have an optimal nucleus-to-cytoplasm ratio and are epithelial cells. As shown in Fig. 8A, NF-κB p65 was constitutively expressed in the cytoplasm of HeLa cells. Incubation of TNF-α (10 ng/ml) with HeLa cells for 30 min already caused p65 translocation into the nucleus (Fig. 8B). Preincubation with L. reuteri alone for 1 or 2 h did not cause p65 translocation into the nucleus (Fig. 8C), but L. reuteri prevented TNF-α-induced p65 translocation and promoted NF-κB condensation around the nucleus (Fig. 8D).
FIG. 8.
Effect of L. reuteri on NF-κB translocation to the nucleus. Immunohistochemical staining for NF-κB p65 constitutively expressed in the cytoplasm of the normal HeLa cells (A). Incubation with TNF-α for 30 min caused p65 translocation (B). Preincubation with L. reuteri for 1 h did not cause p65 translocation (C) but prevented TNF-α-induced translocation and promoted p65 condensation around the nucleus (D).
L. reuteri inhibited TNF-α-induced IκB degradation.
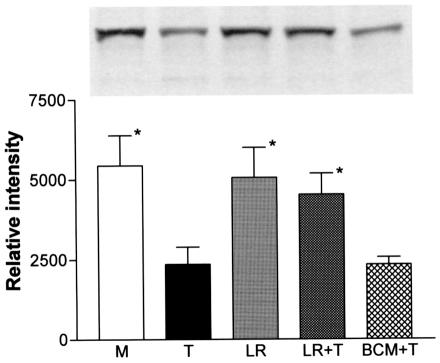
To study the inhibition effect of L. reuteri on NF-κB further, a Western blot analysis of IκB was conducted on whole T84 cell lysates. As shown in Fig. 9, incubation of TNF-α with T84 cells for 2 h caused a significant reduction of the amount of IκBα compared with the media control without the added cytokine (P < 0.05). There were no differences in the amounts of IκBα between L. reuteri-pretreated T84 cells and media controls. Preincubation of L. reuteri with T84 significantly (P < 0.05) blocked the TNF-α-induced IκBα reduction. Preincubation of BCM with T84 cells for 2 h did not block the TNF-α effect on IκB.
FIG. 9.
Effect of L. reuteri on IκB degradation. L. reuteri (LR), BCM, or media (M) was incubated with T84 cells for 2 h, followed by TNF-α (T) challenge for 2 h. Whole-cell lysates were used for IκB Western blotting. IκB-specific band intensity was significantly decreased by TNF-α. BCM did not block the effect, but L. reuteri significantly inhibited the TNF-α effect. *, P < 0.01, compared with TNF-α-treated cells (n = 3).
DISCUSSION
Probiotics have been shown to have beneficial health effects (9). Many different activities have been ascribed to probiotics; however, the mechanisms whereby these effects are achieved are poorly understood. The effects include enhanced innate and acquired immunity (6), increased anti-inflammatory cytokine production (IL-10) (30), and reduced intestinal permeability (20). Various strains of lactobacillus have been particularly well studied both in animals and humans. They may be effective in preventing and treating traveler's diarrhea (23), recurrent Clostridium difficile infection (8), rotavirus (36), and Helicobacter infections (28 002). L. Reuteri isolated from mouse intestine inhibited the onset of colitis in IL-10 transgenic knockout mice (22). A clinical trial with a mixture of probiotics has shown significant improvement in chronic pouchitis (7). A number of clinical trials of inflammatory bowel disease are now planned or under way.
In a review on how probiotics may effect bacterial-epithelial cross talk, Lu and Walker (17) concluded that despite information about the competition with pathogen for attachments, strengthening tight junctions between enterocytes, and enhancing mucosal immune responses to pathogens, additional molecular studies are needed to find more precisely the mechanism of probiotic-epithelial cross talk. Almost all probiotics in clinical use have been selected in part on the basis of their capacity to colonize the intestinal epithelium. This is why our experiments were conducted on human intestinal epithelial cell lines.
We show that L. reuteri inhibited the constitutive synthesis of IL-8 and inhibited the synthesis and secretion of IL-8 induced by TNF-α. In this respect our results extend those of Neish et al. (29), who showed that a nonpathogenic Salmonella (Salmonella pullorum) similarly inhibited TNF-α-induced IL-8 production by T84 cells. In contrast, the pathogenic organism S. enterica serovar Typhimurium induced IL-8 synthesis by T84 cells. Given evidence implicating enteric bacteria in the pathogenesis of murine models of colitis and inflammatory bowel disease in humans (31), the demonstrated ability of L. reuteri to inhibit the IL-8 response to serovar Typhimurium may be of particular physiologic relevance. Our results showed that in order to induce these inhibitory effects, L. reuteri must be preincubated with the epithelial cells and be adherent and alive. Furthermore, the effect was not reproduced by conditioned media, bacterial lysates, or heat-killed or gamma-irradiated organisms. Our findings contrast with those of Rachmilewitz et al. (32), who report that the anti-inflammatory effects of probiotics in a murine model of colitis depend on the action of bacterial DNA on Toll-like receptor 9 and that live microorganisms are not required to mediate these effects. The inability of gamma-irradiated L. reuteri to inhibit TNF-α-induced IL-8 secretion suggests that there are a variety of pathways involved in the anti-inflammatory effects of probiotics, not all of which depend on signaling by Toll-like receptor 9. The inhibitory effect appears at least in part to have occurred through the NF-κB pathway, since live L. reuteri prevented TNF-α-induced NF-κB translocation into the nuclei of HeLa cells through the inhibition of IκB degradation induced by TNF-α. Neither CM, bacterial lysates, or killed bacteria had this effect. Kelly et al. (15) have demonstrated that the anti-inflammatory activity of the commensal gut bacterium Bacteroides thetaiotaomicron does not involve inhibition of IκB degradation but selectively antagonizes NF-κB via a previously unknown mechanism. This indicates that there are multiple mechanisms through which a probiotic organism might impinge on NF-κB signaling to mediate an anti-inflammatory response. The mechanisms utilized may vary between probiotic species. NF-κB regulates several important physiological processes, including inflammation. Inhibition of this transduction pathway can block specific gene expression, e.g., IL-8 (4). L. reuteri inhibited NF-κB translocation to the nucleus and thereby prevented NF-κB binding to DNA or its interactions with the basal transcription machinery. NF-κB is clearly involved in the generation of proinflammatory pathways, including IL-8, which is the major chemotactic factor inducing leukocyte infiltration (1). Thus, the inhibition we have seen of IL-8 activation caused by a major probiotic lactobacillus strain may be useful where polymorphonuclear leukocytes are an integral component of the local inflammatory response. The timing of this effect may be crucial, since Lawrence et al. (16) have suggested that the NF-κB pathway may also be an important anti-inflammatory pathway in the resolution of inflammation. Additionally, Miettinen et al. (26) have shown that gram-positive bacteria (including lactobacilli) can cause rapid activation of NF-κB in human primary macrophages and cytokines including IL-10 in peripheral blood monocytes (27). However, this is unlikely to be a major issue in vivo unless lactobacilli are translocated across the epithelium.
Our experiments differ from those of Neish et al. (29) in several ways. While we did not see significant IL-8 secretion induced by TNF-α in T84 cells under 2 h of incubation, we did show dramatic inhibition of mRNA for IL-8 within 30 min of coculture both constitutively (Fig. 4) and in the presence of TNF-α. Recent work on probiotics has shown in vivo that certain lactobacilli (Lactobacillus casei and Lactobacillus bulgaricus) but not Lactobacillus crispatus or nonpathogenic Escherichia coli reduced the secretion of TNF-α release by mucosal explants from patients undergoing surgery for Crohn's disease (2). Coculture with these organisms further reduced the number of CD4+ cells as well as TNF-α expression by intraepithelial lymphocytes. However, no such results were obtained with normal healthy mucosa. These authors did not examine the effect of these organisms on the epithelium itself.
Another lactobacillus (Lactobacillus rhamnosus GG) prevented cytokine-induced apoptosis in both mouse and human epithelial cells. In these experiments, products were found in CM which promoted survival of the intestinal cells (37). Isolauri and associates (14, 30) have shown in a number of studies that in children with severe atopic dermatitis and eczema, this organism promotes intestinal integrity, reduces permeability, increases circulating IL-10, and reduces fecal eosinophil products as well as improving clinical status. Maassen et al. (19) have shown convincingly in a murine model that different organisms and even the culture conditions of individual strains differentially affected cytokine synthesis in murine intestine. However, given the fact that L. reuteri inhibits the colitis which occurs in IL-10-deficient transgenic mice, it is clear that the induction of IL-10 cannot solely explain the inhibitory effects of this or other probiotic organisms (11, 22).
Our experiments show that L. reuteri did not by itself, at different concentrations of bacteria, upregulate mRNA for IL-10 in either of the intestinal cell lines used. This may reflect the findings of Christensen et al. (3) that L. reuteri has a reduced capacity to induce IL-10 production in dendritic cells when compared to other lactobacilli species. Examination of other species within the genus will help clarify this point. It is of particular interest that L. reuteri but not the pathogen serovar Typhimurium selectively upregulated mRNA for NGF, which has been shown to have anti-inflammatory activity in a hapten-induced model of colitis (33) and in a model of acute allergic encephalomyelitis (5). Whether the autocoid reciprocal stimulation of IL-10 by NGF (18) is a factor that is important in this cross talk is currently being studied. Haller et al. (10) showed that some nonpathogenic bacteria can elicit a differential cytokine response depending on the presence of underlying leukocytes in a coculture model with CaCo-2 cells. We have been unable to show an increase in transforming growth factor β (TGF-β) in previous studies with T84, CaCo-2, and HT-29 cells because of the high constitutive level of synthesis of this cytokine and, therefore, cannot exclude the role of TGF-β in our own experiments.
McCarthy et al. (24) have shown recently that both a lactobacillus and bifidobacteria fed to IL-10-deficient mice not only inhibited the onset of colitis but caused a downregulation of synthesis by splenocytes and Peyer's patch lymphocytes of gamma interferon and TNF-α while not affecting TGF-β. So, the immunoregulatory effects of the oral ingestion of probiotics can extend beyond the intestine. We tentatively conclude that certain probiotic organisms have the capacity to locally and directly stimulate activation pathways in the epithelium which inhibit an innate inflammatory response. The direct contact of the bacteria with epithelial cells was essential for the inhibition of TNF-α-induced IL-8 to occur. There is a need to establish the molecular basis of the mechanisms of action of these organisms to explain the beneficial effects which they appear to induce both within the intestine and systemically in different diseases (34).
Acknowledgments
The authors gratefully acknowledge the assistance of Gudrun Goettsche, Pam Lyn, and Beth Dixon and the advice of Fiona Smaill.
Editor: F. C. Fang
REFERENCES
- 1.Baggiolini, M., and P. Loetscher. 2000. Chemokines in inflammation and immunity. Immunol Today 21:418-420. [DOI] [PubMed] [Google Scholar]
- 2.Borruel, N., M. Carol, F. Casellas, M. Antolin, F. de Lara, E. Espin, J. Naval, F. Guarner, and J. R. Malagelada. 2002. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. GUT 51:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen, H. R., H. Frøkiær, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 166:171-178. [DOI] [PubMed] [Google Scholar]
- 4.Epinat, J. C., and T. D Gilmore. 1999. Diverse agents act at multiple levels to inhibit the Rel/NF-kappaB signal transduction pathway. Oncogene 18:6896-6909. [DOI] [PubMed] [Google Scholar]
- 5.Flugel, A., K. Matsumuro, H. Neumann, W. E. Klinkert, R. Birnbacher, H. Lassmann, U. Otten, and H. Wekerle. 2001. Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis: inhibition of monocyte transendothelial migration. Eur. J. Immunol. 31:11-22. [DOI] [PubMed] [Google Scholar]
- 6.Gill, H. S., K. J. Rutherfurd, J. Prasad, and P. K Gopal. 2000. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br. J. Nutr. 83:167-176. [DOI] [PubMed] [Google Scholar]
- 7.Gionchetti, P., F. Rizzello, A. Venturi, P. Brigidi, D. Matteuzzi, G. Bazzocchi, G. Poggioli, M. Miglioli, and M. Campieri. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305-309. [DOI] [PubMed] [Google Scholar]
- 8.Gorbach, S. L. 1987. Bacterial diarrhoea and its treatment. Lancet ii:1378-1382. [DOI] [PubMed] [Google Scholar]
- 9.Gorbach, S. L. 2000. Probiotics and gastrointestinal health. Am. J. Gastroenterol. 95:S2-S4. [DOI] [PubMed] [Google Scholar]
- 10.Haller, D., C. Bode, W. P. Hammes, A. M. Pfeifer, E. J. Schiffrin, and S. Blum. 2000. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. GUT 47:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid, Q. A., E. Schotman, M. R. Jacobson, S. M. Walker, and S. R. Durham. 1997. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J. Allergy Clin. Immunol. 99:254-260. [DOI] [PubMed] [Google Scholar]
- 12.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalliomaki, M., S. Salminen, H. Arvilommi, P. Kero, P. Koskinen, and E. Isolauri. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076-1079. [DOI] [PubMed] [Google Scholar]
- 14.Kalliomaki, M., S. Salminen, T. Poussa, H. Arvilommi, and E. Isolauri. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo controlled trial. Lancet 361:1869-1871. [DOI] [PubMed] [Google Scholar]
- 15.Kelly, D., J. I. Campbell, T. P. King, G. Grant, E. A. Jansson, A. P. G. Coutts, S. Pettersson, and S. Conway. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and ReIA. Nat. Immunol. 5:104-112. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence, T., D. W. Gilroy, P. R. Colville-Nash, and D. A Willoughby. 2001. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 7:1291-1297. [DOI] [PubMed] [Google Scholar]
- 17.Lu, L., and W. A. Walker. 2001. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am. J. Clin. Nutr. 73:1124S-1130S. [DOI] [PubMed] [Google Scholar]
- 18.Ma, D., D. Wolvers, A. Stanisz, and J. Bienenstock. 2003. Interleukin-10 and nerve growth factor have reciprocal upregulatory effects on intestinal epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284:R1323-R1329. [DOI] [PubMed] [Google Scholar]
- 19.Maassen, C. B., C. Holten-Neelen, F. Balk, M. J. Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 20.Madsen, K., A. Cornish, P. Soper, C. McKaigney, H. Jijon, C. Yachimec, J. Doyle, L. Jewell, and C. De Simone. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580-591. [DOI] [PubMed] [Google Scholar]
- 21.Madsen, K. L. 2001. The use of probiotics in gastrointestinal disease. Can. J. Gastroenterol. 15:817-822. [DOI] [PubMed] [Google Scholar]
- 22.Madsen, K. L., J. S. Doyle, L. D. Jewell, M. M. Tavernini, and R. N. Fedorak. 1999. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116:1107-1114. [DOI] [PubMed] [Google Scholar]
- 23.Marteau, P. R., M. de Vrese, C. J. Cellier, and J. Schrezenmeir. 2001. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 73:430S-436S. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy, J., L. O'Mahony, L. O'Callaghan, B. Sheil, E. E. Vaughan, N. Fitzsimons, J. Fitzgibbon, G. C. O'Sullivan, B. Kiely, J. K. Collins, and F. Shanahan. 2003. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. GUT 52:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen, M., A. Lehtonen, I. Julkunen, and S. Matikainen. 2000. Lactobacilli and streptococci activate NF-κB and STAT signaling pathways in human macrophages. J. Immunol. 164:3733-3740. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 64:5403-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukai, T., T. Asasaka, E. Sato, K. Mori, M. Matsumoto, and H Ohori. 2002. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 32:105-110. [DOI] [PubMed] [Google Scholar]
- 29.Neish, A. S., A. T. Gewirtz, H. Zeng, A. N. Young, M. E. Hobert, V. Karmali, A. S. Rao, and J. L. Madara. 2000. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 289:1560-1563. [DOI] [PubMed] [Google Scholar]
- 30.Pessi, T., Y. Sutas, M. Hurme, and E. Isolauri. 2000. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin. Exp. Allergy 30:1804-1808. [DOI] [PubMed] [Google Scholar]
- 31.Podolsky, D. K. 2002. Inflammatory bowel disease. N. Engl. J. Med. 347:417-429. [DOI] [PubMed] [Google Scholar]
- 32.Rachmilewitz, D., K. Katakura, F. Karmeli, T. Hayashi, C. Reinus, B. Rudensky, S. Akira, K. Takeda, J. Lee, K. Takabayashi, and E. Raz. 2004. Toll-like receptor signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126:520-528. [DOI] [PubMed] [Google Scholar]
- 33.Reinshagen, M., H. Rohm, M. Steinkamp, K. Lieb, I. Geerling, A. Von Herbay, G. Flamig, V. E. Eysselein, and G. Adler. 2000. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology 119:368-376. [DOI] [PubMed] [Google Scholar]
- 34.Shanahan, F. 2000. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm. Bowel Dis. 6:107-115. [DOI] [PubMed] [Google Scholar]
- 35.Svanborg, C., G. Godaly, and M. Hedlund. 1999. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 2:99-105. [DOI] [PubMed] [Google Scholar]
- 36.Szajewska, H., M. Kotowska, J. Z. Mrukowicz, M. Armanska, and W. Mikolajczyk. 2001. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J. Pediatr. 138:361-365. [DOI] [PubMed] [Google Scholar]
- 37.Yan, F., and D. B Polk. 2002. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277:50959-50965. [DOI] [PMC free article] [PubMed] [Google Scholar]