Abstract
Squamous cell lung cancer (SQCLC) is an aggressive type of lung cancer and most are diagnosed at advanced stage. Patients with advanced SQCLC tend to be older, current or former smoker, with central type tumour located near large blood vessels and seldom with druggable genetic alternations. Consequently, progress of targeted therapy and antivascular agents available in lung adenocarcinoma could not be duplicated in this subset of patients. The treatment paradigms have long been dominant by cytotoxic agents and posed many therapeutic challenges. Until recent years, immune checkpoint inhibitors, other monoclonal antibodies and afatinib have been approved for treatment of advanced SQCLC, presenting a novel treatment landscape and initiating the era of precision medicine in this subset of patients. This review will summarise the recent treatment progresses in advanced SQCLC with a focus on checkpoint inhibitors of programmed cell death-1 receptor or its ligand, and discuss the emerging challenges in this new era.
Keywords: squamous cell lung cancer, immune checkpoint inhibitors, monoclonal antibodies
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. Non-small cell lung cancer (NSCLC) accounts for ∼85% of new diagnoses and about 20-30% NSCLC cases are squamous cell lung cancer (SQCLC).1 SQCLC is characterised by unique clinicopathological and molecular features that have evolved substantially over time.2 Generally, patients with SQCLC tend to be older, 3 typically at advanced stage,4 strongly associated with smoking,5 most with centrally located tumours that are locally aggressive, and often without actionable genetic alternations. Interestingly, efforts in recent years have revealed an increasing frequency of peripheral SQCLC, with a potential to become as common as central SQCLC,6 7 and identified several potential actionable genetic abnormalities such as FGFR1 and PI3K amplification.8-10 Despite these scientific advances, there is no regulatory approval on the clinical application of corresponding targeted agents in this subset of patients until now.
The abovementioned characteristics of SQCLC have made it a different disease from lung adenocarcinoma. As a result, several recently developed regimens such as pemetrexed, bevacizumab and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) which demonstrate preferable efficacy and tolerability in patients with adenocarcinoma of the lung are unsuitable for or mostly ineffective in lung SQCLC.11-13 Platinum-based chemotherapy has been the dominant regimen for treating SQCLC for years and under such strategy, the median overall survival (OS) in advanced SQCLC has remained static at ∼9-11 months.13 14 In addition to the unsatisfactory efficacy, patients with advanced SQCLC often experienced a higher frequency of adverse events (AEs),15 which in turn might delay treatment plan and success, or even result in supportive care without active anticancer interventions.16 Consequently, compared with advanced lung adenocarcinoma which has benefited from precision medicine, the treatment of advanced SQCLC has been largely lagged behind and represented an unmet clinical need.
Significant advances have been made with the success of immunotherapy and monoclonal antibodies in this subset of patients. Several phase III studies have demonstrated superior efficacy and acceptable AEs of checkpoint inhibitors of programmed cell death-1 (PD1)/programmed cell death-1 ligand (PD-L1) pathway, when compared with traditional chemotherapy in first-line and/or second-line treatment of advanced SQCLC.17-21 Regarding these impressive results, the US Food and Drug administration (FDA) and European Medicines Agency have granted the marketing approval to three checkpoint inhibitors, including: pembrolizumab, nivolumab and atezolizumab (by FDA only) in the treatment of advanced SQCLC with restrictions on PD-L1 selection or lines of treatment. Besides, ramucirumab and afatinib have also been approved in second-line treatment of advanced SQCLC. Necitumumab in combination with gemcitabine and cisplatin has been approved in first-line treatment of advanced SQCLC. These novel progresses have constituted an evolving treatment landscape of advanced SQCLC with more opportunities and challenges. This review will summarise the novel progresses in treatment of advanced SQCLC with a highlight of immunotherapy and discuss the emerging challenges in this new era.
Progress in immunotherapy
Pembrolizumab
Pembrolizumab is PD-1 checkpoint inhibitor that has been approved in the USA and Europe for the first-line treatment of advanced NSCLC with high PD-L1 expression and second-line treatment for PD-L1-positive advanced NSCLC progressed from platinum-based chemotherapy. Preliminary data on safety and efficacy of pembrolizumab were initially demonstrated in the phase I study (KEYNOTE-001) enrolling advanced NSCLC, including SQCLC and non-squamous carcinoma.22 Pembrolizumab demonstrated acceptable safety profile and antitumour activity with an objective response rate (ORR) of 19.4% and a median OS of 12.0 months in total patients. Besides, this study also demonstrated that PD-L1 expression in at least 50% of tumour cells correlated with improved efficacy of pembrolizumab, laying the foundation of PD-L1 selection in further studies.
Second-line setting
Later on, the efficacy of pembrolizumab in advanced SQCLC and non-squamous NSCLC was initially demonstrated in second-line setting in a phase II/III, multicentre randomised study (table 1).17 A total of 1034 patients with PD-L1 expression on at least 1% of tumour cells were enrolled in KEYNOTE 010 with 345 allocated to receive pembrolizumab 2 mg/kg, 346 allocated to pembrolizumab 10 mg/kg and 343 allocated to docetaxel. SQCLC accounts for around 20% of patients in each treatment arms. For total population, the median OS was significantly longer for pembrolizumab 2 mg/kg versus docetaxel (10.4 vs 8.5 months, HR 0.71, p=0.0008) and for pembrolizumab 10 mg/kg versus docetaxel (12.7 vs 8.5 months, HR 0.61, p<0.0001). These significantly different OS and HR results were more pronounced with PD-L1 proportion score =50% (pembrolizumab 2 mg/kg versus docetaxel: 14.9 vs 8.2 months, HR 0.54, p=0.0002; pembrolizumab 10 mg/kg versus docetaxel: 17.3 vs 8.2 months, HR 0.50, p<0.0001). In subgroup analysis of OS based on tumour histology, there was no statistical difference (HR=0.74, 95%CI 0.50 to 1.09) between pembrolizumab and docetaxel for patients with SQCLC which might partially result from the small sample size, but the data suggested a clinical benefit also in this subgroup. Similar to OS, the median progression-free survival (PFS) was significantly longer in pembrolizumab when compared with docetaxel in patients with PD-L1 proportion score =50% (pembrolizumab 2 mg/kg versus docetaxel: 5.0 vs 4.1 months, HR 0.59, p=0.0001; pembrolizumab 10 mg/kg versus docetaxel: 5.2 vs 4.1 months, HR 0.59, p<0.0001), but there was no statistical difference for the overall population. These data contributed to the FDA and EMA approval of pembrolizumab as a new treatment option for second-line treatment of advanced PD-L1-positive NSCLC and validated the use of PD-L1 selection.
Table 1.
The efficacy of programmed cell death-1/programmed cell death-1 ligand checkpoint inhibitors for the second-line treatment of advanced squamous cell lung cancer in phase III randomised controlled trials
| RCTs | Setting | Histology | No. of sqclc/Tpts | Agent/dose | Clinical efficacy |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS |
PFS |
ORR | |||||||||
| Median (months) | HR (sqclc) | 95% CI | Median (months) | HR (sqclc) | 95% CI | ||||||
| CheckMate 017 | Second-line | SQCLC | 135/135 | Nivolumab 3 mg/kg q2w |
9.2 | 0.59 | 0.44 to 0.79 | 3.5 | 0.62 | 0.47 to 0.81 | 20% |
| 137/137 | Docetaxel | 6.0 | / | / | 2.8 | / | / | 9% | |||
| KEYNOTE 010 | Second-line | NSCLC | 76/344 | Pembrolizumab 2 mg/kg q3w |
0.74 | 0.50 to 1.09 | 3.9 | 0.86 | 0.62 to 1.20 | 18% | |
| 80/346 | Pembrolizumab 10 mg/kg q3w |
4.0 | 18% | ||||||||
| 66/343 | Docetaxel | / | 4.0 | / | 9% | ||||||
| OAK | Second-line | NSCLC | 112/425 | Atezolizumab 1200 mg q3w |
8.9 | 0.73 | 0.54 to 0.98 | 2.8 | / | / | |
| 110/425 | Docetaxel | 7.7 | / | / | 4.0 | / | / | ||||
NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; q2w, every 2 weeks; q3w, every 3 weeks; RCTs, randomised controlled trials; SQCLC/sqclc, squamous cell lung cancer; Tpts, total patients.
Second-line pembrolizumab is well tolerated with a relatively different AEs profile when compared with docetaxel.17 Decreased appetite, fatigue, nausea and rash were reported as common AEs attributed to pembrolizumab, similar to that in KEYNOTE 001. Grade 3-5 treatment-related AEs occurred less frequent with pembrolizumab than docetaxel (in 13% of patients given pembrolizumab 2 mg/kg, 16% given pembrolizumab 10 mg/kg, 35% with docetaxel). AEs leading to permanent discontinued treatment were also less common with pembrolizumab versus docetaxel (4% with pembrolizumab 2 mg/kg, 5% with pembrolizumab 10 mg/kg and 10% with docetaxel). Overall, treatment-related death occurred in six patients of the pembrolizumab group and five in the docetaxel group. Besides, the most common special interest based on their likely immune aetiology, irrespective of relation to treatment were hypothyroidism, hyperthyroidism and pneumonitis in the pembrolizumab group.
First-line setting
The first-line efficacy of pembrolizumab was recently reported in a phase III study enrolling 305 treatment-naïve patients without sensitising alternations of EGFR or ALK.18 Patients with PD-L1 expression on at least 50% of tumour cells were randomly assigned to receive pembrolizumab or platinum-based chemotherapy. About 18.8% and 17.9% of patients in the pembrolizumab and chemotherapy group have tumour histology of SQCLC, respectively. The study met its primary end point with significantly prolonged PFS in the pembrolizumab group versus the chemotherapy group (10.3 vs 6.0 months, HR 0.50, p<0.001). The estimated rate of OS at 6 months also favoured pembrolizumab when compared with chemotherapy (80.2% vs 72.4%, HR 0.60, p=0.005). The ORR was 44.8% in the pembrolizumab group, higher than 27.8% in the chemotherapy group. The benefit of pembrolizumab in patients who had SQCLC is notable. In the subgroup analysis based on histological type (squamous vs non-squamous), HR for disease progression or death was 0.35 in the SQCLC subgroup and 0.55 in the non-SQCLC subgroup, respectively, further elucidating the efficacy of pembrolizumab in advanced SQCLC.
The safety profile of first-line pembrolizumab is preferable with most treatment-related AEs appearing to be mild to moderate and could be well managed.18 Generally, treatment-related AEs of pembrolizumab were consistent with that previously observed in advanced NSCLC. Treatment-related AEs of any grade (73.4% vs 90%) and grade 3 or higher (26.6% vs 53.3%) were less frequent with pembrolizumab versus chemotherapy. Grade 3-5 treatment-related AEs that occurred in four or more patients were diarrhoea (3.9%) and pneumonitis (2.6%) in the pembrolizumab group and anaemia (19.3%), neutropenia (13.3%), decreased platelet count (6.0%), thrombocytopenia (5.3%), decreased neutrophil count (4.0%), fatigue (3.3%) and decreased appetite (2.7%) in the chemotherapy group. Immune-mediated AEs occurred more frequently in the pembrolizumab group compared with the chemotherapy (29.2% vs 4.7%), but most of these events were of grade 1 or 2 severity, and none led to death.
Nivolumab
Preliminary results on the efficacy and tolerability of nivolumab in advanced SQCLC were reported in the phase I and II study of nivolumab. Among the previously treated SQCLC at advanced stage, nivolumab demonstrated durable responses and acceptable safety profiles. Nivolumab was associated with ORR of 15% and about 17%, with the median OS of 8.2-9.2 months and survival rates of 41% at 1 year and 19% at 3 years.23 24
Second-line setting
Nivolumab was the first anti-PD-1 agent that was approved by FDA and EMA for second-line treatment of advanced SQCLC in 2015. These approvals were based on the results from phase III study (CHECKMATE 017) comparing the efficacy and safety of nivolumab with docetaxel in advanced SQCLC progressed on or after platinum-based chemotherapy.
In CHECKMATE 017, 272 patients were randomised to receive nivolumab (3 mg/kg) every 2 weeks, or docetaxel (75 mg/m2) every 3 weeks.19 The study reached its primary end point of significantly improved OS (table 1). The median OS was significantly longer with nivolumab versus docetaxel (9.0 vs 6.0 months, HR 0.59, p<0.001). The overall survival rate at 1 year was 42% with nivolumab, when compared with 24% with docetaxel. Besides, nivolumab also significantly prolonged PFS than docetaxel (3.5 vs 2.8 months, HR 0.62, p<0.001). Of note, biomarker analysis suggested that PD-L1expression was neither prognostic nor predictive of benefit from nivolumab.
Treatment-related AEs occurred less frequently in the nivolumab group versus the docetaxel group.19 Grade 3-5 events were reported in 7% of patients with nivolumab and in 57% of patients with docetaxel. The most common treatment-related AEs with nivolumab were fatigue (16%), decreased appetite (11%) and asthenia (10%) and with docetaxel were neutropenia (33%), fatigue (33%), alopecia (22%) and nausea (23%). Treatment-related AEs that led to discontinuation of agents occurred less frequently with nivoluamb than docetaxel (3% vs 10%). The most common treatment-related AEs leading to treatment discontinuation in the nivolumab group were pneumonitis (2%) and in the docetaxel group were peripheral neuropathy (3%) and fatigue (2%), respectively. Also, two additional patients had discontinued treatment due to pneumonitis. No treatment-related death was reported in the nivolumab group, compared with three in the docetaxel group.
Atezolizumab
Atezolizumab is the first PD-L1 checkpoint inhibitor that was recently approved by FDA for the treatment of advanced NSCLC progressed from platinum-based chemotherapy in 2016. In addition, patients with EGFR or ALK-positive advanced NSCLC should have disease progression from FDA-approved agents for these aberrations prior to receive atezolizumab. This approval was based on data from phase II (POPLAR) and phase III (OAK) studies which all demonstrated the superiority of atezolizumab compared with docetaxel on efficacy and tolerability in the treatment of advanced NSCLC failed from platinum-containing chemotherapy.
Second-line setting
Two hundred and eighty-seven advanced NSCLC were enrolled in the POPLAR study and randomly allocated to receive atezolizumab 1200 mg or docetaxel 75 mg/m2 once every 3 weeks.20 Patients were stratified by PD-L1 tumour-infiltrating immune cell status, histology and previous lines of therapy. SQCLC constitute for 34% in the atezolizumab and the docetaxel group. Baseline PD-L1 expression was scored by immunohistochemistry in tumour cells and tumour-infiltrating T cells. The study met its primary end point with significantly prolonged OS in the intention-to-treat population with 12.6 months for atezolizumab, when compared with 9.7 months for docetaxel (HR 0.73, p=0.04). Biomarker analysis suggested that increased improvement in OS was associated with high PD-L1 expression. Besides, exploratory analysis demonstrated that patients with pre-existing immunity, defined by high T-effector-interferon-?-associated gene expression, also had improved OS with atezolizumab. Subgroup analysis demonstrated that in patients with SQCLC, OS also favoured atezolizumab when compared with docetaxel (10.1 vs 8.6 months, HR 0.80).
The OAK study had a similar design to POPLAR.21 Eight hundred and fifty advanced NSCLC (including SQCLC) patients progressed from platinum-based chemotherapy were randomised to receive atezolizumab and docetaxel. Atezolizumab significantly improved the median OS in the intention-to-treat population when compared with docetaxel (13.8 vs 9.6 months, HR 0.73, p=0.0003). Subgroup analysis based on histology further demonstrated the superiority of atezolizumab regardless of tumour histology. In patients with advanced SQCLC, the median OS was also significantly longer with atezolizumab than docetaxel (8.9 vs 7.7 months, HR 0.73, p=0.0383) (table 1). Besides, the OS benefit could be achieved without selection on PD-L1 expression, despite patients with increasing PD-L1 expression were associated with a reduced HR.
Generally, atezolizumab demonstrated a better safety profile when compared with docetaxel in this subset of patients. According to data from POPLAR and OAK, treatment-related AEs of any grade occurred in 64-67% of patients with atezolizumab and in 86-88% of docetaxel.20 21 Grade 3-4 treatment-related AEs were reported in 11-15% of patients with atezolizumab when compared with 39-43% with docetaxel. Based on detailed data from POPLAR, the most common atezolizumab-related grade 3 AEs were pneumonia (2%) and increased aspartate aminotransferase (2%). No grade 4 treatment-related AEs was reported in the atezolizumab group. Eight per cent of patients in the atezolizumab group discontinued because of AEs versus 22% patients in the docetaxel group. Treatment-related death was reported in one patient in the atezolizumab group versus three patients in the docetaxel group.
Advances in monoclonal antibodies
Necitumumab
Necitumumab is a recombinant antibody of human IgG1 EGFR. FDA and EMA approved the use of necitumumab, in combination with gemcitabine and cisplatin in first-line treatment of advanced SQCLC in 2015. This approval was attributed to evidence from a phase III randomised controlled trial (RCT) comparing necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone in this subset of patients.
First-line setting
A total of 1093 patients with advanced SQCLC were randomly assigned to receive necitumumab plus gemcitabine and cisplatin (n=545) or gemcitabine and cisplatin (n=548).25 Necitumumab was administered at a dose of 800 mg on days 1 and 8, and was continued after the end of chemotherapy until disease progression or intolerable toxic side effects occurred. Necitumumab plus gemcitabine and cisplatin significantly prolonged the median OS when compared with gemcitabine and cisplatin (11.5 vs 9.9 months, HR 0.84, p=0.01). The median PFS was also significantly longer in the necitumumab plus gemcitabine and cisplatin group versus the gemcitabine and cisplatin group (5.7 vs 5.5 months, HR 0.85, p=0.02). ORR were reported in similar proportion of patients in the two groups, but necitumumab plus gemcitabine and cisplatin was associated with a significantly higher disease control rate than gemcitabine and cisplatin (Cochran-Mantel-Haenszel p=0.043). HR for OS for necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin was more favourable in patients whose tumour demonstrated high EGFR expression (HR 0.75) than those with low EGFR expression (HR 0.90).
Grade 3 or worse AEs (72% vs 62%) and the incidence of severe AEs (48% vs 38%) were both higher in the necitumumab plus gemcitabine and cisplatin group than the gemcitabine and cisplatin group.25 More patients with necitumumab plus gemcitabine and cisplatin reported grade 3-4 hypomagnesaemia (9% vs 1%) and grade 3 rash (4% vs <1%) than with gemcitabine and cisplatin. Treatment-related death occurred in 3% of patients in the necitumumab plus gemcitabine and cisplatin group and 2% in the gemcitabine and cisplatin group. Overall, the safety profile of necitumumab plus gemcitabine and cisplatin was considered acceptable and in line with expectations.
Ramucirumab
Ramucirumab is a human vascular endothelial growth factor receptor 2 inhibitor. Ramucirumab in combination with docetaxel were approved in USA and Europe in treating advanced NSCLC progressed from platinum-based chemotherapy. This approval is mainly supported by results from a phase III study (REVEL) comparing the efficacy and safety of ramucirumab plus docetaxel versus placebo plus docetaxel in second-line treatment for this subset of patients.
Second-line setting
A total of 1253 advanced NSCLC who progressed from first-line platinum-based chemotherapy were randomly allocated to receive docetaxel 75 mg/m2 and either ramucirumab (10 mg/kg) or placebo every 3 weeks. SQCLC accounted for 25% and 27% in the ramucirumab plus docetaxel and the placebo plus docetaxel group, respectively.26 The primary end point was OS in all patients assigned to treatment. The median OS was significantly longer with ramucirumab plus docetaxel than placebo plus docetaxel (10.5 vs 9.1 months, HR 0.86, p=0.023). Regarding patients with SQCLC, there was a numerical longer median OS with ramucirumab-docetaxel than placebo-docetaxel (9.5 vs 8.2 months). For the overall population, the median PFS was 4.5 months with ramucirumab plus docetaxel, also significantly longer than 3.0 months with the controlled group. Such benefit of PFS was consistent in the subgroup based on tumour histology of SQCLC and non-squamous.
The toxicity of ramucirumab plus docetaxel was relatively higher but acceptable when compared with docetaxel.26 The most common grade 3 or worse AEs were neutropenia (49% with ramucirumab-docetaxel vs 40% with placebo-docetaxel), febrile neutropenia (16% vs 10%), fatigue (14% vs 10%), leucopoenia (14% vs 12%) and hypertension (6% vs 2%). The numbers of AEs leading to death (5% with ramucirumab-docetaxel vs 6% with placebo-docetaxel) and grade 3 or worse pulmonary haemorrhage (1% vs 1%) were similar between the two groups. Of note, unlike a previous study of bevacizumab which demonstrated that 31% of patients with SQCLC had severe haemoptysis,11 patients with SQCLC in this study seemed to derive similar benefit from ramucirumab plus docetaxel without an increase in toxicity, particularly respiratory tract haemorrhage, when compared with the controlled group and non-squamous NSCLC.
Advances in afatinib
Afatinib is an irreversible ErbB kinase inhibitor, also well established as a second-generation EGFR inhibitor. The approval of afatinib in treating patients with advanced SQCLC progressing after platinum-based chemotherapy has been made in USA and Europe in 2016. An open-label phase III RCT (LUX-lung 8) comparing afatinib with erlotinib in second-line treatment of advanced SQCLC laid the foundation for this approval.
Second-line setting
Patients with advanced SQCLC progressed after at least four cycles of platinum-based-chemotherapy were enrolled in LUX-lung 8 and randomly assigned to treatment arms with 398 to afatinib and 397 to erlotinib.27 At the time of primary analysis (median follow-up 6.7 months), the median PFS was 2.4 months with afatinib, significantly longer than 1.9 months with erlotinib (HR 0.82, p=0.0427). At primary analysis of OS (median follow-up 18.4 months), the median OS was significantly greater with afatinib versus erlotinib (7.9 vs 6.8 months, HR 0.81, p=0.007), so were the median PFS (2.6 vs 1.9 months, HR 0.81, p=0.0103). There was no statistical difference on ORR between afatinib and erlotinib groups (6% vs 3%, p=0.0551).
Numbers of grade 3 or higher AEs were similar between two groups (57% with afatinib, 57% with erlotinib).27 Higher incidences of treatment-related grade 3 diarrhoea and grade 3 stomatitis were reported with afatinib than erlotinib (10% vs 2% and 4% vs none). Grade 3 rash or acne was higher with erlotinib versus afatinib (10% vs 6%). Twenty per cent of patients with afatinib had treatment discontinued due to AEs versus 17% with erlotinib. Treatment-related AEs leading to death occurred in six patients with afatinib and five with erlotinib. In general, afatinib demonstrated a manageable safety profile, similar to erlotinib.
Emerging treatment paradigms of advanced SQCLC
With regard to abovementioned progress in SQCLC and the updated NCCN guideline, the emerging treatment paradigms seem to differ from previous algorithm which mainly comprised chemotherapy regimens. To date, treatment-naïve patients with advanced SQCLC should be recommended to receive PD-L1 testing of tumour specimens. For patients with PD-L1 expression in =50% of tumour cells, pembrolizumab is preferable as a first-line option. In the case of disease progression from first-line pembrolizumab, platinum-based chemotherapy should be administered as second-line treatments. Docetaxel alone or in combination with ramucirumab could be tailored in third-line setting. For those treatment-naïve patients with PD-L1 expression in <50%, platinum-based chemotherapy such as gemcitabine plus cisplatin should be recommended. In the case of disease progression from first-line chemotherapy, immune checkpoint inhibitors including nivolumab and atezolizumab are approved for second-line treatment of this subset of patients regardless of PD-L1 expression and pembrolizumab should be administered in PD-L1-positive patients. Docetaxel alone or in combination with ramucirumab could be administered as third-line treatments. Of note, two regimens including necitumumab combined with gemcitabine and cisplatin that were approved by FDA in first-line and afatinib in second-line treatment of advanced SQCLC were not recommended by the updated NCCN guideline with concerns on efficacy and safety when compared with other current available regimens. Therefore, these agents are not included in the present paradigms (figure 1).
Figure 1.
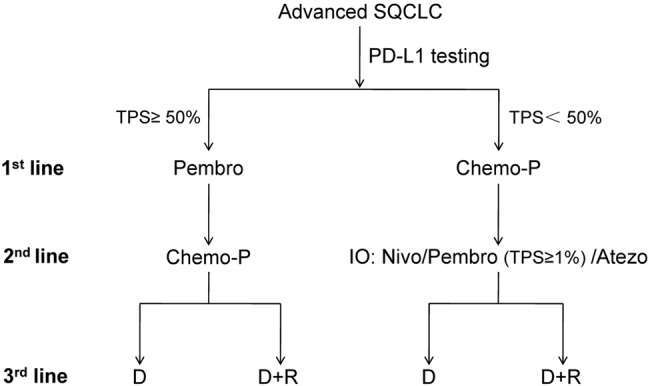
The emerging treatment paradigms for overall management of advanced SQCLC. SQCLC, squamous cell lung cancer; TPS, tumour proportion score; Pembro, pembrolizumab; Chemo-P, platinum-based chemotherapy; IO, immunotherapy; D+R, docetaxel plus ramucirumab; D, docetaxel; Nivo, nivolumab; Atezo, atezolizumab; 1st, first; 2nd, second; 3rd, third; PD-L1, programmed cell death-1 ligand.
Novel challenges for the treatment of advanced SQCLC
Optimisation of checkpoint inhibitors
Just as pembrolizumab moved from second-line to first-line setting with an impressive HR of 0.35 in the subgroup of SQCLC, how to optimise multiple checkpoint inhibitors deserve further investigation in clinical practice. The first question clinicians have to face is whether treatment should be administered based on PD-L1 expression. Two phase III studies comparing pembrolizumab and nivolumab with platinum-based chemotherapy, respectively, might provide preliminary answer to this question.18 28 In KEYNOTE 024, which chose PD-L1 expression =50% as the inclusion criteria, pembrolizumab demonstrated an additional PFS benefit of 4.3 months than chemotherapy. However, in CHECKMATE 026 which defined PD-L1 expression >1% as the inclusion criteria and analysed patients with PD-L1 expression =5%, no superiority of PFS was demonstrated with nivolumab when compared with chemotherapy (4.2 vs 5.9 months).28 Subgroup analysis on SQCLC and non-squamous patients also demonstrated similar negative results. Current evidences suggested that checkpoint inhibitors should be selected based on biomarker such as PD-L1 expression in first-line treatment of advanced SQCLC. As for second or later lines of treatment, there still exist controversies on PD-L1 selection. Moreover, future studies deserve to more precisely define the levels of PD-L1 expression that might predict the efficacy of checkpoint inhibitors. The ongoing study IMpower 131 (NCT02367794) evaluating the efficacy of atezolizumab in first-line treatment of advanced SQCLC which also evaluated PD-L1 expression as inclusion criteria might provide further evidences on biomarker selection.29
Second, whether PD-L1 expression is a sole biomarker for checkpoint inhibitors is still undetermined. Data from several phase III studies evaluating the efficacy of pembrolizumab, nivolumab and atezolizumab in second-line setting all demonstrated significantly prolonged OS when compared with docetaxel.17 19 20 However, only pembrolizumab has selected PD-L1 expression =50% as the inclusion criteria, the other two agents had no restriction on PD-L1 expression.17 Therefore, an emerging highlight might be whether there exist other predictive biomarkers of PD1/PD-L1 blockade. A recent study published in the 2016 European Society for Medical Oncology (ESMO) meeting suggested that HLA-A2 status could influence the outcome of advanced NSCLC patients treated with immune checkpoint inhibitors.30 Preliminary results with 30 patients enrolled in analysis demonstrated that HLA-A2-positive status was associated with longer PFS to immunotherapy versus HLA-A2-negative status (2.04 vs 1.3 months, log-rank; p=0.020). Regarding the complexity of antitumour immunity, other emerging biomarkers such as TIM3 and LAG3 deserve further exploration to identify more predictive and prognostic biomarkers that might influence the outcome of immune checkpoint inhibitors.
Will combinational strategy involving checkpoint inhibitors work?
The combination of immune checkpoint inhibitors with other currently available regimens is of great clinical interest. Current approvals on checkpoint inhibitors all based on clinical trials demonstrating the efficacy and safety of single agents. Whether combinational regimens involving checkpoint inhibitors will provide more benefits with acceptable toxicities is challenging. Trials evaluating the efficacy and safety of these anti-PD-1/PD-L1 inhibitors in combination with chemotherapy, anti-CTLA-4 inhibitor ipilimumab and EGFR monoclonal antibody necitumumab are ongoing. CHECKMATE 227 (NCT02477826), a phase III study which includes three experimental arms including nivolumab plus platinum-based chemotherapy or ipilimumab, respectively, and nivolumab monotherapy and one controlled arm of platinum-based chemotherapy, will demonstrate the efficacy and safety of combinational strategy in advanced SQCLC and also provide direct comparison among combinational, monotherapy of nivolumab and chemotherapy.31 A phase I study (NCT02451930) evaluating the safety and efficacy of pembrolizumab in combination with necitumumab in advanced NSCLC including subgroup of SQCLC is ongoing.32 Other studies such as IMpower 111 (NCT02409355) and IMpower 131 (NCT02367794) evaluating the safety and efficacy of atezolizumab plus chemotherapy in metastatic SQCLC will also provide deeper insights in the future.29 33
Concerns on cost-effectiveness
With the marketing of PD-1/PD-L1 checkpoint inhibitors, another emerging challenge is the increasing cost of these agents when compared with conventional chemotherapy. How to improve the cost-effectiveness and release the economic burden of patients is urgently needed, so as to transform the benefits of these agents from bench to bedside. A recent analysis published in the 2016 ESMO meeting calculated quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICER) to assess the cost-effectiveness and economic impact of nivolumab and pembrolizumab with data from three RCTs and drug acquisition costs from the USA.34 This study demonstrated that among patients with advanced SQCLC, PD-L1 expression improved incremental QALY only for patients with PD-L1 >5% and >10% (by 15% and 18%, respectively), suggesting that the use of PD-L1 expression as a biomarker increases cost-effectiveness and decreases the economic treatment burden with immune checkpoint inhibitors. Further endeavours in biomarker selection, health insurance promotion and charity project (especially in developing countries) will help improve the cost-effectiveness of checkpoint inhibitors and bring benefit to as many advanced SQCLC as possible in clinical practice.
Conclusion
The treatment paradigm of advanced SQCLC has evolved from the previous chemotherapy-dominance to a novel era of immune checkpoint inhibitors along with multiple regimens such as monoclonal antibodies. PD1/PD-L1 checkpoint inhibitors have demonstrated superior efficacy and more tolerable safety profile when compared with traditional chemotherapy in first and second-line treatment of advanced SQCLC. Under such shift, the median PFS of first-line pembrolizumab has reached 10.3 months in advanced NSCLC with PD-L1 expression =50%. This superiority was more evident in patients with SQCLC with an HR of 0.35. To go further, the identification of PD-L1 expression as a predictive biomarker for efficacy of checkpoint inhibitors has been translating the treatment paradigm from the histology-driven pattern to precision medicine. Along with the precision medicine come novel challenges. These incremental advances demonstrated by clinical trials still deserve further validation in clinical practice. Moreover, how to optimise multiple checkpoint inhibitors based on biomarkers, how to maximise clinical efficacy with combinational strategy and how to improve cost-effectiveness of these novel agents need more investigation in future, so as to yield more benefits to our patients.
Footnotes
Contributors: Y-LW designed the manuscript, Y-CZ, QZ and Y-LW wrote the manuscript.
Funding: Supported by the Key Technologies Research and Development Program of Guangzhou (2011Y2-00014), the Key Laboratory Program of Guangdong (2012A061400006) and the Special Fund for Research in the Public Interest from the National Health and Family Planning Commission of the People's Republic of China (grant 201402031).
Competing interests: Y-LW has received speaker fees from AstraZeneca, Roche, Boerhinger Ingelman, Eli Lilly, Pfizer and Sanofi.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669–92. 10.1016/j.ccm.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 2. Drilon A, Rekhtman N, Ladanyi M, et al. . Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol 2012;13:e418–26. 10.1016/S1470-2045(12)70291-7 [DOI] [PubMed] [Google Scholar]
- 3. Subramanian J, Morgensztern D, Goodgame B, et al. . Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23–8. 10.1097/JTO.0b013e3181c41e8d [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 5. American Cancer Society. What is non-small cell lung cancer? http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-celllung-cancer-what-is-non-small-cell-lung-cancer (accessed 8 Nov 2016).
- 6. Funai K, Yokose T, Ishii G, et al. . Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am J Surg Pathol 2003;27:978–84. 10.1097/00000478-200307000-00013 [DOI] [PubMed] [Google Scholar]
- 7. Sakurai H, Asamura H, Watanabe S, et al. . Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann Thorac Surg 2004;78:222–7. 10.1016/j.athoracsur.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 8. Weiss J, Sos ML, Seidel D, et al. . Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93 10.1126/scitranslmed.3001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okudela K, Suzuki M, Kageyama S, et al. . PIK3CA mutation and amplification in human lung cancer. Pathol Int 2007;57:664–71. 10.1111/j.1440-1827.2007.02155.x [DOI] [PubMed] [Google Scholar]
- 10. Ji M, Guan H, Gao C, et al. . Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC). BMC Cancer 2011;11:147 10.1186/1471-2407-11-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DH, Fehrenbacher L, Novotny WF, et al. . Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184–91. 10.1200/JCO.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 12. Hanna N, Shepherd FA, Fossella FV, et al. . Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97. 10.1200/JCO.2004.08.163 [DOI] [PubMed] [Google Scholar]
- 13. Scagliotti GV, Parikh P, von Pawel J, et al. . Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 14. Socinski MA, Bondarenko I, Karaseva NA, et al. . Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055–62. 10.1200/JCO.2011.39.5848 [DOI] [PubMed] [Google Scholar]
- 15. Gronberg BH, Sundstrom S, Kaasa S, et al. . Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer 2010;46:2225–34. 10.1016/j.ejca.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 16. Langer CJ, Obasaju C, Bunn P, et al. . Incremental innovation and progress in advanced squamous cell lung cancer: current status and future impact of treatment. J Thorac Oncol 2016;11:2066–81. 10.1016/j.jtho.2016.08.138 [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Baas P, Kim DW, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 18. Reck M, Rodriguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med Epub ahead of print: 9 Oct 2016. doi: 10.1056/NEJMoa1606774 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 19. Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fehrenbacher L, Spira A, Ballinger M, et al. . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 21. Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLCPresented at 2016 European Society for Medical Oncology meeting Abstract LBA44. [Google Scholar]
- 22. Garon EB, Rizvi NA, Hui R, et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 23. Gettinger SN, Horn L, Gandhi L, et al. . Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004–12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizvi NA, Mazieres J, Planchard D, et al. . Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257–65. 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thatcher N, Hirsch FR, Luft AV, et al. . Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–74. 10.1016/S1470-2045(15)00021-2 [DOI] [PubMed] [Google Scholar]
- 26. Garon EB, Ciuleanu TE, Arrieta O, et al. . Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665–73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 27. Soria JC, Felip E, Cobo M, et al. . Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897–907. 10.1016/S1470-2045(15)00006-6 [DOI] [PubMed] [Google Scholar]
- 28. Socinski M, Creelan B, Horn L, et al. . CHECKMATE 026: a phase 3 trial of nivolumab vs investigator's choice of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage IV/recurrent programmed death ligand 1(PD-L1)-positive NSCLC Presented at 2016 European Society for Medical Oncology Meeting LBA7_PR. [Google Scholar]
- 29. National Institutes of Health. Clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02367794?term=IMpower+131&rank=1 (accessed 12 Nov 2016).
- 30. Mezquita L, Charrier M, Lahmar J, et al. . HLA-A2 and immune checkpoints inhibitors in advanced non-small cell lung cancer (NSCLC) patients [abstract]. Ann Oncol 2016;27 (Suppl 6): vi426. [Google Scholar]
- 31. National Institutes of Health. Clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02477826?term=NCT02477826&rank=1(accessed 12 Nov 2016).
- 32. National Institutes of Health. Clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02451930?term=NCT02451930&rank=1 (accessed 12 Nov 2016).
- 33. National Institutes of Health.Clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02409355?term=NCT02409355&rank=1(accessed 12 Nov 2016).
- 34. Junior PNA, Mello RD, Tadokoro H, et al. . Cost effectiveness and estimate of economical impact of immune checkpoint inhibitors for NSCLC relative to PD-L1 expression [abstract]. Ann Oncol 2016;27(Suppl6):vi427. [Google Scholar]


