Abstract
The most common type of infective endocarditis is bacterial endocarditis. However, fungal infections have been seen more frequently, mostly in the immunocompromised population. We report a case of invasive Aspergillus fumigatus native mitral valve endocarditis. The patient received appropriate empiric antifungal treatment with a combination of liposomal amphotericin B and flucytosine, associated with surgical debridement, valve replacement and chordae tendineae repair. Despite receiving the standard treatment of Aspergillus endocarditis, and susceptibility of the microorganism to the antifungal regimen, the patient, unexpectedly, developed early-onset septic emboli. It is surprising to see that the patient had developed such complications early, despite attempts to eliminate the source of infection with surgical intervention.
Background
Fungal endocarditis is unfortunately associated with considerable morbidity and mortality.1 2 The management of fungal endovascular infections routinely require early surgical debridement associated with medical treatment.2 However, the survival rate still appears to be <20%, and thus, rapid diagnosis and treatment are crucial.2 Given that none of the fungal pathogens are considered ‘typical microorganisms’ according to Duke's criteria, and Aspergillus spp (unlike Candida spp) rarely produces positive blood cultures, the diagnosis of this type of endocarditis is usually challenging and requires high index of suspicion.2 Combination of early surgical intervention and antifungal therapy is considered as standard management for Aspergillus endocarditis. In this manuscript, we present a case of Aspergillus endocarditis with severe complications early after treatment. To the best of our knowledge, early-onset septic embolisation despite combination of immediate surgical debridement and standard antifungal treatment has not been explained in the medical literature. This complication was not expected due to full susceptibility of the microorganism to the antifungal therapy used. We also review the current medical literature related to the management of Aspergillus endocarditis.
Case presentation
The patient was a 64-year-old woman who was immunocompromised secondary to a recent diagnosis of acute promyelocytic leukaemia (APL). She initially presented with generalised fatigue over 6–8 weeks, associated with dyspnoea and fever. Her physical examination revealed a systolic heart murmur loudest at the apex, and radiating to the right axilla. There was no evidence of rash, joint pain, stiffness, splinter haemorrhage, Osler's nodes or Janeway lesions. Two months earlier, the patient was initiated on immunosuppressive chemotherapy for APL including idarubicin along with all-trans-retinoic acid (ATRA). Her medical history included hypertension and hypothyroidism due to thyroidectomy after the diagnosis of a malignant thyroid nodule. She also had a 15-pack-year history of smoking and social casual drinking of alcohol. She had no history of illicit drug use. A month prior to her presentation, she had developed severe pancytopenia and febrile neutropenia, likely secondary to chemotherapy.
Investigations
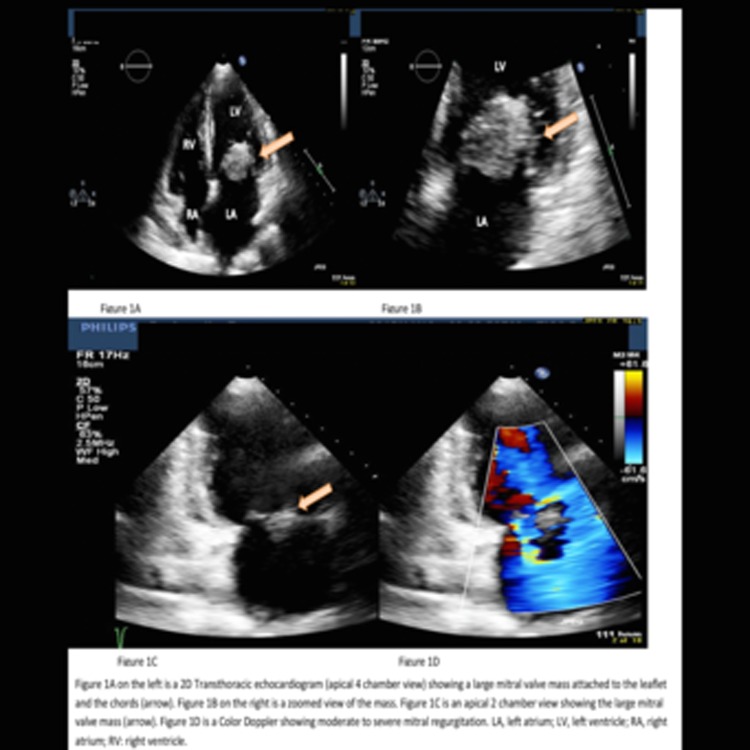
Initial investigation revealed a WCC of 18.9×109/L, Hgb of 72 g/L and Plt of 59×109/L. Transthoracic echocardiogram showed a mitral valve and mitral chords masses with moderate to severe mitral regurgitation, suspicious for infective endocarditis (figure 1). A transoesophageal echocardiogram (with 3D video capture) confirmed mobile masses on both mitral valve leaflets, with the largest being 2.0×1.2 cm in diameter.
Figure 1.
A transthoracic echocardiogram showing a mitral valve and mitral chords masses with significant mitral regurgitation.
Her blood cultures, legionella urinary antigen, anti-Brucella antibody, Coxiella burnetii IgG and Bartonella henselae IgG were all negative. Chest X-ray (CXR) showed diffuse interstitial pulmonary oedema. Her chest CT showed bilateral effusion and pulmonary oedema. Brain CT revealed multiple septic emboli involving the anterior limb of the left internal capsule, adjacent caudate nucleus head and periventricular area (figure 2).
Figure 2.
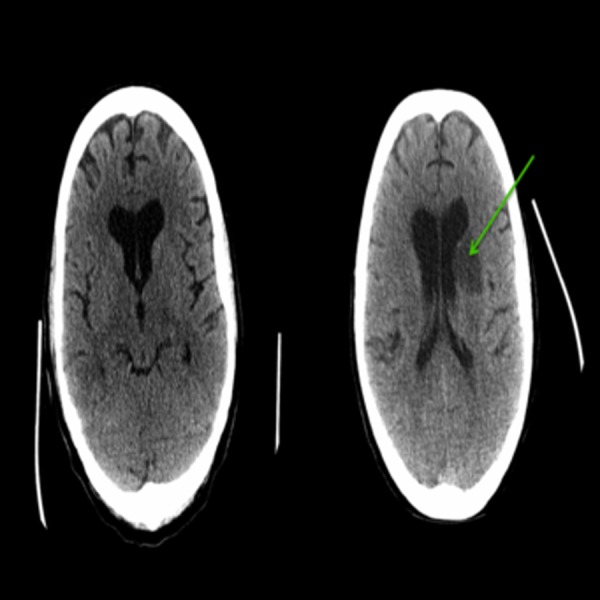
A CT-head showing an ill-defined hypodensities suspicious for acute infarcts involving the anterior limb of the left internal capsule, adjacent caudate nucleus head and periventricular area later identified as secondary to septic emboli of Aspergillus endocarditis.
A repeat intraoperative transthoracic echocardiogram assessment captured an excellent view of the mitral valve mobile mass (video 1). Intraoperative tissue cultures grew Aspergillus fumigatus. Tracheal aspirate did not show infection with fungal microorganisms. Histopathologic assessment showed evidence of endomyocarditis secondary to mould infection compatible with Aspergillus spp.
Video 1.
An intraoperative transthoracic echocardiogram capturing a view of the mobile nature of the mitral valve mass.
While being on antifungal therapy and following surgical debridement, her repeat brain CT revealed multifocal new intraparenchymal haemorrhage within the left cerebral hemisphere dissecting into the ventricular system associated with diffuse cerebral oedema. There was also a 1.1 cm midline shift to the right complicated by uncal herniation (figure 3).
Figure 3.
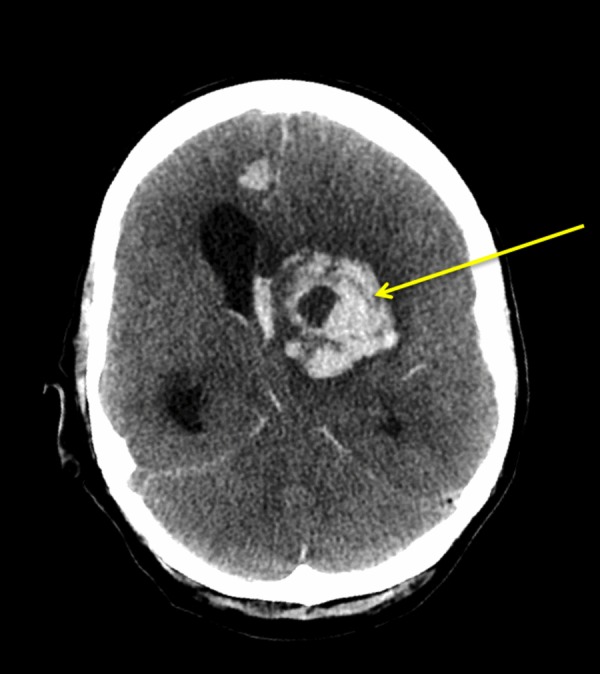
A CT head showing multifocal intraparenchymal haemorrhages within the left cerebral hemisphere dissecting into the ventricular system, with diffuse cerebral oedema and a 1.1 cm midline shift to the right.
Differential diagnosis
Differential diagnosis of culture-negative endocarditis: acute infective endocarditis with fastidious microorganisms, nonbacterial thrombotic endocarditis, vasculitis, acute rheumatic fever, antiphospholipid syndrome, connective tissue diseases, cholesterol emboli syndrome, temporal arteritis and atrial myxoma.
The patient's initial presentation of fever, leucocytosis, and newly appreciated murmur consistent with mitral regurgitation suggested endocarditis. Since no improvement occurred with broad-spectrum antibiotics, other differentials were then considered. Endocarditis remained high on the differential especially given that repeated echocardiograms confirmed a rapidly growing mass with signs of early septic emboli, despite the initial negative cultures. Although initial echocardiograms of the mobile masses did not produce images consistent with marantic endocarditis or atrial myxoma, it could not be absolutely confirmed as infectious in nature until tissue biopsy confirmed it. The culprit pathogen responsible was then diagnosed as Aspergillus fumigatus.
Treatment
Initial empiric antibiotic regimen included intravenous ceftriaxone and vancomycin. The patient failed to show any sign of improvement despite persistently negative blood cultures. Her imaging studies showed evidence of septic brain emboli, while her general condition was deteriorating. We repeated her echocardiogram, which showed an increase in size of the mitral valve masses. As a result, surgical intervention was expedited with suspicious diagnosis of fungal endocarditis. Initial staining of tissue specimens indicated fungal elements and empiric intravenous liposomal Amphotericin B (AMB) 5 mg/kg/dose plus oral flucytosine (5-FC) 25 mg/kg/dose every 6 hours were initiated. Culture results indicated Aspergillus fumigatus. We continued antifungal therapy and closely monitored kidney function tests and serum electrolytes.
Outcome and follow-up
The patient initially showed signs of improvement after her surgical intervention of mitral valve replacement and chordae tendineae repair along with antifungal therapy. Unfortunately, despite lack of vegetation on subsequent echocardiogram over the 2 weeks following her surgery, she developed intracranial haemorrhage and uncal herniation leading to her death.
Discussion
Infective endocarditis is usually caused by bacterial infection.1 Fungal microorganisms are the causes of infective endocarditis with relative frequency of only ∼2–4%.1 It is important to note that fungal endocarditis is mostly seen in patients with prosthetic cardiac valves and native valve fungal endocarditis is rare.1 However, the frequency of fungal endocarditis seems to be rising.3 Candida spp account for ∼52% of fungal endocarditis cases, and it appears to be the most common causative organism.1 Less common organisms include Aspergillus spp, which account for ∼24%, Histoplasma capsulatum ∼6% and others combined for the remaining 17%.2 4 More importantly, Aspergillus endocarditis may be associated with significant morbidity and mortality.5
The diagnostic criteria for infective endocarditis depend on history, physical examination and certain laboratory, microbiological and imaging findings. There is strong evidence supporting the use of the modified Duke criteria for the diagnosis of infective endocarditis.6 Diagnosis for fungal endocarditis applies the same criteria. However, fungal microorganisms usually cause culture-negative endocarditis (negative blood cultures), and the diagnosis is challenging.
This report represents a case of endocarditis with persistent negative blood cultures despite fulfilling criteria of infective endocarditis. Blood culture may be negative in ∼2.5–31% of patients with infective endocarditis, but most are secondary to initiating empiric antibiotics prior to obtaining blood cultures.7 However, there are still times of true culture-negative cases even in the setting of excellent technique of obtaining blood cultures prior to initiating antibiotics.7
In practice, patients usually receive empiric treatment once blood cultures are drawn.1 The empiric therapy typically consists of only antibiotics to cover bacterial endocarditis. It is important to consider the possibility of fungal endocarditis specifically in patients with history of diabetes mellitus, prolonged indwelling intravenous lines and those who are immunocompromised.3 Unfortunately, empiric antifungal treatment is usually delayed due to lack of suspicion and difficulty in confirming the diagnosis.4
A common and serious complication of infective endocarditis is septic embolisation to vital organs, which occurs in 13–44% of cases.8–10 The most common organs that could be affected by septic emboli are lungs, in right-sided infective endocarditis, and brain, in left-sided endocarditis.8 10 In the current case report, the patient developed fatal brain emboli that caused a severe haemorrhagic stroke and uncal herniation despite susceptibility of the causative microorganism to the antifungals used and surgical treatment to remove the source of infection; the mitral valve vegetation. This complication could be explained by the progressive microvascular invasion in pathophysiology of systemic infection with Aspergillus spp.
Evidence for treatment of Aspergillus fumigatus endocarditis is limited to case reports. The majority of case reports are related to prosthetic valve endocarditis.1 Combination of liposomal AMB plus 5-FC, in addition to surgical valve replacement is considered the standard therapy for empiric fungal endocarditis.11–13 The efficacy of 5-FC is more evident in Candida spp. Endocarditis, and it is known to have a synergistic effect with AMB.14 AMB deoxycholate has been described in the limited literature as it is highly nephrotoxic and physicians prefer using the liposomal form of AMB instead, especially in renal dysfunction, as was the case with the patient explained in this manuscript.1 The treatment dose for liposomal AMB is 3–5 mg/kg/day.1 Initial treatment course of at least 6 weeks with subsequent prolonged oral suppressive therapy with an azole is highly recommended.15–17 It is important to note that there are some case reports supporting the initial use of voriconazole instead of AMB in the antifungal regimens, given the high toxicity of the latter.18 19
We have conducted a literature review for treatment cases of Aspergillus fumigatus endocarditis. We limited the results to those focused on humans and were available in English, yielding a total of 13 case reports, which are summarised in table 1.20–31 Table 1 describes the clinical presentations, whether or not the patient was immunocompromised, cardiac valve involved, complications from infective endocarditis, blood culture results, treatment regimens, whether or not the patient underwent early surgical intervention, and final outcomes of these cases. Although invasive pulmonary aspergillosis is almost strictly seen in immunocompromised patients, infective endocarditis with Aspergillus spp may still affect those who are immunocompetent1 32 33 (table 1). Second, although the mortality outcome seems to be similar between immunocompromised and immunocompetent patients, early surgical intervention appears to be crucial in the effective management of Aspergillus endocarditis. Third, as table 1 shows, Aspergillus fumigatus endocarditis is a serious infection associated with high mortality. The table shows 5 out of 13 patients did not survive despite antifungal therapy. Fourth, looking at the antifungal treatment, it seems combination therapy with antifungals had similar outcomes to those with monotherapy. Fifth, table 1 also shows 4 out of 5 patients who passed away did not receive voriconazole. Whether voriconazole provides protective effects against mortality in fungal endocarditis is not clear. This finding seems to be inconclusive as severity of endocarditis may have been different among the 13 cases reviewed. Further case reports and case series are required to improve our understanding of Aspergillus endocarditis. Comparative studies can determine the role of voriconazole in decreasing the mortality associated with Aspergillus endocarditis.
Learning points.
High index of suspicion for fungal endocarditis is required, specifically in patients with negative blood cultures, those who are immunocompromised, or patients who have failed to improve with empiric broad-spectrum antibiotics therapy.
In addition to early diagnosis and source elimination by surgical measures, early initiation of antifungal therapy is crucial.
Despite appropriate management, septic embolisation may occur. This risk should be explained to the patient before surgical intervention.
Current data on optimal treatment for fungal endocarditis, and more specifically Aspergillus endocarditis, is limited. Further case reports and other studies are required.
Table 1.
Summary of case reports of Aspergillus fumigatus endocarditis
| Case report (reference number) | Initial patient presentation | Immune-compromised status | Valve affected on echo | Complication | Blood culture result | Early surgical intervention | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1.20 | A 53-year-old male patient with multilobar nodular pneumonia | No | Native tricuspid valve | Pulmonary septic emboli | Negative | None | Intravenous AMB. | Patient passed away 16 days after admission while on antifungal therapy. |
| 2.21 | A 68-year-old man with cavitary pneumonia, involving left lung | Yes | Native mitral valve | Multiple liver abscesses, brain haemorrhage, acute renal failure, and endophthalmitis | Negative | Mitral valve vegetectomy | Initial treatment with micafungin, which was switched to AMB and subsequently voriconazole. | Patient survived with no sign of recurrence after 24 months follow-up. |
| 3.22 | An 18-year-old man status post bone marrow transplantation, chronic pneumonia | Yes | Native tricuspid valve extending into right atrium, interatrial septum and superior vena cava, and pacemaker wire. | None | Negative | Valvuloplasty and pacemaker removal | Intravenous course of voriconazole followed by indefinite oral voriconazole. | Patient survived with no sign of recurrence after 6 months follow-up. |
| 4.23 | A 65-year-old man with intermittent fever for 4 months | No | Native Tricuspid valve and interatrial septum | None | Negative | Valvuloplasty | Intravenous course of voriconazole followed by indefinite oral voriconazole. | Patient survived with no sign of recurrence after 6 months follow-up. |
| 5.24 | An 8 month-old infant status post liver transplantation, pneumonia with A. fumigatus | Yes | Native mitral valve chordae and papillary muscle, interventricle septum, left lateral ventricular wall, and apical right ventricle | None | Negative | None | Combination of voriconazole plus caspofungin; subsequently. AMB was added due to no clinical response. Her treatment followed by indefinite oral voriconazole. |
Patient survived with no sign of recurrence after 20 months follow-up. |
| 6.25 | A 52-year-old man status post pancreas-kidney transplantation, pneumonia | Yes | Native tricuspid valve anterior leaflet | Diffuse pulmonary septic emboli with pleural-based wedge-shaped lesions | Negative | Tricuspid valve replacement | Caspofungin plus oral voriconazole and aerosolized AMB deoxycholate. | Patient passed away from fatal haemoptysis 61 days after valve replacement surgery while still on antifungal therapy. |
| 7.26 | A 57-year-old man with a myocardial infarction, and cardiogenic shock found to have A. fumigatus endocarditis. | Yes | Native mitral valve anterior leaflet | Left circumflex coronary artery and first obtuse marginal artery mycotic aneurysms | Positive | Mitral valve replacement | Liposomal AMB, plus caspofungin. Voriconazole was only added during the first week of therapy. | Patient survived with no sign of recurrence after 3 months follow-up. |
| 8.18 | A 25-year-old man with pneumonia and haemoptysis. | No | Native tricuspid valve | Septic pulmonary emboli | Negative | Valvuloplasty | Liposomal AMB and oral voriconazole followed by suppressive treatment for 6 months with oral voriconazole. | Patient survived with no sign of recurrence after 6 months follow-up. |
| 9.27 | A 32-year-old man status post renal transplantation, thromboembolic events involving renal, iliac and common femoral arteries | Yes | Native mitral valve, posterior leaflet | Spondylodiscitis and cutaneous lesions | Negative | None (planned intention was to undergo surgical valve replacement) | Intravenous AMB for 12 days. | Patient passed away prior to surgical intervention. |
| 10.28 | A 64-year-old man with blurry vision, erythematous and swollen right eye, associated with tender nodules on his right palm (Osler's nodes) | Yes | Native aortic valve | Endophthalmitis | Negative | Aortic valve replacement | Intraocular AMB and intravenous liposomal AMB. | Patient survived with no sign of recurrence after 18 months follow-up. |
| 11.29 | A 10 month-old infant with acute respiratory distress 3 months after cardiac surgery. | No | Non-valvular. Vegetation on inferior aspect of ventricular septal defect patch. | Acute hepatic and renal failure with septic emboli and abscess formation | Negative | Surgical debridement and repair | Liposomal AMB plus flucytosine. | Patient survived with no sign of recurrence after 15 months follow-up. |
| 12.30 | A 66-year-old man with decreased vision and pain in the left eye, severe pain in the left calf followed by numbness and weakness in the left foot | No | Native mitral valve, subsequently recurrence with the prosthetic mitral valve involvement (post valve replacement) | Left femoral artery septic embolus | Negative | Mitral valve replacement | Intravenous AMB and. oral flucytosine. The second medication (flucytosine) was discontinued 4 days postoperation. | Patient passed away due to recurrence of endocarditis following valve replacement. |
| 13.31 | A 13-year-old woman with fever, acute severe pain of the right lower extremity | No | Prosthetic mitral valve | Septic emboli to the brain, right common iliac and femoral arteries | Negative | Mitral prosthetic valve replacement | Intravenously AMB. | Patient passed away 13 days after admission while on antifungal therapy. |
Footnotes
Contributors: RR, SMH-M, DM and YT contributed to this manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gould FK, Denning DW, Elliott TS et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012;67: 269–89. 10.1093/jac/dkr450 [DOI] [PubMed] [Google Scholar]
- 2.Baddour LM, Wilson WR, Bayer AS et al. , American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435–86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 3.Giamarellou H. Nosocomial cardiac infections. J Hosp Infect 2002;50:91–105. 10.1053/jhin.2001.1144 [DOI] [PubMed] [Google Scholar]
- 4.Ellis ME, Al-Abdely H, Sandridge A et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001;32:50–62. 10.1086/317550 [DOI] [PubMed] [Google Scholar]
- 5.El-Hamamsy I, Dürrleman N, Stevens LM et al. Aspergillus endocarditis after cardiac surgery. Ann Thorac Surg 2005;80:359–64. 10.1016/j.athoracsur.2004.08.070 [DOI] [PubMed] [Google Scholar]
- 6.Li JS, Sexton DJ, Mick N et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 7.Kupferwasser LI, Darius H, Müller AM et al. Diagnosis of culture negative endocarditis: the role of the Duke criteria and the impact of transesophageal echocardiography. Am Heart J 2001;142:146–52. 10.1067/mhj.2001.115586 [DOI] [PubMed] [Google Scholar]
- 8.Steckelberg JM, Murphy JG, Ballard D et al. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med 1991;114:635–40. 10.7326/0003-4819-114-8-635 [DOI] [PubMed] [Google Scholar]
- 9.De Castro S, Magni G, Beni S et al. Role of transthoracic and transesophageal echocardiography in predicting embolic events in patients with active infective endocarditis involving native cardiac valves. Am J Cardiol 1997;80:1030–4. 10.1016/S0002-9149(97)00598-5 [DOI] [PubMed] [Google Scholar]
- 10.Snygg-Martin U, Gustafsson L, Rosengren L et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis 2008;47:23–30. 10.1086/588663 [DOI] [PubMed] [Google Scholar]
- 11.Rao K, Saha V. Medical management of Aspergillus flavus endocarditis. Pediatr Hematol Oncol 2000;17:425–7. 10.1080/08880010050034382 [DOI] [PubMed] [Google Scholar]
- 12.Gumbo T, Taege AJ, Mawhorter S et al. Aspergillus valve endocarditis in patients without prior cardiac surgery. Medicine (Baltimore) 2000;79:261–8. 10.1097/00005792-200007000-00007 [DOI] [PubMed] [Google Scholar]
- 13.Wagner DK, Werner PH, Bonchek LI et al. Successful treatment of post-mitral valve annuloplasty Aspergillus flavus endocarditis. Am J Med 1985;79:777–80. 10.1016/0002-9343(85)90532-7 [DOI] [PubMed] [Google Scholar]
- 14.Pappas PG, Kauffman CA, Andes D r et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503–35. 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert HM, Peters ED, Lang SJ et al. Successful treatment of fungal prosthetic valve endocarditis: case report and review. Clin Infect Dis 1996;22:348–54. 10.1093/clinids/22.2.348 [DOI] [PubMed] [Google Scholar]
- 16.Melgar GR, Nasser RM, Gordon SM et al. Fungal prosthetic valve endocarditis in 16 patients. An 11-year experience in a tertiary care hospital. Medicine (Baltimore) 1997;76:94–103. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen MH, Nguyen ML, Yu VL et al. Candida prosthetic valve endocarditis: prospective study of six cases and review of the literature. Clin Infect Dis 1996;22:262–7. 10.1093/clinids/22.2.262 [DOI] [PubMed] [Google Scholar]
- 18.Vassiloyanakopoulos A, Falagas ME, Allamani M et al. Aspergillus fumigatus tricuspid native valve endocarditis in a non-intravenous drug user. J Med Microbiol 2006;55:635–8. 10.1099/jmm.0.46398-0 [DOI] [PubMed] [Google Scholar]
- 19.Walsh TJ, Hier DB, Caplan LR. Aspergillosis of the central nervous system: clinicopathological analysis of 17 patients. Ann Neurol 1985;18:574–82. 10.1002/ana.410180511 [DOI] [PubMed] [Google Scholar]
- 20.Vohra S, Taylor R, Aronowitz P. The tell-tale heart: Aspergillus fumigatus endocarditis in an immunocompetent patient. Hosp Pract (1995) 2013;41:117–21. 10.3810/hp.2013.02.1017 [DOI] [PubMed] [Google Scholar]
- 21.Kuroki K, Murakami T. Aspergillus endocarditis in a native valve without prior cardiac surgery. Gen Thorac Cardiovasc Surg 2012;60:771–3. 10.1007/s11748-012-0076-5 [DOI] [PubMed] [Google Scholar]
- 22.Kalokhe AS, Rouphael N, El Chami MF et al. Aspergillus endocarditis: a review of the literature. Int J Infect Dis 2010;14:e1040–7. 10.1016/j.ijid.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 23.Kothari A, Pillai BS, Bhan A. Pacing lead endocarditis due to Aspergillus fumigatus. Indian J Med Microbiol 2010;28:72–3. 10.4103/0255-0857.58737 [DOI] [PubMed] [Google Scholar]
- 24.Mourier O, Durand P, Lambert V et al. Aspergillus fumigatus endocarditis in a pediatric liver transplant recipient: favorable outcome without cardiac surgery. Pediatr Transplant 2009;13:636–40. 10.1111/j.1399-3046.2008.00929.x [DOI] [PubMed] [Google Scholar]
- 25.Van Meensel B, Meersseman W, Bammens B et al. Fatal right-sided endocarditis due to Aspergillus in a kidney transplant recipient. Med Mycol 2007;45:565–8. 10.1080/13693780701496517 [DOI] [PubMed] [Google Scholar]
- 26.Saxena P, Clarke B, Dunning J. Aspergillus endocarditis of the mitral valve in a lung-transplant patient. Tex Heart Inst J 2007;34:95–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Marín P, García-Martos P, García-Doncel A et al. Endocarditis by Aspergillus fumigatus in a renal transplant. Mycopathologia 1999;145:127–9. 10.1023/A:1007006122591 [DOI] [PubMed] [Google Scholar]
- 28.Keating MR, Guerrero MA, Daly RC et al. Transmission of invasive aspergillosis from a subclinically infected donor to three different organ transplant recipients. Chest 1996;109:1119–24. 10.1378/chest.109.4.1119 [DOI] [PubMed] [Google Scholar]
- 29.Hosking MC, MacDonald NE, Cornel G. Liposomal amphotericin B for postoperative Aspergillus fumigatus endocarditis. Ann Thorac Surg 1995;59:1015–7. 10.1016/0003-4975(94)00743-Q [DOI] [PubMed] [Google Scholar]
- 30.Vishniavsky N, Sagar KB, Markowitz SM. Aspergillus fumigatus endocarditis on a normal heart valve. South Med J 1983;76:506–8. 10.1097/00007611-198304000-00027 [DOI] [PubMed] [Google Scholar]
- 31.Kammer RB, Utz JP. Aspergillus species endocarditis. The new face of a not so rare disease. Am J Med 1974;56:506–21. [DOI] [PubMed] [Google Scholar]
- 32.Rana M, Fahad B, Abid Q. Embolic Aspergillus endophthalmitis in an immunocompetent patient from aortic root Aspergillus endocarditis. Mycoses 2008;51:352–3. 10.1111/j.1439-0507.2008.01491.x [DOI] [PubMed] [Google Scholar]
- 33.Reichenberger F, Habicht JM, Gratwohl A et al. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J 2002;19:743–55. 10.1183/09031936.02.00256102 [DOI] [PubMed] [Google Scholar]