Abstract
Streptococcus suis capsular type 2 is an important zoonotic agent of meningitis. Previous studies reported that, in contrast to nonencapsulated mutants, encapsulated S. suis is able to resist phagocytosis. However, the mechanisms by which S. suis avoids phagocytosis are unknown. To elucidate the signaling pathway(s) involved in S. suis antiphagocytosis, we compared the ability of an encapsulated strain and its nonencapsulated mutant to induce the activation of Akt and protein kinase C (PKC), which are downstream kinases of the phosphatidylinositol 3-kinase (PI-3K) pathway, known to be involved in the phagocytosis processes. The results demonstrated high levels of Akt and PKCα phosphorylation after infection of J774 macrophages with the nonencapsulated mutant, whereas the encapsulated strain showed reduced activation of PI-3K/Akt/PKCα signaling pathway, as well as several protein tyrosine events. These results correlated with the number of intracellular bacteria. Macrophages pretreated with specific PI-3K or PKC inhibitors showed reduced levels of Akt and PKCα phosphorylation, resulting in 50% reduction of phagocytosis. The role of phosphatases in the antiphagocytic mechanisms was evaluated by using phosphatase inhibitors, as well as SHP-1-deficient macrophages. Only in the absence of SHP-1 did the phagocytosis of encapsulated S. suis significantly increase, leading to Akt phosphorylation levels similar to those observed with the nonencapsulated strain, indicating activation of this important SH2 domain-containing tyrosine phosphatase by encapsulated S. suis. Finally, when purified S. suis capsular polysaccharide (CPS) was added to macrophages, no phosphorylation events were observed. In addition, CPS and encapsulated S. suis were able to inhibit the uptake of the nonencapsulated mutant. These results suggest the importance of CPS in the mechanisms, whereby S. suis downmodulates phagocytosis.
Streptococcus suis is not only one of the most important swine pathogens worldwide, but it is also a zoonotic agent. Among the capsular types described, type 2 is the most frequently associated with disease in animals, as well as in humans (18). The most important clinical feature associated with S. suis is meningitis; however, other pathologies have also been described (19). Knowledge on virulence factors and the pathogenesis of S. suis infection is still limited. S. suis is transmitted via the respiratory route and remains localized in the palatine tonsils. From this site, bacteria should travel and persist throughout the bloodstream and reach the central nervous system (16). An early theory suggested uptake of bacteria by monocytes, intracellular survival, and invasion of the central nervous system by the “Trojan horse theory” (51). However, most studies carried out during the last decade suggest that bacteria may also use another mechanism (or mechanisms) to disseminate (16). In fact, S. suis is a well-encapsulated bacterium, and it has been proposed that the capsular polysaccharide (CPS) confers antiphagocytic properties on it (5, 44, 45). The CPS is thus far the only proven critical virulence factor, based on the studies with nonencapsulated isogenic mutants. The absence of CPS correlates with increased hydrophobicity and phagocytosis with murine and porcine phagocytes. In addition, nonencapsulated mutants were shown to be avirulent and cleared from circulation rapidly in both mouse and pig models of infection (5, 45). A recent report described surface adhesion, without phagocytosis, of S. suis to murine macrophages. Adhesion is mediated, at least in part by the sialic acid component of the CPS (41). Hence, it could be hypothesized that surface adherence to phagocytes with impaired uptake is a key step for a successfully infection, as suggested for Haemophilus influenzae type b, another important meningeal pathogen (34).
Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection have been reported for a diverse range of microorganisms, including Leishmania and Mycobacteria spp. (14, 29, 32, 35). On the other hand, mechanisms of phagocytosis avoidance by extracellular pathogens have been described for some gram-negative bacteria such as Escherichia coli, Yersinia sp., and Helicobacter pylori (10). This requires a functional type III or type IV (for the later species) secretion system (11, 15, 38). In contrast, little or no information is available regarding the mechanisms involved in phagocytosis impairment by typically extracellular gram-positive pathogens.
Several bacterial pathogens require or activate phosphatidylinositol 3-kinase (PI-3K)-mediated signaling pathways to gain entry into host cells. PI-3K and its downstream effector molecules, such as the protein kinase Akt, as well as the protein kinase C (PKC) family member, are involved in several cellular processes such as cytoskeleton rearrangements and macrophage activation (4, 33). Thus, modulation of these important signaling pathways may have an impact on bacterial survival and dissemination by enhancing their pathogenic lifestyles, as recently described for enteropathogenic E. coli (EPEC) (3). In the present study, the role of bacterial modulation of signaling pathways in S. suis resistance to phagocytosis was investigated by comparing the ability of a well-encapsulated virulent S. suis strain and its nonencapsulated isogenic mutant to activate Akt and PKCα signaling molecules after infection of mouse J774 macrophages. The role of the CPS in this process, as well as the possible mechanisms of PI-3K pathway modulation, such as phosphatase activation, were analyzed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. suis capsular type 2 virulent and encapsulated strain 735-SM (ATCC 43765) and its nonencapsulated isogenic mutant 2A, obtained by mutagenesis with the self-conjugative transposon Tn916 (5), were used in comparative studies (kindly provided by M. Gottschalk, GREMIP, University of Montreal). Molecular and phenotypic characterization of mutant 2A has been performed, as reported elsewhere (5) (N. Fittipaldi et al., submitted for publication). Bacteria were maintained as stock cultures in 50% glycerol-Todd-Hewitt broth (THB; Difco Laboratories, Detroit, Mich.) at −80°C. The THB was supplemented with 10 μg of tetracycline (Sigma-Aldrich, Oakville, Ontario, Canada)/ml for growing the mutant (5). Bacteria were grown overnight onto bovine blood agar plates at 37°C, and isolated colonies were used as inocula for THB, which were incubated for 18 h at 37°C. Working cultures for infection assays were made by inoculating 400 μl of these cultures in 10 ml of THB at 37°C with agitation until they reached the mid-log phase (4 to 6 h of incubation, optical density of 0.5 at 540 nm). Bacteria were washed twice in phosphate-buffered saline (PBS; pH 7.4) and appropriately diluted (see below). An accurate determination of the CFU/milliliter in the final suspension was made by plating onto THB agar. Purified S. suis type 2 CPS was kindly provided by M. Gottschalk (GREMIP, University of Montreal). The CPS purification protocol has been previously reported (9), and no protein contamination was detected by using a modification of the Lowry procedure reported by Markwell et al. (30). In addition, no nucleic acid contamination was detected (data not shown).
Cell line and cell culture.
J774.A1 murine (BALB/c) macrophage cell line (ATCC TIB67; American Type Culture Collection, Rockville, Md.) was used in the present study. Cells were grown at 37°C in 5% CO2 in Dulbecco Modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum (hiFBS; Gibco, Burlington, Vt.). This cell line has been previously used for evaluation of S. suis interactions with phagocytes (41, 42, 44) and has been shown to present levels of phagocytosis similar to those of normal peritoneal macrophages (44). In some experiments, the bone marrow-derived macrophage cell lines me-3 and LM-1 were used. These cell lines were previously generated in our laboratory (13) from phosphotyrosine phosphatase SHP-1-deficient motheaten (me) mice and their wild-type littermates (LMme). LM-1 and me-3 cells were maintained in Dulbecco modified Eagle medium 10% hiFBS as described above.
Phagocytosis assay.
For evaluation of bacterial phagocytosis, 48 h culture cells were scraped up, washed once, and resuspended in antibiotic-free medium at ∼105 cells/ml. Then, 1 ml of this suspension was distributed into 24-well Falcon tissue culture plates (VWR CanLab, Montreal, Quebec, Canada) and incubated for 3 h at 37°C, 5% CO2 to allow cell adhesion before assays. Phagocytosis assays were performed as previously described (44). Briefly, plates were infected with S. suis strains by removing culture medium and adding 1 ml of 107 bacterial suspension per well in cell culture medium supplemented with 10% hiFBS to obtain a ratio of ca. 100 bacteria per macrophage. Phagocytosis was left to proceed for 15, 30, and 90 min at 37°C, 5% CO2. After incubation, cell monolayers were washed twice with warm PBS and reincubated for 1 h with medium containing penicillin G (5 μg/ml) and gentamicin (100 μg/ml; Sigma) to kill extracellular bacteria. It has been demonstrated that these antibiotics do not penetrate eukaryotic cells under these conditions (44). Supernatant controls were taken in every test to confirm that antibiotics effectively killed extracellular bacteria. After antibiotic treatment, cell monolayers were washed three times, and the medium was replaced with 1 ml of sterile distilled water to lyse the macrophages. After vigorous pipetting to ensure complete cell lysis, viable intracellular bacteria were determined by quantitative plating of serial dilutions of the lysates on THB agar. Each test was done four times in independent experiments, and the number of CFU recovered per well (mean number ± the standard deviation [SD]) was determined.
Cell activation assays.
For evaluation of cellular activation, 48 h culture of macrophages were scraped up, washed once, and resuspended in antibiotic-free medium at ∼106 cells/ml. Next, 3 ml of this suspension was distributed into six-well tissue culture plates (Falcon; VWR) and incubated for 3 h at 37°C, 5% CO2 to allow cell adhesion before assays. Plates were then infected with S. suis strains by removing the culture medium and adding 3 ml of 108 bacterial suspension per well in cell culture medium to obtain a ratio of ca. 100 bacteria per macrophage. Plates were centrifuged at 130 × g for 10 min to enhance the contact of bacteria with the surface of cells (41) and were then incubated at 37°C in 5% CO2. At different time intervals (see Results) the plates were washed three times with PBS, and cell lysates were prepared by disrupting adherent cells in cold lysis buffer containing 20 mM Tris-HCl (pH 8.0), 0.14 M NaCl, 10% glycerol (vol/vol), 1% IGEPAL CA-630 (vol/vol), 1 mM phenylmethylsulfonyl fluoride, 50 mM sodium fluoride, 2 mM sodium orthovanadate, and 20 μg of leupeptin and aprotinin/ml, as previously described (36). It should be noted that cell viability after S. suis infection under the present assay conditions is higher than 85 to 90% as previously reported (41).
Western blotting.
Protein samples from the resulting cell lysates (25 μg/line) were boiled in Laemmli loading buffer containing sodium dodecyl sulfate before being subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore, Netean, Ontario, Canada) as previously described (36). After 2 h of blocking in Tris-buffered saline-Tween-5% skimmed milk, membranes were washed and incubated overnight at 4°C with anti-Phospho-Akt (Ser473), anti-Phospho-PKCα/βII (Thr638/641), or anti-Phospho-PTEN (Ser380) antibodies (Cell Signaling Technology/New England Biolabs, Beverly, Mass.). After a washing step, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies, and proteins were visualized by enhanced chemiluminescence (Western Lightning Chemiluminescence Reagent Plus; Gibco). Western blots were then stripped by using Restore Western blot stripping buffer (Pierce, Rockford, Ill.), and membranes were reprobed with anti-Akt polyclonal antibody or anti-PTEN monoclonal antibody 26H9 (Cell Signaling) or anti-PKCα monoclonal antibody (clone 3; BD Transduction Laboratories/Pharmingen, Mississauga, Ontario, Canada). To evaluate total tyrosine phosphorylation, membranes were blotted with anti-Tyr(P) (clone 4G10; Upstate Biotechnology, Inc., Lake Placid, N.Y.).
Inhibition assays.
In selected experiments, J774A1 cells were pretreated with the PI-3K inhibitor LY-294002 at 5, 10, 25, or 50 μM (BIOMOL Research Laboratories, Plymouth Meeting, Pa.) or with the PKCα/βI inhibitor Gö6976 at 1, 10, 25, or 50 nM (Cedarlane, Hornby, Ontario, Canada) 1 h before infection. Phagocytosis assays and Western blot analysis of Akt and PKCα activation were done as described above after 15 min of incubation in presence of bacteria. The serine/threonine phosphatase inhibitor okadaic acid at 1, 10, or 100 nM (BIOMOL) (7), and the tyrosine phosphatase (PTP) inhibitors bpV(phen) and bpV(pic) (two peroxovanadium [pV] compounds) at 10 μM were also used 1 h before infection as previously described (36), followed by 15-min bacterial infection. These inhibitors have been shown not to be toxic for macrophages at the doses used in the present study (12, 28, 36).
CPS blockade of phagocytosis in coinfection model.
J774.A1 cells were pretreated with purified CPS at 100 μg/ml or preinfected with the wild-type S. suis strain 735 (at 107 CFU/ml) for 15 min. Thereafter, pretreated macrophages were reinfected with the nonencapsulated S. suis mutant 2A, the nonencapsulated GBS mutant COH1-13, or the encapsulated GBS parent strain COH1 at 107 CFU/ml for an additional 15 min. GBS strains were kindly provided by C. E. Rubens, Children's Hospital and Medical Center, Seattle, Wash. The phagocytosis assay then proceeded as described above. Mutants 2A and COH1-13 were differentiated from strain 735 in coinfected cells by plating them on THB agar containing 10 μg of tetracycline/ml (since these two isogenic mutants were derived by using Tn916 transposition (5, 39). GBS strain COH1 was differentiated from strain 735 in coinfected cells on the basis on beta-hemolysis production on sheep blood agar. Each test was done three times in independent experiments, and the number of CFU recovered per well (mean number ± the SD) was determined. The results were expressed as the percentage of phagocytosis with respect to control cells infected with individual strains (considered 100% of phagocytosis).
Statistical analysis.
Each test was done at least in triplicate. Differences were analyzed for significance by using the Student unpaired t test (two-tailed P value). A P value of <0.05 was considered significant.
RESULTS
S. suis phagocytosis correlates with Akt and PKCα activation.
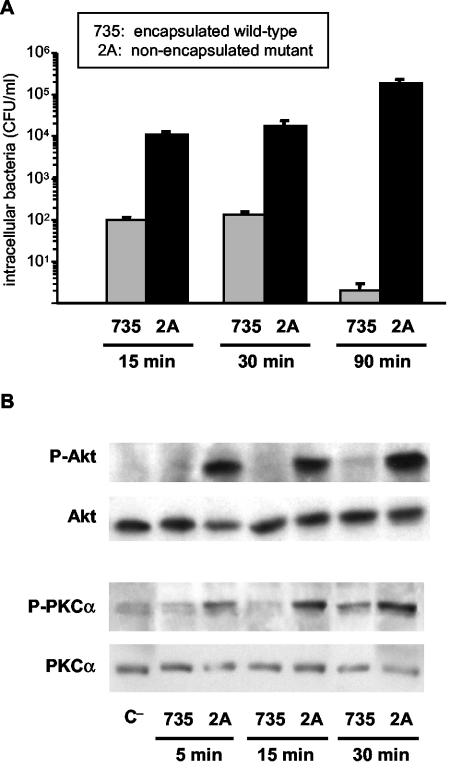
Fig. 1A shows levels of phagocytosis of S. suis wild-type strain 735-SM and its nonencapsulated mutant 2A by J774 macrophages. As previously reported (44), phagocytosis of the nonencapsulated mutant occurred rapidly and increased with incubation time. In contrast, the wild-type strain is only slightly phagocytosed and is completely eliminated after 90 min of incubation, as also reported with primary macrophages (44). To further understand the differences observed between encapsulated and nonencapsulated strains, the induction of the PI-3K downstream kinase Akt, as well as the induction of PKCα, was analyzed. As shown in Fig. 1B, the nonencapsulated mutant rapidly activated Akt and PKCα phosphorylation in macrophages (as early as 5 min after infection), whereas wild-type S. suis-infected macrophages showed weak activation of both second messengers. No further activation of the two evaluated kinases was observed after 90 min of infection with the wild-type strain (data not shown). Since phagocytosis is dependent on the ability of the microorganism to bind to the cell surface, these differences may just be related to reduced adhesion of the encapsulated strain. In this regard, we previously demonstrated that wild-type S. suis adheres at high numbers to J774 cells. The adhesion increases with bacterial doses and incubation time (41), whereas phagocytosis decreases (Fig. 1). Thus, the lack of Akt or PKC activation by wild-type strain is not related to lower levels of adhesion.
FIG. 1.
(A) Kinetics of S. suis phagocytosis by J774 macrophages. Intracellular bacteria (encapsulated strain 735 and nonencapsulated mutant 2A) were recovered from cell lysates 15, 30, or 90 min postinfection. The results are expressed as means ± the SD of CFU of recovered bacteria/ml. (B) Western blot analysis of Akt and PKCα phosphorylation kinetics after S. suis infection of J774 macrophages. Lysates obtained from untreated control cells (C−) and cells infected with encapsulated strain 735 and nonencapsulated mutant 2A were subjected to Western blotting at different times postinfection (5, 15, and 30 min), and Akt and PKCα phosphorylation levels were revealed by using anti-Phospho-Akt (P-Akt) or anti-Phospho-PKCα (P-PKCα). Stripped filters were then reprobed with anti-Akt polyclonal antibody or anti-PKCα monoclonal antibody to confirm equal levels of protein. The results are representative of three individual experiments.
Phagocytosis of S. suis is dependent on PI-3K upstream of Akt and PKCα.
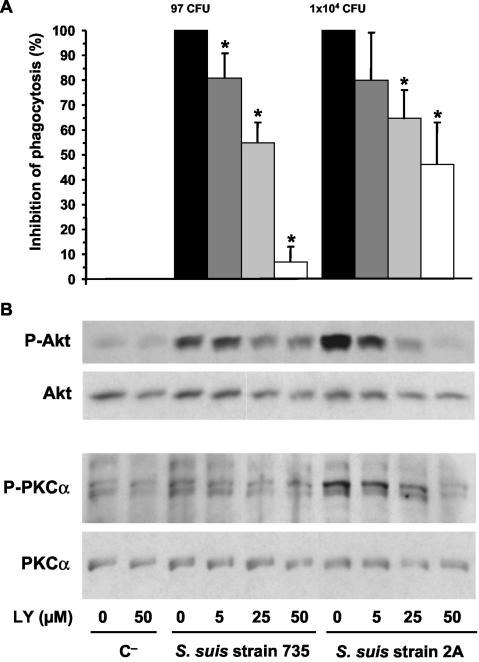
To evaluate the role of PI-3K in phagocytosis of S. suis, J774 macrophages were pretreated with the inhibitor LY-294002 (as described in Materials and Methods). LY-294002 partially but significantly inhibited phagocytosis of mutant 2A. The observed inhibition was dose dependent, reaching 46% ± 17% at an inhibitor concentration of 50 μM (P < 0.001). The few numbers of ingested wild-type bacteria were also inhibited by LY-294002 (Fig. 2A). To confirm the specific inhibition of PI-3K, phosphorylation of the downstream kinase Akt was evaluated by Western blotting. Both, the weak activation and the strong activation of Akt in wild type- and mutant-infected macrophages, respectively, were inhibited by LY-294002 in a dose-dependent fashion (Fig. 2B).
FIG. 2.
(A) S. suis phagocytosis inhibition by pretreatment of J774 macrophages with the PI-3K inhibitor LY-294002 (LY). Macrophages were pretreated 1 h before infection with 5, 25, or 50 μM doses of inhibitor (as indicated in the bottom panel of the figure). After 15 min of bacterial infection, phagocytosis inhibition was quantified by plating intracellular bacteria (encapsulated strain 735 and nonencapsulated mutant 2A) recovered from cell lysates, and the results are expressed as means ± the SD of the percent inhibition with respect to the phagocytosis values of untreated control cells (considered 100% phagocytosis and shown as CFU of recovered bacteria/milliliter in their corresponding histograms). An asterisk indicates a significant difference versus the untreated control cells (P < 0.01). (B) J774 macrophages were pretreated 1 h before infection with 5, 25, or 50 μM doses of inhibitor, and lysates obtained from noninfected control cells (C−) and 15-min-infected cells with encapsulated strain 735 and nonencapsulated mutant 2A were subjected to Western blotting. Akt and PKCα phosphorylation levels were revealed by using anti-Phospho-Akt (P-Akt) or anti-Phospho-PKCα (P-PKCα). Stripped filters were then reprobed with anti-Akt polyclonal antibody or anti-PKCα monoclonal antibody to confirm equal levels of protein. The results are representative of three individual experiments.
Since several isoforms of the PKC family have been shown to be regulated by the PI-3K pathway, probably directly or indirectly via the intermediate kinase PDK1 (for phosphoinositide-dependent kinase 1) (47), we evaluated the phosphorylation of PKCα after treatment of macrophages with the inhibitor LY-294002. As shown in Fig. 2B, PKCα phosphorylation induced by S. suis strains was also significantly reduced by increasing doses of the inhibitor. Thus, PKCα phosphorylation seems to occur, at least in part, downstream of PI-3K activation by S. suis.
PKCα also plays an important role in S. suis phagocytosis.
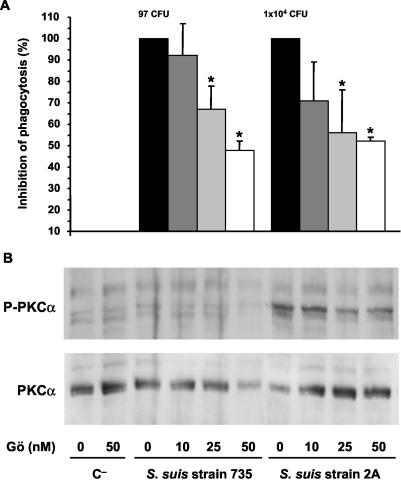
When J774 macrophages were pretreated with the PKC inhibitor Gö6976, a partial, but significant inhibition of phagocytosis rates of mutant 2A were observed, reaching ∼50% inhibition with a concentration of 25 nM of inhibitor (P < 0.005). No further increase of inhibition was observed at higher inhibitor concentrations (Fig. 3A) or when we used a combination of both inhibitors (LY-294002 and Gö6976) at maximal concentrations (52% ± 2%, 46% ± 17%, or 53 ± 5% inhibition by Gö6976 [50 nM], LY-294002 [50 μM], or a combination of both inhibitors, respectively [P > 0.1]). The few numbers of ingested wild-type bacteria were also partially inhibited by Gö6976 (Fig. 3A). To confirm the specificity of the inhibition, PKCα phosphorylation was evaluated by Western blotting. Activation of PKCα in infected macrophages was significantly reduced by Gö6976 in a dose-dependent fashion, as shown in Fig. 3B, whereas Akt activation was not modified by Gö6976 (data not shown).
FIG. 3.
(A) S. suis phagocytosis inhibition by pretreatment of J774 macrophages with the PKCα/βI inhibitor Gö6976 (Gö). Macrophages were pretreated 1 h before infection with 10, 25, or 50 nM doses of inhibitor (as indicated in the bottom panel of the figure). After a period of 15 min of bacterial infection, phagocytosis inhibition was quantified by plating intracellular bacteria (encapsulated strain 735 and nonencapsulated mutant 2A) recovered from cell lysates. The results are expressed as means ± the SD of the percent inhibition versus the phagocytosis values of untreated control cells (considered 100% phagocytosis and shown as the number of CFU of recovered bacteria/milliliter in their corresponding histograms). An asterisk indicates a significant difference versus the phagocytosis values of untreated control cells, (P < 0.01). (B) J774 macrophages were pretreated 1 h before infection with 10, 25, or 50 nM doses of inhibitor, and lysates obtained from noninfected control cells (C−) and 15-min-infected cells with encapsulated strain 735 and nonencapsulated mutant 2A were subjected to Western blotting. PKCα phosphorylation levels were revealed by using anti-Phospho-PKCα (P-PKCα). Stripped filters were then reprobed with anti-PKCα monoclonal antibody to confirm equal levels of protein. The results are representative of three individual experiments.
Role of PTPs in phagocytosis impairment by S. suis.
Since it has been described that tyrosine dephosphorylation of host proteins correlates with the onset of antiphagocytosis and/or macrophage deactivation by other pathogens (2, 15), the total tyrosine phosphorylation (P-Tyr) profiles induced by the wild-type and the mutant strains were compared on infected J774 cells (Fig. 4). Mutant 2A induces phosphorylation of several macrophage proteins (between 50 and 160 kDa). P-Tyr occurs as early as 1 min after infection and decreases with incubation time. In contrast, the wild-type strain induces P-Tyr profiles similar to those observed in uninfected control cells. It is necessary for cells that both the protein tyrosine phosphatases (PTPs) and protein tyrosine kinases maintain their physiological balance to sustain a normal regulation of their P-Tyr-dependent events (52). Thus, activation of host PTP was shown to represent an important mechanism whereby pathogens, such as Leishmania spp., alter host cell functions (2). Of particular interest in this regard is the cytosolic SH2-domain-containing PTP, SHP-1, an important negative regulator of several P-Tyr-dependent signaling cascades involved in the immune response and shown to be modulated in Leishmania infection (2). In the present study, the possibility that SHP-1 modulation by encapsulated S. suis contributes to host protein dephosphorylation and phagocytosis impairment was investigated by using the SHP-1-deficient me-3 cell line and the normal counterpart LM-1 cell line. An average threefold increase of encapsulated S. suis uptake by SHP-1 deficient me-3 cells was observed with respect to normal LM-1 cells after 15 min of infection (Fig. 5A, P < 0.01). This increased phagocytosis correlates with increased levels on Akt activation by the wild-type strain in me-3 SHP-1-deficient cells (Fig. 5B). Differences between these two cell lines in phagocytosis levels of strain 735 were also observed by increasing the infection time; however, these differences were not statistically significant (345 ± 198 versus 616 ± 290 at 30 min; and 1,860 ± 650 versus 2,542 ± 946 at 90 min of recovered intracellular bacteria after infection of LM-1 and me-3 cells, respectively; P > 0.05). It should be noted that these levels and kinetics of phagocytosis of encapsulated S. suis strain 735 by the wild-type LM-1 cells were different from those observed with J774 cells (Fig. 1) and from those previously reported with primary peritoneal macrophages (44), since the bacteria were eliminated at 90 min by these two later phagocytic cells (44). One possible explanation is a reduced bacterial killing capacity of the LM-1 cell line compared to J774 or primary macrophages over time, and for that reason a 15-min incubation time was used for the comparative studies displayed in Fig. 5. On the other hand, no differences were observed in the phagocytosis levels of the nonencapsulated mutant by me-3 and LM-1 cells or in a comparison with mutant uptake by J774 macrophages (Fig. 1 and 5A). Together, these data indicate that SHP-1 may play, at least in part, a role in S. suis modulation of phagocytosis during the initial contact between the bacterium and the cell.
FIG. 4.
S. suis induced levels of tyrosine phosphorylation [Tyr(P)]. J774 macrophages were infected for 1, 5, 15, or 30 min with encapsulated strain 735 and nonencapsulated mutant 2A. Cell lysates (total proteins) from noninfected control cells (C−) and infected cells were subjected to Western blotting, and macrophage Tyr(P) protein levels were revealed by using an anti-Tyr(P) (clone 4G10) monoclonal antibody. The results are representative of three individual experiments.
FIG. 5.
Role of phosphatases in phagocytosis resistance by encapsulated S. suis. (A) Comparative phagocytosis levels (expressed as CFU of intracellular recovered bacteria/milliliter) after 15 min of infection of control J774 cells, wild-type LM-1 macrophages, or SHP-1-deficient me-3 cell lines by S. suis encapsulated 735 or nonencapsulated mutant 2A strains. An asterisk indicates a significant difference versus the phagocytosis values of J774 or LM-1 (P < 0.01; n = 4). (B) Comparative Akt phosphorylation levels after 15 min of infection of J774 cells, LM-1, or SHP-1-deficient me-3 cell lines by S. suis encapsulated 735 or nonencapsulated mutant 2A strains. Western blotting was performed as described for Fig. 1. Stripped filters were reprobed with anti-Akt polyclonal antibody to confirm equal levels of protein. The results are representative of three individual experiments. (C) Effect of phosphatase inhibitors on encapsulated S. suis phagocytosis levels. J774 cells were pretreated for 1 h with the serine/threonine phosphatase inhibitor okadaic acid at 1, 10, or 100 nM or with the tyrosine phosphatases inhibitors bpV(phen) and bpV(pic) (two peroxovanadium [pV] compounds) at 10 μM before 15 min of infection with S. suis strain 735. Phagocytosis was quantified by plating intracellular bacteria recovered from cell lysates, and the results are expressed as means ± the SD of the percent phagocytosis values obtained with untreated control cells (considered 100% phagocytosis). (D) Levels of phosphorylation of the PTEN lipid phosphatase analyzed by Western blotting after 5, 15, 30, or 90 min of J774 cells infection with S. suis encapsulated 735 or nonencapsulated mutant 2A strains. C−, noninfected control cells. The results are representative of three individual experiments.
To further elucidate the role of PTP in the mechanisms involved in S. suis resistance to phagocytosis, we pretreated J774 macrophages with the general PTP inhibitor, bpV(phen) before infection. Despite the fact that the effectiveness of this compound in controlling Leishmania infection was previously demonstrated (36), no increase in phagocytosis rates of encapsulated S. suis was observed. Since the importance of the nature of the ancillary ligand of pV compounds has already been already reported (36), we evaluated the effect of another pV derivate, bpV(pic), in S. suis phagocytosis. However, bpV(pic) also failed to modulate encapsulated S. suis uptake (Fig. 5C).
Role of other phosphatases in the modulation of phagocytosis by encapsulated S. suis.
In addition to PTP, the serine/threonine family of protein phosphatases has been shown to play an important role in regulating cellular functions by modulating the phosphorylation state of critical proteins (6). Okadaic acid is a naturally occurring polyether toxin produced by marine dinoflagellates. It is a potent and selective inhibitor of protein phosphatase 1 (PP1) and PP2A and, to a lesser extent, PP2B (>50 nM), which are major cytosol protein phosphatases that dephosphorylate proteins on their serine and threonine residues (7). To test this hypothesis, several doses (ranging from 1 to 100 nM) of the inhibitor okadaic acid were used to treat J774 macrophages 1 h before infection with the encapsulated S. suis strain 735 for an additional 15 min. Despite the use of several doses, phagocytosis levels of encapsulated S. suis were similar to those of untreated cells (Fig. 5C). Doses higher than 100 nM were toxic for the cells; thus, the concentration of the inhibitor could not be further increased.
Finally, PI-3K-generated D3-phosphorylated phosphoinositides function as important intracellular second messengers and contribute to the activation of the serine/threonine kinase Akt and PKC isoforms (4). Thus, lipid phosphatases may also play an important regulatory function at this level (23). One important member of this family of phosphatases is the protein encoded by the tumor suppressor gene PTEN, which targets the D3 phosphate of the inositol ring (4, 27). To elucidate the possible role of this phosphatase, levels of PTEN phosphorylation were analyzed by Western blotting after J774 infection with S. suis strains at different time intervals (ranging from 5 to 90 min). As shown in Fig. 5D, no significant modulation of PTEN was observed with respect to the uninfected control cells at any of the incubation times tested, suggesting that this particular member of the lipid phosphatase family does not seem to be modulated during S. suis infection of macrophages.
S. suis type 2 CPS is a poor inductor of Akt and PKCα activation.
In order to elucidate the contribution of the capsule in the differences observed between phagocytosis levels of encapsulated and nonencapsulated strains, purified CPS at several concentrations was added to J774 macrophages for 15 min (Fig. 6). Levels of Akt and PKCα phosphorylation were then evaluated by Western blotting. Purified CPS did not induce activation of these kinases even at high concentrations. This lack of activation is not related to lost of cell viability since purified CPS has previously been shown not to be toxic for J774 cells (42).
FIG. 6.
S. suis type 2 purified CPS effect on Akt and PKCα phosphorylation levels. Lysates obtained from 15-min-infected cells with encapsulated strain 735, nonencapsulated mutant 2A, or purified CPS (1, 10, or 100 μg/ml) were subjected to Western blotting, and the Akt and PKCα phosphorylation levels were revealed by using anti-Phospho-Akt (P-Akt) or anti-Phospho-PKCα (P-PKCα). Stripped filters were then reprobed with anti-Akt polyclonal antibody or anti-PKCα monoclonal antibody to confirm equal levels of protein. The results are representative of three individual experiments.
S. suis type 2 CPS reduces phagocytosis levels of related and nonrelated prays.
To further understand the role of the CPS in phagocytosis resistance, macrophages were pretreated with purified CPS (100 μg/ml) or preinfected with the encapsulated S. suis strain 735 for 15 min before reinfection with the nonencapsulated mutant 2A for additional 15 min. As shown in Fig. 7, preinfection with the encapsulated strain significantly inhibited the phagocytosis levels of mutant 2A (P < 0.001); this effect could be mimicked by the pretreatment of macrophages with purified CPS material (P < 0.001). To evaluate whether this inhibitory effect extended to other nonrelated prays, phagocytosis inhibition was assessed by using an encapsulated group B streptococcus (GBS) strain and its nonencapsulated isogenic mutant. Both strains are normally phagocytosed at high levels by J774 macrophages (44). After preinfection of the macrophages with the encapsulated S. suis strain 735, the phagocytosis rates of the nonencapsulated GBS mutant strain were significantly inhibited by at least 50% (P < 0.05), and the uptake of the encapsulated GBS parent strain was partially reduced (30% inhibition, P > 0.1) (Fig. 7).
FIG. 7.
Phagocytosis inhibition by pretreatment of J774 macrophages with purified CPS at 100 μg/ml or by preinfection with the S. suis type 2 encapsulated strain 735 (S.s) for 15 min before reinfection with S. suis nonencapsulated mutant 2A, GBS nonencapsulated mutant COH1-13, or GBS encapsulated parent strain COH1 (at 107 CFU/ml). Intracellular bacteria were recovered from cell lysates after 15 min of reincubation. The results are expressed as means ± the SD of the percent phagocytosis versus untreated control cells (1 × 104 CFU for strain 2A, 4 × 105 CFU for strain COH1, and 2 × 105 CFU for strain COH1-13; considered 100% phagocytosis in their respective histograms). An asterisk indicates a significant difference versus the phagocytosis values of untreated control cells (P < 0.05).
DISCUSSION
Mechanisms of bacterial phagocytosis avoidance have just begun to be elucidated and in most cases are limited to gram-negative bacteria (10). In the present study, signaling pathways involved in phagocytosis resistance by S. suis, a gram-positive meningeal pathogen, were analyzed. S. suis is a well-encapsulated bacterium, and the CPS has been proven to be a critical virulence factor (5, 45). Here, the importance of CPS in bacterial resistance to macrophage uptake was further characterized. The presence of CPS would be responsible for weak activation of the serine/threonine kinases Akt and PKCα, as well as several tyrosine phosphorylation events, which correlates with low levels of phagocytosis. On the other hand, the absence of CPS leads to high activation of these kinases, with consequent phagocytosis of the nonencapsulated strain. The antiphagocytosis effect occurs rapidly and persists for at least 90 min postinfection.
This rapid interaction of S. suis with its host cell is in agreement with a previous report that describes high adherence levels of S. suis to J774 cells after a short period of infection (41). In contrast to the effect seen with S. suis, the onset of the EPEC antiphagocytic effect is detectable after 120 min of infection, a finding that correlates with adhesion levels and several tyrosine dephosphorylation events of J774 cells (15). Nevertheless, for both pathogens there is a direct correlation between the kinetics of bacterial adhesion to the macrophage surface and the antiphagocytic activity. This finding argues for a role of intimate attachment in antiphagocytosis (15).
The importance of the capsules has been largely appreciated as a physical barrier that protects bacteria from the immune system (22), and for most streptococcal species, such as S. pyogenes, S. uberis, S. equi, and S. suis, these capsules are related to phagocytosis resistance (26, 31, 44, 50). However, the mechanisms involved have for the most part never been addressed. The old dogma stating that the antiphagocytic effect of CPS is due to their net electrostatic charge may be questionable, since well-encapsulated GBS strains are known to adhere and easily enter phagocytic cells (44, 49). Thus, other mechanisms are probably more important overall in the antiphagocytic effect of CPS. First, encapsulated S. suis is able to adhere to the surface of phagocytes, and the CPS itself is involved in such adhesion (41). Second, encapsulated S. suis is able to induce the release of proinflammatory cytokines by phagocytes by an adhesion-dependent process (42, 43). However, the levels of induced cytokines are lower than those induced by the nonencapsulated mutant (42, 48). Indeed, the results of the present study suggest that the capsule may play a more important role by “modulating” activation of the phagocytic signaling machinery of professional phagocytes. Since the CPS by itself is a poor activator of phosphorylation processes and since CPS pretreatment of macrophages inhibits phagocytosis of the nonencapsulated mutant, it is possible that the capsule prevents other bacterial cell wall components from interacting with receptors connected to phagocytic pathways or that the CPS itself interacts with receptors that do not signal for phagocytosis. This would result in blockade of the phagocytic pathway, leading to impaired uptake of the encapsulated bacteria. This effect could even be extended to nonencapsulated GBS and, at least in part, to encapsulated GBS, which probably bind to different receptors. In this regard, it has been reported that pretreatment of macrophages or neutrophils with purified S. uberis CPS blocks phagocytosis of whole bacteria, as well as the uptake of other nonrelated bacterial species, such as GBS and Staphylococcus aureus. Direct interaction of CPS with macrophages was required for this inhibitory effect (1, 26). However, signaling pathways involved in this event have not been evaluated thus far. These observations and our results support the concept that CPS from gram-positive bacteria plays an important role by modulating signaling pathways involved in phagocytosis.
Indeed, the use of a nonencapsulated strain allowed us to partially characterize signaling pathways involved in the uptake of the gram-positive S. suis bacterium under nonopsonic conditions, which has been previously shown by cytochalasin inhibition studies to be an active process (42). Modulation of Akt and PKCα by S. suis occurs downstream of PI-3K, as demonstrated by inhibition studies with LY294002 (Fig. 2) and wortmannin (unpublished observations), two specific PI-3K inhibitors. The activation of PI-3K increases the production of 3-phosphoinositides, which act as important signaling intermediates in the downstream activation of Akt (4, 20). In addition, activation of atypical and novel PKC isoforms was also reported to occur in a PI-3K-dependent fashion, whereas the activation of classical PKC isoforms by this pathway is still controversial (4, 20, 25, 47). In the present study, we demonstrated by using inhibitors of PI-3K the dependence of classical PKCα on the PI-3K pathway after S. suis infection of macrophages. Thus, modulation of PI-3K by S. suis not only has a direct impact on Akt phosphorylation but also affects classical PKCα activation. This finding is in contrast to the reported PI-3K-independent mechanism of classical PKC activation by PDK-1 in COS-7 cells (46) but is in agreement with the LY294002-sensitive activation of PKCα reported in multiple myeloma cell migration (37).
PI-3K-mediated pathway inhibition by a bacterial pathogen was first reported for EPEC as a mechanism of host cell cytoskeleton subversion (3). PI-3K activation has been related to both FcγR- and CR3-mediated phagocytosis pathways (8, 10). Our results further involve the PI-3K/Akt signaling pathway in the nonopsonic phagocytosis of a S. suis nonencapsulated mutant. Similarly, it has been reported that phagocytosis of secretion-deficient EPEC engages as-yet-uncharacterized opsonin-independent receptors, which also trigger PI-3K-mediated signaling (3). On the other hand, and despite the fact that PKC is known to be important in FcγR-mediated phagocytosis (21, 24, 40), bacterial modulation of this kinase by an extracellular pathogen under nonopsonic conditions has not been addressed before. In the present study, PI-3K-dependent activation of PKCα is observed after macrophage infection with the nonencapsulated mutant. Inhibition of both PI-3K and PKCα partially inhibits its uptake. In contrast, encapsulated S. suis antiphagocytosis correlates with low PKCα activation. Since only partial inhibition of phagocytosis was observed when we used both PI-3K and PKC inhibitors, the involvement of other unknown signaling pathways during the phagocytosis process cannot be ruled out. Similarly, PI-3K requirement was reported as not absolute since its inhibition reduced secretion-deficient EPEC uptake by 50 to 55%. It has been suggested that other PI-3K-independent phagocytic pathways initiated by different nonopsonic receptor(s) may be involved (3).
Dephosphorylation events with consequent inhibition of phagocytosis could be related to phosphatase activation, as already reported for other pathogens (10, 15). Indeed, the reduced levels of total protein tyrosine phosphorylation suggest a possible role for tyrosine phosphatases as part of the mechanisms used by encapsulated S. suis to avoid phagocytosis. In contrast to findings reported for EPEC, peroxovanadium compounds cannot reverse the S. suis antiphagocytic phenotype (15). However, by using SHP-1 deficient macrophages, a role for this important SH2 domain-containing tyrosine phosphatase could be elucidated during the initial interaction of S. suis with the phagocytic cell. In the absence of SHP-1 the phagocytosis of encapsulated S. suis significantly increases, leading to Akt phosphorylation levels that are similar to those observed with the nonencapsulated strain. SHP-1 has also been shown to negatively regulate the CD66-mediated phagocytosis of Neisseria gonorrhoeae (17). Besides SHP-1, the activation of other phosphatases cannot be ruled out. Indeed, PTEN, a major tumor suppressor that converts the PI-3K lipid product PIP3 into PIP2 and thus antagonizes PI-3K-mediated signals, has been proposed as a mediator of EPEC negative regulation of PI-3K signaling (3). Despite the fact that activation of PTEN cannot be observed by Western blotting of S. suis-infected cells, the role of PTEN requires further elucidation.
In conclusion, reduced activation of PI-3K/Akt/PKCα signaling pathway, as well as several protein tyrosine events seems to be related to phagocytosis avoidance by encapsulated S. suis. The importance of this pathway for phagocytosis modulation under nonopsonic conditions could be extended to other encapsulated streptococci that are known to poorly activate the complement (22). SHP-1 phosphatase modulation by encapsulated S. suis would be related, at least in part, to the dephosphorylation events and reduced phagocytosis. Research is ongoing to further elucidate the mechanisms by which encapsulated S. suis mediates its antiphagocytic phenotype.
Acknowledgments
This study was supported by grants from the Canadian Research Network on Bacterial Pathogens of Swine and from the Canadian Institutes in Health Research (CIHR) to M.O. M.O. is member of the CIHR Group in Host-Pathogen Interactions. M.O. is the recipient of a CIHR Investigator award and a Burroughs-Wellcome Fund award in molecular parasitology.
Editor: J. T. Barbieri
REFERENCES
- 1.Almeida, R. A., and S. P. Oliver. 1993. Antiphagocytic effect of the capsule of Streptococcus uberis. Zentbl. Veterinarmed. [B] 40:707-714. [DOI] [PubMed] [Google Scholar]
- 2.Blanchette, J., N. Racette, R. Faure, K. A. Siminovitch, and M. Olivier. 1999. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-γ-triggered JAK2 activation. Eur. J. Immunol. 29:3737-3744. [DOI] [PubMed] [Google Scholar]
- 3.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathway. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. Akt/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965-1014. [DOI] [PubMed] [Google Scholar]
- 5.Charland, N., J. Harel, M. Kobish, S. Lacasse, and M. Gottschalk. 1998. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144:325-332. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, P. 1989. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 58:453-508. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, P., C. F. B. Holmes, and Y. Tsukitani. 1990. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci. 15:98-102. [DOI] [PubMed] [Google Scholar]
- 8.Cox, D., B. M. Dale, M. Kashiwada, C. D. Helgason, and S. Greenberg. 2001. A regulatory role for Src homology 2 domain-containing inositol 5′-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18). J. Exp. Med. 193:61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Campo Sepulveda, E. M., E. Altman, M. Kobisch, S. D'Allaire, and M. Gottschalk. 1996. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet. Microbiol. 52:113-125. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, J. D. 2000. Bacterial inhibition of phagocytosis. Cell. Microbiol. 2:379-386. [DOI] [PubMed] [Google Scholar]
- 11.Fallman, M., K. Andersson, S. Hakansson, K. E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foey, A. D., and F. M. Brennan. 2004. Conventional protein kinase C and atypical protein kinase Cζ differentially regulate macrophage production of tumor necrosis factor alpha and interleukin-10. Immunology 112:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forget, G., K. A. Siminovitch, S. Brochu, S. Rivest, D. Radzioch, and M. Olivier. 2001. Role of host phosphotyrosine phosphatase SHP-1 in the development of murine leishmaniasis. Eur. J. Immunol. 31:3185-3196. [DOI] [PubMed] [Google Scholar]
- 14.Gomis, S. M., D. L. Godson, T. Beskorwayne, G. A. Wobeser, and A. A. Potter. 1997. Modulation of phagocytic function of bovine mononuclear phagocytes by Haemophilus somnus. Microb. Pathol. 22:13-21. [DOI] [PubMed] [Google Scholar]
- 15.Goosney, D. L., J. Celli, B. Kenny, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun. 67:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 75:59-71. [DOI] [PubMed] [Google Scholar]
- 17.Hauck, C. R., E. Gulbins, F. Lang, and T. F. Meyer. 1999. Tyrosine phosphatase SHP-1 is involved in CD66 mediated phagocytosis of Opa52-expressing Neisseria gonorrhoeae. Infect. Immun. 67:5490-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins, R., and M. Gottschalk. 2001. Distribution of Streptococcus suis capsular types in 2000. Can. Vet. J. 42:223. [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, R., and M. Gottschalk. 1999. Streptococcal diseases, p. 563-570. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine. Iowa State University, Ames.
- 20.Jiang, G., and B. B. Zhang. 2002. PI 3-kinase and its up- and down-stream modulators as potential targets for the treatment of type II diabetes. Front. Biosci. 7:d903-917. [DOI] [PubMed] [Google Scholar]
- 21.Karimi, K., and M. R. Lennartz. 1995. Protein kinase C activation precedes arachidonic acid release during IgG-mediated phagocytosis. J. Immunol. 155:5786-5794. [PubMed] [Google Scholar]
- 22.Kasper, D. L. 1986. Bacterial capsule: old dogmas and new tricks. J. Infect. Dis. 153:407-415. [DOI] [PubMed] [Google Scholar]
- 23.Krystal, G. 2000. Lipid phosphatases in the immune system. Semin. Immunol. 12:397-403. [DOI] [PubMed] [Google Scholar]
- 24.Larsen, E. C., J. A. DiGennaro, N. Saito, S. Mehta, D. J. Loegering, J. E. Mazurkiewicz, and M. R. Lennartz. 2000. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J. Immunol. 165:2809-2817. [DOI] [PubMed] [Google Scholar]
- 25.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 26.Leigh, J. A., and T. R. Field. 1994. Streptococcus uberis resists the bactericidal action of bovine neutrophils despite the presence of bound immunoglobulin. Infect. Immun. 62:1854-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leslie, N. R., and C. P. Downes. 2002. PTEN: the down side of PI 3-kinase signalling. Cell. Signal 14:285-295. [DOI] [PubMed] [Google Scholar]
- 28.Mansell, A., N. Khelef, P. Cossart, and L. A. O'Neill. 2001. Internalin B activates nuclear factor-κB via Ras, phosphoinositide 3-kinase, and Akt. J. Biol. Chem. 276:43597-43603. [DOI] [PubMed] [Google Scholar]
- 29.Mansfield, J., and M. Olivier. 2002. Evasion and latency: evasion by parasites, p. 379-392. In A. Sher, R. Ahmed, and S. H. E. Kaufmann (ed.), Immunology of infectious diseases. ASM Press, Washington, D.C.
- 30.Markwell, M.-A. K., S. M. Hass, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 31.Moses, A. E., M. R. Wessels, K. Zalcman, S. Alberti, S. Natanson-Yaron, T. Menes, and E. Hanski. 1997. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect. Immun. 65:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandan, D., K. L. Knutson, R. Lo, and N. E. Reiner. 2000. Exploitation of host signaling machinery: activation of macrophage phosphotyrosine phosphatases as a novel mechanism of molecular microbial pathogenesis. J. Leukoc. Biol. 67:464-470. [DOI] [PubMed] [Google Scholar]
- 33.Newton, A. C. 1995. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 270:28495-28498. [DOI] [PubMed] [Google Scholar]
- 34.Noel, G. J., S. K. Hoiseth, and P. J. Edelson. 1992. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J. Infect. Dis. 166:178-182. [DOI] [PubMed] [Google Scholar]
- 35.Olivier, M., N. Racette, J. Blanchette, C. Matte, H. Kaur, I. Hardy, J.-F. Marquis, and G. Forget. 2003. Role of host phosphotyrosine phosphatase SHP-1 in the development of leishmaniasis. J. Parasitol. 89:S169-S173. [Google Scholar]
- 36.Olivier, M., B. J. Romero-Gallo, C. Matte, J. Blanchette, B. I. Posner, M. J. Tremblay, and R. Faure. 1998. Modulation of interferon-γ-induced macrophage activation by phosphotyrosine phosphatase inhibition. J. Biol. Chem. 273:13944-13949. [DOI] [PubMed] [Google Scholar]
- 37.Podar, K., Y.-T. Tai, B. K. Lin, R. P. Narsimhan, M. Sattler, T. Kijima, R. Salgia, D. Gupta, D. Chauhan, and K. C. Anderson. 2002. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with β1 integrin- and phosphatidylinositol 3-kinase-dependent PKCα activation. J. Biol. Chem. 277:7875-7881. [DOI] [PubMed] [Google Scholar]
- 38.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389-1404. [DOI] [PubMed] [Google Scholar]
- 39.Rubens, C. E., L. M. Heggen, R. F. Haft, and M. R. Wessels. 1993. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol. Microbiol. 8:343-855. [DOI] [PubMed] [Google Scholar]
- 40.Schagat, T. L., M. J. Tino, and J. R. Wright. 1999. Regulation of protein phosphorylation and pathogen phagocytosis by surfactant protein A. Infect. Immun. 67:4693-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segura, M., and M. Gottschalk. 2002. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect. Immun. 70:4312-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura, M., J. Stankova, and M. Gottschalk. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segura, M., N. Vadeboncoeur, and M. Gottschalk. 2002. CD14-dependent and independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 127:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segura, M. A., P. Cléroux, and M. Gottschalk. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21:189-195. [DOI] [PubMed] [Google Scholar]
- 45.Smith, H. E., M. Damman, J. Van der Velde, F. Wagenaar, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnenburg, E. D., T. Gao, and A. C. Newton. 2001. The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isozymes by a mechanism that is independent of phosphoinositide 3-kinase. J. Biol. Chem. 276:45289-45297. [DOI] [PubMed] [Google Scholar]
- 47.Toker, A., and A. C. Newton. 2000. Cellular signaling: pivoting around PDK-1. Cell 103:185-188. [DOI] [PubMed] [Google Scholar]
- 48.Vadeboncoeur, N., M. Segura, D. Al-Numani, G. Vanier, and M. Gottschalk. 2003. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 35:49-58. [DOI] [PubMed] [Google Scholar]
- 49.Valentin-Weigand, P., P. Benkel, M. Rohde, and G. S. Chhatwal. 1996. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect. Immun. 64:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wibawan, I. W., F. H. Pasaribu, I. H. Utama, A. Abdulmawjood, and C. Lammler. 1999. The role of hyaluronic acid capsular material of Streptococcus equi subsp. zooepidemicus in mediating adherence to HeLa cells and in resisting phagocytosis. Res. Vet. Sci. 67:131-135. [DOI] [PubMed] [Google Scholar]
- 51.Williams, A. E., and W. F. Blakemore. 1990. Pathogenesis of meningitis caused by Streptococcus suis type 2. J. Infect. Dis. 162:474-481. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Z.-Y. 2002. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu. Rev. Pharmacol. Toxicol. 42:209-234. [DOI] [PubMed] [Google Scholar]