Abstract
Introduction
Non-selective β-blockers (NSBBs) are widely prescribed in patients with cirrhosis for primary and secondary prophylaxis of bleeding oesophageal varices. Furthermore, it has been suggested that the clinical benefits of NSBBs may extend beyond their haemodynamic effects. Recently, a potentially harmful effect has been described in patients with refractory ascites or spontaneous bacterial peritonitis.
Methodology
A comprehensive literature search on β-blockers and cirrhosis survival using the electronic databases PubMed/MEDLINE, AMED, CINAHL and the Cochrane Central Register of Controlled Trials. Full-text manuscripts published over more than 35 years, from 1980 to April 2016 were reviewed for relevance and reference lists were cross-checked for additional pertinent studies regarding potential NSBB effects, especially focused on those concerned with survival and/or acute kidney injury (AKI).
Discussion
The proposed review will be able to provide valuable evidence to help decision making in the use of NSBB for the treatment of advanced cirrhosis and highlights some limitations in existing evidence to direct future research.
Keywords: CIRRHOSIS, ASCITES, OESOPHAGEAL VARICES, HEPATORENAL SYNDROME
Introduction
Bleeding oesophageal varices in cirrhosis are associated with a 40% 1-year mortality.1 Non-selective β-blockers (NSBBs) reduce the hepatic venous pressure gradient (HVPG)2 and remain the mainstay for primary prophylaxis in combination with endoscopic band ligation for secondary prevention of variceal bleeding in cirrhosis.3 4 Since the seminal work by Lebrec et al5 35 years ago, pharmacological prophylaxis of portal hypertensive haemorrhage has experienced little variation and these drugs are still widely prescribed today in at-risk patients.6 7 Although HVPG should be measured at baseline and 4–8 weeks after starting NSBB treatment to identify those patients who are most likely to benefit from continuing prophylaxis with NSBBs (HVPG reduction to <12 mm Hg or a 20% decrease in HVPG vs the baseline value), this procedure is not widely available in clinical practice.8 This haemodynamic response may also help prevent the development of ascites and hepatorenal syndrome (HRS).9 Several other drugs designed to reduce portal pressure have been tested over the past 30 years, such as selective β-blockers, nitrates, angiotensin receptor antagonists, α-receptor antagonists and endothelin receptor antagonists. None of them, however, have revealed any advantages over NSBBs in terms of their safety profiles in the prophylaxis against variceal bleeding.8 In addition to the clinical benefits derived from their haemodynamic effects, it has been suggested that NSBBs might provide non-haemodynamic-mediated benefits such as preventing spontaneous bacterial peritonitis (SBP)10 or hepatocellular carcinoma (HCC).11 Although several high-quality studies have demonstrated the benefits of NSBBs in both the prophylaxis of variceal bleeding, either alone or combined with endoscopic procedures, and in overall survival,12 13 only a few studies have reported patients with advanced cirrhosis, particularly decompensated patients with refractory ascites (RA).14
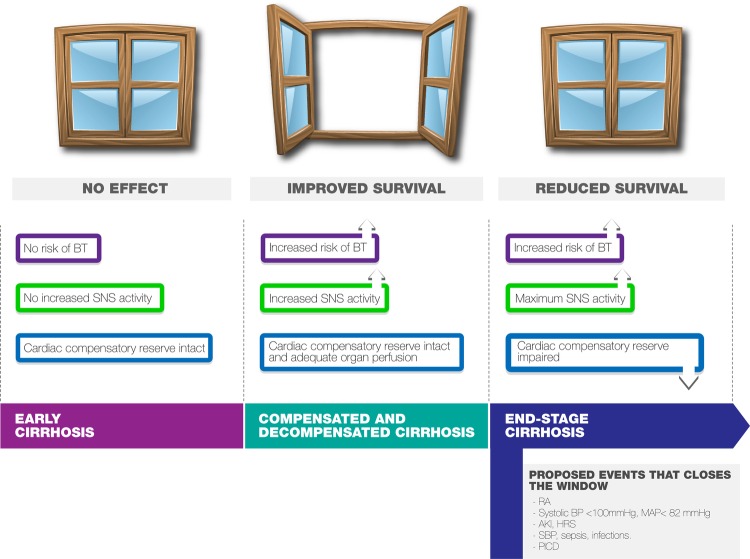
The β-blocker therapeutic window hypothesis in cirrhosis
The findings reported by Lebrec’s group led to the formulation of the β-blockers ‘therapeutic window’ hypothesis in cirrhosis15 (figure 1). According to their theory, NSBBs have no apparent effects in early cirrhosis, where clinically significant portal hypertension (HVPG≥10 mm Hg) has not yet been reached; medium and large varices have still not developed and sympathetic nervous system (SNS) activity has not yet increased.16 As the cirrhosis progresses, portal pressure increases, splanchnic hyperaemia develops, SNS activity increases and bacterial translocation (BT) occurs. This is the point when the therapeutic window opens and current guidelines recommend prophylaxis with NSBBs.17 More advanced cirrhosis is associated with profound peripheral arterial vasodilation and blood pressure (BP) and organ perfusion integrity become critically dependent on cardiac output, so at this stage patients who maintained a high cardiac output showed better survival rates.18–20 Furthermore, it has been proposed that patients with RA experience a reduced sensitivity to the β-adrenergic blockade in favour of the α-adrenergic blockade secondary to increased levels of splanchnic proinflammatory cytokines, with a subsequent reduction in NSBBs' beneficial effects21 (figure 2). At this stage NSBBs might be detrimental and the therapeutic window closes (figure 1). However, there have been several studies challenging this view and the exact role of NSBBs needs further clarification.
Figure 1.
Effects of NSBBs on advanced cirrhosis: hypothesis of the ‘therapeutic window’. Adapted from Krag et al.15AKI, acute kidney injury; BT, bacterial translocation; BP, blood pressure; HRS, hepatorenal syndrome; MAP, mean arterial pressure; SNS, sympathetic nervous system; RA, refractory ascites; SBP, spontaneous bacterial peritonitis; PICD, postparacentesis-induced circulatory dysfunction.
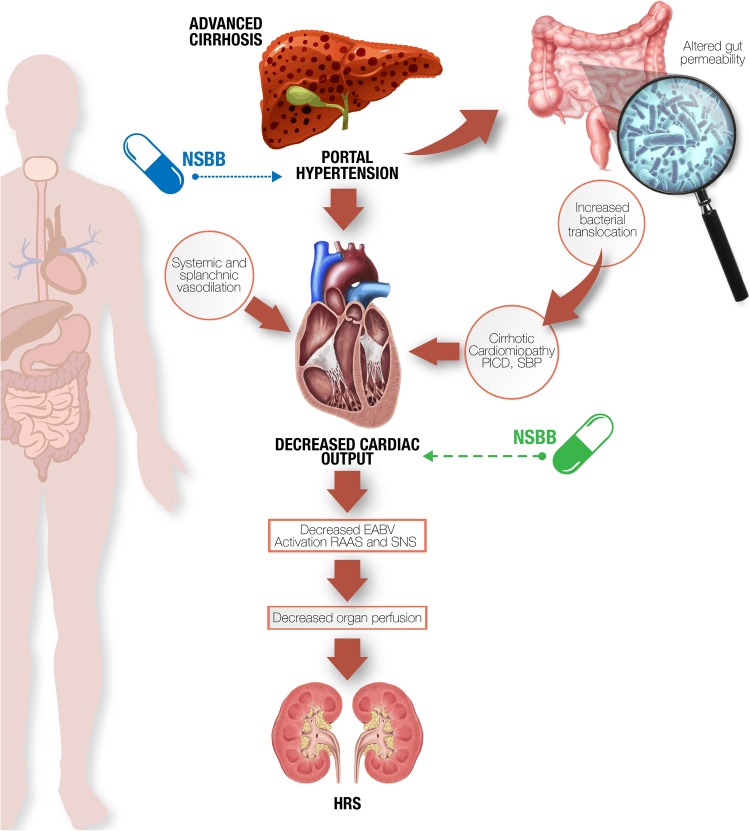
Figure 2.
Beneficial effects (dotted line), deleterious effects (discontinued line). In advanced cirrhosis, especially in presence of cardiomyopathy, NSBB therapy decreases cardiac output leading to EABV reduction which in turn contributes to HRS. Conversely, NSBBs' positive effects include reduction of gut permeability and the risk of SBP. EABV, effective arterial blood volume; HRS, hepatorenal syndrome; NSBBs, non-selective β-blockers; SBP, spontaneous bacterial peritonitis.
Aim and methods
This review aims to provide an extensive examination of the available evidence regarding the effectiveness and safety of NSBBs in terms of overall survival and renal function in different scenarios of advanced cirrhosis. To this end, we carried out a comprehensive literature search on β-blockers and cirrhosis survival using the electronic databases PubMed/MEDLINE, AMED, CINAHL and the Cochrane Central Register of Controlled Trials. Full-text manuscripts published over more than 35 years, from 1980 to April 2016, were reviewed for relevance and reference lists were cross-checked for additional pertinent studies regarding potential NSBB effects (including non-haemodynamic ones), but especially focused on those concerned with survival and/or acute kidney injury (AKI).22 Since the previous meta-analysis by Chirapongsathorn et al23 included a thorough review up until January 2015, we particularly focused on those articles published or indexed after that date. This consisted of peer-reviewed journal articles, retrospectives studies, post hoc analysis and expert opinions.
All selected literature had to meet the research objective by complying with specific end points and the following key words used for the search: ascites, advanced cirrhosis, non-specific β-blockers, AKI, mortality and outcome. The search returned 168 articles that addressed the required criteria of which 26 were published after the aforementioned meta-analysis. A complete position paper from the Baveno VI workshop,17 held in April and published in September 2015, was also included in the review.
Beneficial and protective effects of NSBBs
Variceal bleeding
The protective effects of NSBBs in preventing variceal haemorrhage are known to be mediated by several mechanisms, including decreased cardiac output and splanchnic vasoconstriction—the classic pathways leading to a reduction in portal pressure. Patients who reach these objectives are usually classified as ‘responders’. Nevertheless other NSBB effects are thought to be essential even in classic NSBB non-responders, such as a decrease in azygous or collateral blood flow and a reduction in variceal pressure.24 In spite of these expected benefits, the size of varices must be considered before deciding treatment. Qi et al25 conducted a meta-analysis and concluded that NSBBs should not be recommended for patients with no or small varices. The study included six randomised controlled trials (RCTs) comparing NSBBs (propranolol, timolol and nadolol) with placebo. The NSBBs did not show any significant benefits in terms of preventing the development of large varices, decreasing the incidence of first bleeding, or improving survival. In contrast, they found a significantly higher incidence of adverse events in the NSBBs group compared with the placebo group.25 Despite the limitations (limited number of studies, small sample sizes and high rates of patients lost to follow-up), it is reasonable to accept Qi et al's contraindication as being correct. On the other hand, some recent trial data have suggested that carvedilol delays the progression of small varices with no adverse effects in two similar baseline arms.26 In a very recent RCT, Bhardwaj et al26 showed that carvedilol was effective in delaying the progression from small-to-large oesophageal varices in patients with cirrhosis compared with placebo, despite maintaining a modest 8–10% portal pressure reduction over a long period (24 months of follow-up). There were no deaths related to variceal bleeding or liver disease in either of the groups, reaffirming the relative safety of the long-term use of carvedilol in this subset of patients with cirrhosis. This again raises the question as to which NSBB-treated patients should begin treatment and with what variceal sizes.
Infections and gut permeability
Patients with advanced cirrhosis develop some immunological disturbances, such as altered phagocytic activity, low serum levels of complement factors and decreased bactericidal and opsonic activity, all of which may predispose to bacterial infections.10 27–30 Bacterial infections in cirrhosis are a prominent cause of acute-on-chronic liver failure (ACLF) and NSBBs have shown to improve 28-day and 90-day survival in patients with ACLF.31 However, these data were obtained from a post hoc analysis of the CANONIC cohort and the baseline NSBB group had fewer rates of multiorgan failures and a less advanced grade of ACLF compared with the non-NSBB group, which might represent a limitation to extrapolate these effects to an advanced, stable cirrhosis population. In addition, these results have been questioned by other studies with an NSBB group presenting a higher frequency of kidney and cerebral failure despite a reduction in sepsis; while in data analysis, NSSBs did not modify in-hospital or 3-month outcomes.32
It has been suggested that endotoxaemia is more frequent in advanced liver disease, regardless of the size and number of varices, and can exacerbate hyperdynamic circulation.33 NSBB reduction of gut permeability and BT may be partly independent of portal pressure reduction.34 35 Accordingly, the SNS helps regulate systemic inflammatory response which is overactivated in decompensated cirrhosis; it may also play a key role in BT and abnormal activation of intestinal macrophages, resulting in deregulation of gut barrier permeability.36 37 NSBB-mediated sympathetic blockade therefore attenuates this effect and decreases the risk of infection and host response. These consequences also explain the descent in white cell count and C reactive protein, both involved in the progression to more severe stages of infection and/or ACLF and both independent mortality predictors.32 38
Moreover, propranolol accelerates the intestinal transit which has been shown to prevent bacterial gut overgrowth in murine models and subsequent BT leading to SBP.39–41 These non-haemodynamic beneficial effects have been confirmed in post hoc analysis of controlled trials in patients with decompensated cirrhosis, although these results should be considered within the limitations of the primary trials.10
Hepatocelullar carcinoma
NSBBs have been associated with a reduction in HCC incidence, a leading cause of mortality in cirrhosis.42 43 The proposed mechanism is the inhibition of angiogenesis and the reduction of BT.11 In addition to NSBB antiangiogenic effects,44 a reduction in gut permeability may cause a decrease in the portal load of proinflammatory bacterial by-products, BT and carcinogenesis.40 45 Thiele and colleagues hypothesised that NSBB, catecholamine antagonism prevents cell migration, tumour angiogenesis, invasiveness and proliferation in gastric, breast and pancreatic cancer.46–48 In a recent long-term follow-up study of hepatitis C virus patients with cirrhosis conducted over 11 years, NSBB therapy was the only variable associated with a lower cumulative risk of HCC.49
Portal vein thrombosis
It has been suggested that NSBBs might contribute to portal vein thrombosis (PVT) by reducing cardiac output and through splanchnic vasoconstriction due to the blockade of β2 receptors: both effects may reduce portal venous blood flow velocity which eventually precipitates PVT.50 Nevertheless, in a recent, large longitudinal cohort analysis involving 1243 patients (without HCC) NSBB use was not an independent factor associated with PVT.51
Should the therapeutic window be closed at any point?
Numerous studies have been conducted since Sersté et al questioned the benefits of NSBBs in end-stage cirrhosis. The most relevant studies for and against the use of NSBBs in patients with advanced cirrhosis are described below.
Studies that do not recommend the use of NSBBs in advanced cirrhosis
In 2010, Sersté et al52 observed that patients with cirrhosis and RA receiving NSBB therapy had a greater all-cause mortality. They analysed the differences on the haemodynamic effects and the impact of NSBBs on survival in 151 patients with cirrhosis. More than half of the patients with oesophageal varices were treated with NSBBs. A median survival time of 5 months was observed in patients treated with propranolol compared with 20 months in non-NSBB patients. Factors associated with mortality derived from the multivariate analysis were NSBB therapy, Child-Pugh class C and RA associated with hyponatraemia, hepatic encephalopathy and/or renal failure (intractable ascites). So it is conceivable that patients with intractable RA could be more vulnerable to the deleterious effects of NSBBs than those with diuretic-resistant RA. The authors also stated that low BP in NSBB-treated patients with RA might underlie their detrimental effect.
However, this study was an observational analysis and the NSBB patients appeared to have more severe liver disease at baseline, as reflected by higher serum bilirubin levels, than the non-NSBB patients, hence it is difficult to establish a causal link between NSBB therapy and the main reasons for death. This point is particularly important because all the NSBB patients had oesophageal varices, while only 4% of patients not taking NSBB had oesophageal varices. Therefore, the apparent deleterious effect of NSBB on the survival of patients with clinically significant portal hypertension, reflected by the presence of varices, which is an indication for NSBBs, could be due to higher portal hypertension itself and may impact on prognosis independently of the Model for End-stage Liver Disease (MELD) or Child-Push Turcotte (CPT) scores.53 A further criticism is that the non-NSBB group also had a greater proportion of patients with alcoholic liver disease and no information was given regarding the prevalence of acute alcoholic hepatitis or abstinence from alcohol, or why patients were receiving or not receiving NSBB treatment.54 Moreover, a significant difference in portal pressure cannot be excluded since HVPG measurements were performed in only 37% of patients. Another concern was a higher mortality rate than in previous comparable studies and the causes of death.10 55 The causes of death in the two groups were unclear as 17% of patients died of unspecified causes. Although the mechanism underlying these negative effects was unknown, Sersté et al suggested that NSBBs hampered the cardiac response to postparacentesis-induced circulatory dysfunction (PICD), a syndrome characterised by an exacerbation of splanchnic vasodilation after large-volume paracentesis, which further decreases the effective arterial blood volume and is associated with reduced survival.19 56–59 In addition, cirrhotic cardiomyopathy (CC) with reduced cardiac indices might be further compromised by NSBBs, thus they potentially have a role in adverse outcomes in patients with severe ascites.60–63 This entity is often associated with other portal hypertension complications such as splanchnic and peripheral vasodilation, which lead to decreased renal perfusion, precipitation of HRS and death.60 61 64 65 However, the influence of NSBBs on CC, and whether the latter is related to the severity of liver disease, is still unclear. In keeping with this observation, Krag et al21 demonstrated that the development of HRS and a poor outcome was related to a cardiac systolic dysfunction in patients with advanced cirrhosis and ascites. Low cardiac output was a reflection of organ dysfunction in advanced cirrhosis, and a better predictor of HRS and mortality than MELD score. The proposed mechanism to explain the poorer survival in patients with advanced cirrhosis treated with NSBBs is the blunting of cardiac compensatory response.
To investigate their hypothesis on PICD further, Sersté et al performed a self-controlled cross-over study evaluating the effect of NSBB therapy on the emergence of PICD.66 In this small pilot study, 10 patients with cirrhosis with RA receiving NSBBs were enrolled and monitored before, immediately after and 1 week after a large-volume paracentesis. NSBBs were progressively discontinued (after endoscopic variceal band ligation) and paracentesis and clinical evaluations were repeated. The incidence of PICD after NSBB discontinuation decreased from 80% to 10%, supporting a potentially deleterious effect of NSBBs in patients with RA after large-volume paracentesis. The mechanism underlying PICD may help explain the reported mortality increase observed in these patients. These results should be extrapolated with precaution, since it was a small, non-RCT.
In a very recent study, Kalambokis et al67 evaluated the impact of NSBBs on survival according to CPT classes. They retrospectively evaluated 96 CPT B and 75 CPT C patients with newly diagnosed cirrhosis, of whom 56 CPT B and 45 CPT C patients had oesophageal varices and initiated propranolol. None of the patients had previously been treated with NSBBs, had serum creatinine >1.5 mg/dL, HCC or systolic BP<90 mm Hg, nor were any lost to follow-up. After 2 years of follow-up they observed a higher mortality in CPT B patients on NSBBs compared with non-treated CPT B patients. Another relevant observation is that propranolol use beyond 6 months in CPT C patients with ascites or with MELD score ≥18 was also associated with increased mortality. The mean survival was 10 months in propranolol-treated and 15 months in non-treated CPT C patients (p=0.03). This study is particularly interesting as practically none of the individual RCTs stratified by stage. These results justify the concerns raised over the use of NSBBs in patients with decompensated cirrhosis.
Along the hypothesis that in susceptible patients NSBBs may reduce perfusion of vital organs, including the kidneys, Kim et al68 presented a nested case–control study conducted to evaluate any association between NSBB use and the incidence of AKI in abstract form. Out of a total cohort of 2250 patients waiting for transplant, 202 patients had AKI (they considered only AKI ≥ stage 2). The authors observed that the impact of NSBBs on AKI incidence depended on the presence of ascites, concluding that these drugs increased the risk of AKI in patients with ascites and likely contributed to the mortality increase.
Consistent with the above, a recent multicentre study by the North American Consortium for the Study of End-stage Liver Disease (NACSELD) published at the American Association for the Study of Liver Diseases (AASLD) 2015 Annual Meeting analysed the impact of NSBBs on AKI in 981 non-elective inpatients with cirrhosis, as well as the role of bacterial infections in exacerbating this risk.69 They prospectively enrolled 410 patients taking NSBBs and 571 patients not taking these agents. Patients receiving NSBBs were more frequently diabetic and often with a history of variceal bleeding. NSBB therapy in decompensated cirrhosis increased the likelihood of developing AKI (NSBBs 49% vs non-NSBBs 41%). Furthermore, among patients on NSBBs, renal dysfunction was significantly worse when infected (57% infected NSBBs vs 45% uninfected NSBBs). The fact that patients receiving NSBBs were more frequently diabetic and therefore predisposed to renal complications represents a potential bias in this study. However, NSBB therapy was not a predictor of death, while MELD (OR 1.13) and multiorgan failure (OR 2.37) were significant predictors (p<0.0001). Consequently, the study concluded that the development of infection and the presence of diabetes close the window of benefit derived from β-blocker therapy in patients with decompensated cirrhosis.
Studies recommending the use of NSBBs in advanced cirrhosis
Recently, in a retrospective study including 607 patients at their first paracentesis, Mandorfer et al found an association between NSBB treatment and an increased risk of HRS and AKI in patients with SBP. They also observed, again among patients with SBP, a greater proportion of haemodynamic derangement, longer hospitalisation and reduced transplant-free survival.70 However, patients on NSBBs without SBP had an increased transplant-free survival and required fewer days of non-elective hospitalisation. The higher mortality of NSBB-treated patients with SBP could be explained by a fragile haemodynamic state, reflected in a significantly lower mean BP. The main criticism of this study arises from the retrospective nature of data collection, but also because it is an unmatched, single-centre study.
Leithead et al71 reached opposite conclusions in a single-centre retrospective study including 322 patients with ascites listed for liver transplantation, 159 of whom received NSBBs, while117 had RA (there were no baseline differences between the groups). A mortality reduction in patients receiving NSBBs compared with non-treated patients was observed regardless of the presence of RA. It is noteworthy that increased survival was also observed in patients with lower systolic BP; therefore, these results do not support the suggestions that NSBBs have detrimental haemodynamic effects in patients with RA. However, it was a non-randomised observational study; hence, a selection bias cannot be excluded. Patients in this study, including those with RA, had slightly lower MELD scores than the study by Sersté et al, but they had considerably lower BP, reflecting greater vasodilation. Another potential bias was that the cohort included highly selected candidates awaiting liver transplantation. Finally, NSBB-treated patients were on relatively low doses of NSBBs compared with Sersté et al's study, which might partly explain these conflicting results.
Aligned with the aforementioned study, Aday et al72 performed a retrospective analysis of 2419 patients with cirrhosis and portal hypertension with or without ascites, oesophageal varices or both. Given the retrospective nature of this study the authors subsequently undertook a propensity matching analysis according to the following variables: gender, ethnicity, age, systolic and diastolic BP, MELD, platelets, bilirubin, international normalised ratio, creatinine, haemoglobin, alkaline phosphatase, aminotransferases, albumin and sodium. They selected 865 matched patients, of which patients who did not use NSBBs had a mortality rate more than twice that of patients who were on NSBB therapy. This finding was highly consistent across different categories of patients with cirrhosis, including those with only varices or in combination with ascites (regardless of the severity of the ascites: mild, moderate or severe). As acknowledged by the authors, the inclusion of patients with mild or moderate ascites could be a potential bias in favour of a beneficial effect of NSBBs. Furthermore, patients with varices were not categorised into patients who had bled or not, and therefore there could be a bias associated with patients not receiving β-blockers who presented with variceal haemorrhage. However, the large number of patients is the main strength of this study.
Two additional small retrospective studies supported the benefits of β-blockers at modest doses in patients with cirrhosis with RA.73 74 In the initial study by Sersté et al, nearly half of the patients, and in the consecutive cross-over study, 7 out of 10 patients received 160 mg/day of propranolol. High NSBB doses are linked with more harmful effects to the systemic circulation and with less tolerance, particularly in patients with advanced cirrhosis. Robins and colleagues observed no survival differences between a cohort of patients undergoing elective paracentesis when comparing patients on propranolol with those not receiving β-blockade. These results suggested that propranolol, within a total daily dose of 40–80 mg, is safe among patients with RA. Nevertheless, deleterious effects at higher doses cannot be excluded.
Bossen et al’s75 post hoc analysis of three RCTs involving 1198 patients with ascites is noteworthy for its analysis of the efficacy and safety of the aquaretic agent satavaptan. NSBB-treated patients were more likely than non-NSBB-treated patients to have a history of variceal bleeding but less likely to have CPT class C cirrhosis, hyponatraemia or RA. The 1-year mortality rate was similar in the NSBB group and non-NSBB group; moreover, NSBBs did not increase mortality in the subgroup of patients with RA, SBP or in any other subgroup. It should be noted that 29% of NSBB-treated patients discontinued treatment following a relevant event requiring hospitalisation, such as variceal bleeding, bacterial infection and/or development of HRS. In other words, patients who discontinued NSBB therapy were sicker. These findings conflict with those of Sersté et al and Mandorfer et al.52 70 The reasons for these discrepancies are not entirely clear, but might be explained by a longer follow-up, more advanced disease and data obtained from a single centre in the two latter studies. In Bossen et al's study, severity of portal hypertension could act as a confounder since data on HVPG or gastro-oesophageal varices were missing. Another limitation was the high rate of NSBB discontinuation. Mortality could have been even higher if none of the patients had stopped NSBB treatment. While the main strength of this study was the prospective collection of data from randomised clinical trials, its weakness was that it was only a post hoc analysis of these trials.
Very recently, Bhutta et al76 published data in abstract form from non-elective hospitalised patients with cirrhosis with ascites from the NACSELD database. The cohort consisted of 717 patients, 51% had RA, 19% SBP and 43% were on NSBBs at admission. They compared NSBB-treated to non-NSBB patients to determine their effect on survival and the effect/predictors of NSBB discontinuation. Survival was significantly greater in patients on NSBBs at admission (median survival 58 vs 32 days) and only MELD, but not NSBB therapy, independently predicted survival. They also observed that NSBB use was associated with a lower rate of inflammation biomarkers. Of 308 patients on NSBB therapy, 48% discontinued, mainly due to infection, acute AKI, low systolic BP, hyponatraemia and higher MELD. There was no difference in survival between those who did or did not discontinue. Systolic BP and AKI independently predicted death in a model that also evaluated MELD, Na, infection and NSBB discontinuation.
At the same congress, Onali et al77 published a retrospective study supporting the beneficial role of these drugs, from a cohort of 316 patients assessed for liver transplant. Patients were classified according to whether they received NSBBs or not at the time of transplant. RA was present in 40% of patients. NSBBs were associated with reduced mortality (HR=0.511, 95% CI 0.3 to 0.87, p=0.014), even when considering only patients with RA (NSBB use: HR=0.257, 95% CI 0.1 to 0.66, p=0.005).77
Recently, Chirapongsathorn et al23 conducted a systematic review and meta-analysis to evaluate the effect of NSBBs on all-cause mortality in patients with cirrhosis and RA. The meta-analysis included 3145 patients from three RCTs and eight observational studies of propranolol, carvedilol, nadolol and metoprolol up until January 2015, reporting 1206 deaths. In contrast to some previous studies, they concluded that the use of NSBBs was not associated with a significant increase in all-cause mortality in patients with cirrhosis and ascites or RA.21 66 68 70 This disparity may be partly explained by the heterogeneity across studies, the lack of individual participant data, the effect of the dose and duration of NSBB use or the absence of data differentiating liver-related mortality from non-liver-related deaths. This meta-analysis included studies of metoprolol, which is, however, a cardioselective β-blocker. It is important to note that patients with ascites had a poor prognosis regardless of NSBB therapy. Furthermore, since NSBBs have been clearly shown to decrease the risk of variceal bleeding, regardless of the severity of liver disease and taking into account an ∼15% risk of mortality within 6 weeks associated with variceal bleeding, NSBB withdrawal should cautiously be appraised.3 78–82
Table 1 summarises some of the most relevant publications dealing with the effects of NSBBs on different outcomes.
Table 1.
Studies on the beneficial or deleterious effect of NSBBs on advanced cirrhosis
| Author, year, Ref | Design (n) | End point | Characteristics (RA) | Presence of oesophageal varices non-BB vs BB | CPT-C non-BB vs BB (%) | MAP non-BB vs BB | Doses BB | Follow-up (month) | HR | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Sertsé et al 201052 | Observational Prospective, 151 |
Long-term survival | RA (100%) | 4% vs 100% | 61% vs 74% | 123 vs 103 | 114 mg/day | 8 m | HR 2.61 (1.63 to 4.19) | 1-year probability survival propranolol 19%vs 64% p <0.0001 |
| Mandorfer et al 201470 | Observational Retrospective, 607 (182 SBP) |
Impact of SBP on BB on survival | SBP (NS) | 60% vs 94% | 53% vs 67% | 83 vs 77 | NS | 9.6 (147 person year) | HR 1.64 (1.1 to 2.3) | Patients with SBP on BB increase in mortality risk of 58% |
| Leithead et al 201571 | Observational Retrospective, 322 (208 matched) |
Mortality | Ascites on transplant list (117 (76 matched, 36.5%)) | Previous variceal haemorrhage 29% vs 29% |
NS | 89 vs 86 | 74.8% P (80 mg/day) 25.2% C (6.25 mg/day) |
2.4 (72 days) | HR 0.55 (0.32 to 0.95) RA: HR 0.35 (0.14 to 0.86) |
Mortality after listing 23.2% BB vs 34.8% no-BB |
| Bossen et al 201575 | Post hoc Analysis of 3 RCT, 1188 |
Mortality or hospitalisation | Ascites in RCT of satavaptan/placebo 588 (49%) 559 BB users (46%) |
Previous variceal haemorrhage 13% vs 30% |
28% vs 24% | 85 vs 83 | 159 high dose (>80 mg/day) P or >6.25 mg/day C |
12 (52 weeks) | 0.92 (0.72 to 1.18) RA: 1.02 (0.74 to 1.40) |
HR high dose vs no-BB users 0.8 (0.55 to 1.20) HR low dose vs no-BB users 0.98 (0.72 to 1.13) |
| Mookerjee et al 201631 | Observational prospective, 349 | Mortality at 28 days | ACLF (NS) | NS. Previous gastrointestinal bleeding 17% vs 58% | NS | 79 vs 78 | 40 mg/day (68%) | 12 (56 weeks) | 0.60 (0.36 to 0.98) | 1-year mortality NSBB vs no-NSBB 52% vs 56% p=0.35 |
| Gianelli et al 201682 | Observational, retrospective 526 | Cirrhotic cardiomyopathy | Transplant waitlist (NS) | NS | NS | NS | NS | NS | NS | Systolic dysfunction was higher in MELD>25 with BB, and similar in MELD<25 regardless BB |
| Aday et al 201672 | Retrospective, 2419 Propensity matching score on |
In-hospital mortality | Portal hypertension (100%) | 51% vs 49% | Severe ascites no BB 62% vs 37% on BB | NS | NS | NS | NS | The highest mortality was among those with cirrhosis and severe ascites no-BB (23.2%, compared with 6.5% BB) |
| Robins et al 201474 | Observational retrospective, 114 | Survival | Cirrhosis undergoing elective paracentesis (100%) | 54% vs 100% | 64% vs 64% | NS. | 48.9 mg/day | Median 10 (2–72) | NS | Median survival BB vs no-BB 18 vs 11 months p=0.93 |
| Kim et al 201468 | Nested case–control, 2250 | Association BB-AKI | RA on transplant list (NS) | NS | NS | NS | NS | Median 20.3 (3–201) | NS | |
| Bhutta et al 201676 | Prospective analysis, 717 | Survival | Ascites | 17% vs 31% | NS | NS | NS | NS | NS | Survival 58 days in BB vs 32 days n-BB |
| Chirapongsathorn et al 201623 | Meta-analysis 3 RCT and 8 observational studies, 3145 | Mortality | Ascites 443 (14%) |
NS | NS | NS | NS | NS | RR: 0.95 (0.67 to 1.35) RA: 0.95 (0.57 to 1.61). |
Mortality rate 6 months BB vs no-BB 52% vs 42.5% RR 1.37 (0.94 to 1.98) |
| Kalambokis et al 201667 | Observational retrospective, 171 | Mortality | Ascites | NS | NS | NS | NS | 3 years | NS | Median survival |
| Kimer et al 201573 | Retrospective cohort, 61 | In-hospital mortality | Ascites | 31% vs 82%* | NS | NS | Median 80 mg/day |
∼3.5 years | NS | No difference in survival. Complications 76% no-BB vs 78% BB |
*Thirty-seven per cent of patients in non-BB group and 13% in the BB group were not characterised with oesophageal endoscopy.
ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; BB, β-blockers; C, carvedilol; CP, Child-Pugh Class; CPT, Child-Push Turcotte; MAP, mean arterial pressure; MELD, Model for End-stage Liver Disease; NS, not stated; NSBB, non-selective β-blocker; P, propranolol; RA, refractory ascites; RCT, randomised controlled trial; RR, relative risk; SBP, spontaneous bacterial peritonitis.
RA encompasses a heterogeneous population including diuretic-intractable and diuretic-resistant RA. These differences may underlie discrepancies among different cohorts, while in patients with diuretic-resistant RA NSBBs can be safely prescribed, in those with intractable RA frequently associated with dilutional hyponatraemia, renal dysfunction and/or hepatic encephalopathy are often associated to low cardiac index and a worse circulatory reserve21 52 67 in this setting, the NSBBs detrimental effects may outweigh the benefits and these drugs should not be prescribed. Furthermore, the highest mortality of patients receiving NSBBs occurred within 6 months after the first episode of SBP69 and the fact that a low BP and advanced liver disease (CPT C) were independently associated with higher mortality65 suggests that these drugs are better avoided in patients with CPT C or MELD score ≥25 and within the first 6 months after a SBP episode if associated with haemodynamic deterioration (SBP<90 mm Hg). In addition, patients with a MELD≥18 should receive propranolol at a maximal dose of 40–80 mg/day.74 Therefore, we suggest the therapeutic window should be closed in some specific clinical situations listed in box 1.
Box 1. Suggested clinical situations in which non-selective β-blockers (NSBBs) should be withheld.
Child-Push Turcotte (CPT)-C or Model for End-stage Liver Disease (MELD) ≥25, and
-
▸
Diuretic-intractable refractory ascites (renal dysfunction, hyponatraemia, hepatic encephalopathy),
-
▸
Cardiac index ≤1.5 L/min/m2,
-
▸
Systolic blood pressure (BP) ≤90 mm Hg, either spontaneous or NSBB-induced,
-
▸
Within 6 months after first episode of SBP, as long as haemodynamic deterioration is sustained (eg, BP>90 mm Hg and/or cardiac index ≤1.5 L/min/m2),
-
▸
Consider setting maximal dose of propranolol at 40–80 mg/day if MELD 18–24.
Closing remarks
Although the clinical benefits of NSBBs in patients with cirrhosis have been historically demonstrated in high-quality trials, recent studies have warned against their use in some specific situations with poor circulatory reserve, such as large-volume paracentesis in RA or SBP. Conflicting results may arise from heterogeneous populations (diuretic-intractable RA vs diuretic-resistant RA) and different NSBB dosage. Therefore, it is vitally important to carry out a careful individual risk–benefit analysis in these clinical settings as suggested in box 1. In all other situations, these drugs should not be withheld. However, a large-scale, multicentre, well-controlled study comparing the use of NSBBs with variceal band ligation in patients with advanced cirrhosis would clearly determine the precise role of NSBBs.
Acknowledgments
The authors thank Eulalia Grifol for providing bibliographic support and Jose M Ruiz-Alcántara for graphic design assistance.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Stokkeland K, Brandt L, Ekbom A, et al. Improved prognosis for patients hospitalized with esophageal varices in Sweden 1969–2002. Hepatology 2006;43:500–5. doi:10.1002/hep.21089 [DOI] [PubMed] [Google Scholar]
- 2.Mills PR, Rae AP, Farah DA, et al. Comparison of three adrenoreceptor blocking agents in patients with cirrhosis and portal hypertension. Gut 1984;25:73–8. doi:10.1136/gut.25.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19:475–505. doi:10.1055/s-2007-1007133 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R, Zamora J, Gomez-Camarero J, et al. Meta-analysis: combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med 2008;149:109–22. [DOI] [PubMed] [Google Scholar]
- 5.Lebrec D. Medical treatment of portal hypertension. Presse Med 1991;20:750–5. [PubMed] [Google Scholar]
- 6.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med 1988;319:983–9. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–32. doi:10.1056/NEJMra0901512 [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G, Garcia-Pagan JC, Luca A, et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131:1611–24. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Gea V, Aracil C, Colomo A, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol 2012;107:418–27. doi:10.1038/ajg.2011.456 [DOI] [PubMed] [Google Scholar]
- 10.Senzolo M, Cholongitas E, Burra P, et al. Beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 2009;29:1189–93. doi:10.1111/j.1478-3231.2009.02038.x [DOI] [PubMed] [Google Scholar]
- 11.Thiele M, Albillos A, Abazi R, et al. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int 2015;35:2009–16. doi:10.1111/liv.12782 [DOI] [PubMed] [Google Scholar]
- 12.Abraldes JG, Tarantino I, Turnes J, et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 2003;37:902–8. doi:10.1053/jhep.2003.50133 [DOI] [PubMed] [Google Scholar]
- 13.Bosch J, Berzigotti A, Garcia-Pagan JC, et al. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol 2008;48(Suppl 1):S68–92. doi:10.1016/j.jhep.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 14.Wong F, Salerno F. Beta-blockers in cirrhosis: friend and foe? Hepatology 2010;52:811–13. doi:10.1002/hep.23852 [DOI] [PubMed] [Google Scholar]
- 15.Krag A, Wiest R, Albillos A, et al. The window hypothesis: haemodynamic and nonhaemodynamic effects of b-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61:967–9. doi:10.1136/gutjnl-2011-301348 [DOI] [PubMed] [Google Scholar]
- 16.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005;353:2254–61. doi:10.1056/NEJMoa044456 [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. doi:10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Villanueva C, Albillos A, Genesca J, et al. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology 2016;63:197–206. doi:10.1002/hep.28264 [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-del-Arbol L, Urman J, Fernandez J, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 2003;38:1210–18. doi:10.1053/jhep.2003.50447 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–47. doi:10.1002/hep.20766 [DOI] [PubMed] [Google Scholar]
- 21.Krag A, Bendtsen F, Henriksen JH, et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59:105–10. doi:10.1136/gut.2009.180570 [DOI] [PubMed] [Google Scholar]
- 22.Lebrec D, Nouel O, Corbic M, et al. Propranolol—a medical treatment for portal hypertension? Lancet 1980;2:180–2. [DOI] [PubMed] [Google Scholar]
- 23.Chirapongsathorn S, Valentin N, Alahdab F, et al. Nonselective β-blockers and survival in patients with cirrhosis and ascites: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1096–104.e9. doi:10.1016/j.cgh.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 24.Thalheimer U, Bosch J, Burroughs AK. How to prevent varices from bleeding: shades of grey—the case for nonselective β blockers. Gastroenterology 2007;133:2029–36. [DOI] [PubMed] [Google Scholar]
- 25.Qi XS, Bao YX, Bai M, et al. Nonselective beta-blockers in cirrhotic patients with no or small varices: a meta-analysis. World J Gastroenterol 2015;21:3100–8. doi:10.3748/wjg.v21.i10.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhardwaj A, Kedarisetty CK, Vashishtha C, et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut 2016; ▪▪▪doi:10.1136/gutjnl-2016-311735 [DOI] [PubMed] [Google Scholar]
- 27.Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis 2001;33:41–8. [DOI] [PubMed] [Google Scholar]
- 28.Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246–56, 1256.e1–5 doi:10.1053/j.gastro.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 29.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol 2001;34:32–7. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen P, Vilstrup H, Møller JK, et al. Prognosis of patients with liver cirrhosis and spontaneous bacterial peritonitis. Hepatogastroenterology 2003;50:2133–6. [PubMed] [Google Scholar]
- 31.Mookerjee RP, Pavesi M, Thomsen KL, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol 2016;64:574–82. doi:10.1016/j.jhep.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 32.Pereira G, Baldin C, Victor L, et al. Use of non-selective beta blockers (NSBB) in cirrhotic patients with bacterial infections is associated with lower frequency of sepsis, but not of acute-on-chronic liver failure (ACLF) or survival. Results of a prospective study. J Hepatol 2016;64(Suppl 2):S263. [Google Scholar]
- 33.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol 1995;22:165–72. doi:10.1016/0168-8278(95)80424-2 [DOI] [PubMed] [Google Scholar]
- 34.Reiberger T, Ferlitsch A, Payer BA, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol 2013;58:911–21. doi:10.1016/j.jhep.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 35.Worlicek M, Knebel K, Linde HJ, et al. Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut 2010;59:1127–34. doi:10.1136/gut.2009.185413 [DOI] [PubMed] [Google Scholar]
- 36.Schäper J, Wagner A, Enigk F, et al. Regional sympathetic blockade attenuates activation of intestinal macrophages and reduces gut barrier failure. Anesthesiology 2013;118:134–42. doi:10.1097/ALN.0b013e3182784c93 [DOI] [PubMed] [Google Scholar]
- 37.de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol 2015;6:223 doi:10.3389/fimmu.2015.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038–47. doi:10.1016/j.jhep.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Riordan SM, Williams R. The intestinal flora and bacterial infection in cirrhosis. J Hepatol 2006;45:744–57. doi:10.1016/j.jhep.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 40.Almeida J, Galhenage S, Yu J, et al. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol 2006;12:1493–502. doi:10.3748/wjg.v12.i10.1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Paramo M, Muñoz J, Albillos A, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 2000;31:43–8. doi:10.1002/hep.510310109 [DOI] [PubMed] [Google Scholar]
- 42.Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma. World J Hepatol 2016;8:421–38. doi:10.4254/wjh.v8.i9.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. doi:10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 44.Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 2015;372:735–46. doi:10.1056/NEJMoa1404710 [DOI] [PubMed] [Google Scholar]
- 45.Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut 2012;61:297–310. doi:10.1136/gutjnl-2011-300779 [DOI] [PubMed] [Google Scholar]
- 46.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010;1:628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin Cancer Biol 2008;18:164–70. doi:10.1016/j.semcancer.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao X, Che X, Zhao W, et al. The beta-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor kappaB signaling. Oncol Rep 2010;24:1669–76. [DOI] [PubMed] [Google Scholar]
- 49.Herrera I, Pascual S, Zapater P, et al. The use of β-blockers is associated with a lower risk of developing hepatocellular carcinoma in patients with cirrhosis. Eur J Gastroenterol Hepatol 2016;28:1194–7. doi:10.1097/MEG.0000000000000677 [DOI] [PubMed] [Google Scholar]
- 50.Qi X-S, Bai M, Fan D-M. Nonselective β-blockers may induce development of portal vein thrombosis in cirrhosis. World J Gastroenterol 2014;20:11463–6. doi:10.3748/wjg.v20.i32.11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology 2015;61:660–7. doi:10.1002/hep.27546 [DOI] [PubMed] [Google Scholar]
- 52.Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017–22. doi:10.1002/hep.23775 [DOI] [PubMed] [Google Scholar]
- 53.Thevenot T, Cervoni JP, Monnet E, et al. Is this really the end of beta-blockers in patients with cirrhosis and refractory ascites? Hepatology 2011;53:715–16. doi:10.1002/hep.23839 [DOI] [PubMed] [Google Scholar]
- 54.Thalheimer U, Bosch J, Burroughs AK. An apology for beta blockers. J Hepatol 2014;61:450–1. doi:10.1016/j.jhep.2014.03.040 [DOI] [PubMed] [Google Scholar]
- 55.Senzolo M, Nadal E, Cholongitas E, et al. Is hydrophobia necessary for the hepatologist prescribing nonselective beta-blockers in cirrhosis? Hepatology 2011;53:2149–50. doi:10.1002/hep.24176 [DOI] [PubMed] [Google Scholar]
- 56.Gines A, Fernandez-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996;111:1002–10. doi:10.1016/S0016-5085(96)70068-9 [DOI] [PubMed] [Google Scholar]
- 57.Pozzi M, Osculati G, Boari G, et al. Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology 1994;106:709–19. doi:10.1016/0016-5085(94)90706-4 [DOI] [PubMed] [Google Scholar]
- 58.Panos MZ, Moore K, Vlavianos P, et al. Single, total paracentesis for tense ascites: sequential hemodynamic changes and right atrial size. Hepatology 1990;11:662–7. doi:10.1002/hep.1840110420 [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-del-Arbol L, Monescillo A, Jimenez W, et al. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology 1997;113:579–86. doi:10.1053/gast.1997.v113.pm9247479 [DOI] [PubMed] [Google Scholar]
- 60.Pall A, Czifra A, Vitalis Z, et al. Pathophysiological and clinical approach to cirrhotic cardiomyopathy. J Gastrointest Liver Dis 2014;23:301–10. [DOI] [PubMed] [Google Scholar]
- 61.Wiese S, Hove JD, Bendtsen F, et al. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014;11:177–86. doi:10.1038/nrgastro.2013.210 [DOI] [PubMed] [Google Scholar]
- 62.Chayanupatkul M, Liangpunsakul S. Cirrhotic cardiomyopathy: review of pathophysiology and treatment. Hepatol Int 2014;8:308–15. doi:10.1007/s12072-014-9531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong F. Cirrhotic cardiomyopathy. Hepatol Int 2009;3:294–304. doi:10.1007/s12072-008-9109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol 2007;21:125–40. doi:10.1016/j.bpg.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 65.Karagiannakis DS, Papatheodoridis G, Vlachogiannakos J. Recent advances in cirrhotic cardiomyopathy. Dig Dis Sci 2015;60:1141–51. doi:10.1007/s10620-014-3432-8 [DOI] [PubMed] [Google Scholar]
- 66.Serste T, Francoz C, Durand F, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol 2011;55:794–9. doi:10.1016/j.jhep.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 67.Kalambokis GN, Christodoulou D, Baltayiannis G, et al. Propranolol use beyond 6 months increases mortality in patients with CPT C cirrhosis and ascites. Hepatology 2016;64:1806–8. doi:10.1002/hep.28575 [DOI] [PubMed] [Google Scholar]
- 68.Kim S, Kim W, Larson J, et al. The effect of long-term use of nonselective beta-blocker on the development of acute kidney injury in patients with liver cirrhosis. Hepatology 2014;60:277A. [Google Scholar]
- 69.Wong F, O'Leary JG, Reddy KR, et al. North American Consortium for the Study of End-Stage Liver Disease. The association of non-selective beta-blocker use and acute kidney injury in patients with decompensated cirrhosis admitted into hospital—a study from the North American Consortium for the Study of End Stage Liver Disease (NACSELD). Hepatology 2015;62:66A. [Google Scholar]
- 70.Mandorfer M, Bota S, Schwabl P, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146:1680–90.e1. doi:10.1053/j.gastro.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 71.Leithead JA, Rajoriya N, Tehami N, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015;64:1111–19. doi:10.1136/gutjnl-2013-306502 [DOI] [PubMed] [Google Scholar]
- 72.Aday AW, Mayo MJ, Elliott A, et al. The beneficial effect of beta-blockers in patients with cirrhosis, portal hypertension and ascites. Am J Med Sci 2016;351:169–76. doi:10.1016/j.amjms.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 73.Kimer N, Feineis M, Moller S, et al. Beta-blockers in cirrhosis and refractory ascites: a retrospective cohort study and review of the literature. Scand J Gastroenterol 2015;50:129–37. doi:10.3109/00365521.2014.948053 [DOI] [PubMed] [Google Scholar]
- 74.Robins A, Bowden A, Watson W, et al. Beta-blockers in cirrhosis patients with refractory ascites. Hepatology 2014;59:2054–5. doi:10.1002/hep.26676 [DOI] [PubMed] [Google Scholar]
- 75.Bossen L, Krag A, Vilstrup H, et al. Non-selective beta-blockers do not affect mortality in cirrhosis patients with ascites: Post hoc analysis of three RCTs with 1198 patients. Hepatology 2016;63:1968–76. doi:10.1002/hep.28352 [DOI] [PubMed] [Google Scholar]
- 76.Bhutta A, Garcia-Tsao G, Reddy R, et al. Beta-blocker use in hospitalized cirrhotic patients with ascites is associated with a lower MELD, less inflammation and an improved survival. J Hepatol 2016;64(Suppl 2):S245 doi:10.1016/S0168-8278(16)00253-1 [Google Scholar]
- 77.Onali S, Kalafateli M, Majumdar A, et al. Non-selective beta blockers (NSBBS) use is associated with improved survival in cirrhotic patients with ascites: a single centre retrospective study. J Hepatol 2016;64(Suppl 2):S668 doi:10.1016/S0168-8278(16)01259-9 [Google Scholar]
- 78.Poynard T, Cales P, Pasta L, et al. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med 1991;324:1532–8. [DOI] [PubMed] [Google Scholar]
- 79.Bhutta A, Garcia-Tsao G. The role of medical therapy for variceal bleeding. Gastrointest Endosc Clin N Am 2015;25:479–90. doi:10.1016/j.giec.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 80.Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev 2012;8:CD004544 doi:10.1002/14651858.CD004544.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pagliaro L, D'Amico G, Tine F, et al. Prevention of upper gastrointestinal bleeding from portal hypertension in cirrhosis: rationale for medical treatment. Dig Dis 1992;10(Suppl 1):56–64. [DOI] [PubMed] [Google Scholar]
- 82.Giannelli V, Roux O, Ratou P, et al. Prevalence of cardiomyopathy and impact of the use of non selective beta blockers in end-stage liver disease. J Hepatol 2016;64:S167. [Google Scholar]