Abstract
LuxS plays a role in the synthesis of an extracellular signaling molecule, autoinducer 2 (AI-2). To analyze a possible role of AI-2 in regulating Helicobacter pylori gene expression, we constructed a panel of transcriptional reporter strains. We show that the expression of H. pylori flaA is growth phase dependent and that flaA transcription increases in association with increased culture density. Mutating the luxS gene eliminates growth-phase-dependent control of flaA, and this growth phase dependence is restored when the luxS mutant strain is complemented with the wild-type luxS gene.
Quorum sensing is a mechanism used by bacteria to regulate expression of genes in response to changes in population density (reviewed in reference 21). Quorum-sensing processes depend on the production, secretion, and recognition of diffusible signaling molecules that accumulate with bacterial growth. Among the gram-negative bacteria, two quorum-signaling mechanisms have been identified. The LuxI/LuxR system uses an acetylated homoserine lactone signal molecule (reviewed in reference 16), and the LuxS system uses a signal molecule termed autoinducer 2 (AI-2) (4).
Synthesis of AI-2 is dependent on the activity of the LuxS protein, which functions as an AI-2 synthase (31). The pathway for AI-2 production has been linked to the methyl metabolic cycle (25), which allows recycling between S-adenosyl methionine and methionine. A by-product of this pathway is the toxic metabolite S-ribosylhomocysteine, which LuxS converts to 2,3-pentanedione (DPD) and homocysteine. DPD cyclizes in the presence of boron, forming AI-2 (24, 25). To date, the best characterized LuxS-based quorum-sensing system is that of Vibrio harveyi. In this organism, AI-2 regulates gene expression by activating an elaborate network of two-component signal transduction systems (14, 15, 21).
Various gram-negative and gram-positive bacterial species, including Helicobacter pylori (13, 18, 20), Campylobacter jejuni (10), enterohemorrhagic Escherichia coli (EHEC) (27), Salmonella enterica serovar Typhimurium (30), Clostridium perfringens (23), and Bacillus anthracis (19) produce AI-2 molecules that can stimulate luminescence in an AI-2-specific V. harveyi reporter strain. AI-2 plays a role in the regulation of multiple processes relevant to bacterial pathogenesis, including hemin uptake in Porphyromonas gingivalis (5), iron uptake in Actinobacillus actinomycetemcomitans (11), and flagellar production and type III secretion processes in EHEC (26, 28). Although H. pylori contains a luxS orthologue and produces AI-2 (13, 18, 20), previous studies have not identified any H. pylori genes that are regulated by AI-2. In the present study, we sought to investigate further whether AI-2 plays a role in regulating gene expression in H. pylori.
Two previous studies have reported that H. pylori flaA is transcribed at low levels when the density of bacterial cultures is low, and it is transcribed at higher levels when the bacterial cultures approach late log or stationary phase (22, 33). We hypothesized that growth-phase-dependent transcriptional regulation of flaA might be dependent on a luxS regulatory pathway. To monitor expression of flaA, we constructed reporter strains in which either promoterless chloramphenicol acetyltransferase (cat) or β-galactosidase (lacZ) cassettes were introduced into flaA. As controls, we constructed reporter strains to monitor expression of two genes (i.e., HP0609 and HP0689) that are reportedly transcribed in a growth-phase-independent manner (33). To construct these reporter strains, the coding regions of the following genes from H. pylori strain J99, a strain for which the entire genome sequence is known (1), were PCR amplified and cloned into plasmid pGEMT (Promega) with the following oligonucleotide primers: for flaA (i.e., gene designations HP0601 and JHP0548), 5′-TGGGCAAACTACGGAATCTCG-3 and 5′-GCAGGGGCGTTTTTCAATAAATCC-3′; for HP0609 (JHP0556), 5′-CTTCTTAGGGCTAGGGTTCGTTTC-3′ and 5′-TGGGTGTTGTTATTGGAAGTGGC-3′; and for HP0689 (JHP0628), 5′-CCACCACATTGAGAATGAGATCG-3′ and 5′-GCCTGCCAATAGAGCTTGCTATC-3′.
Transcriptional reporters for flaA, HP0609, and HP0689 were generated by insertion of blunt-ended HindIII fragments harboring either a promoterless E. coli chloramphenicol acetyltransferase (cat) gene from plasmid pCM7 (American Type Culture Collection no. 37173) (6) or aphA-3 (encoding kanamycin resistance) and a promoterless lacZ gene from plasmid pBW (9) into unique restriction sites within the coding regions of the cloned genes (i.e., NheI for flaA, BglII for HP0609, and BamHI for HP0689). These insertions are predicted to disrupt the encoded proteins at amino acids 404 (FlaA), 342 (HP0609), and 167 (HP0689). Introduction of the transcriptional reporters into the H. pylori chromosome was accomplished by natural transformation and allelic exchange as previously described (9, 12). Transformants were initially selected on the basis of antibiotic resistance, and insertion of reporters into the desired sites was confirmed by PCR analyses of genomic DNA. The cat transcriptional reporters were successfully introduced into H. pylori J99 and 26695. Multiple attempts to introduce the lacZ transcriptional fusions into H. pylori J99 were unsuccessful. These fusions were, however, successfully introduced into H. pylori 1061 (17), a strain in which the lacZ reporter has been used previously (9, 35).
H. pylori luxS sequences were PCR amplified with the primers 5′-CGATCAACGACAAAGTGGTGG-3′ and 5′-AACTCATCGGCTTCTAAATCTCCC-3′. Cloning of the H. pylori J99 luxS gene (i.e., JHP0097) and the strain 26695 luxS gene (i.e., HP0105) into pGEMT resulted in plasmids pGEMTluxS1 and pGEMTluxS2, respectively. The luxS genes were disrupted via introduction of the aph3 cassette from pUC4K (Pharmacia) into a unique XcmI site, resulting in pGEMTluxSKm1 and pGEMTluxSKm2, respectively. These insertions are predicted to disrupt the encoded LuxS proteins at amino acid 75. Transformation of H. pylori strains J99, 26695, and 1061 with plasmids pGEMTluxSKm1, pGEMTluxSKm2, and pGEMTluxSCAT (13), respectively, resulted in the luxS mutants J99luxSKm, 26695luxSKm, and 1061luxSCAT.
Additional luxS mutant plasmids were constructed by XcmI/NheI restriction digestion of pGEMTluxS2, followed by insertion of a sacB-kan cassette (7) into the blunt-ended XcmI/NheI site (yielding pGEMTluxSXN396) or by recircularization of the XcmI/NheI-digested pGEMTluxS2 (yielding pGEMTluxSXN). pGEMTluxSXN codes for a C-terminal-truncated LuxSΔ75-156. H. pylori 26695 was transformed with pGEMTluxSXN396, and introduction of the disrupted luxS gene into the chromosome resulted in the luxS mutant 26695luxSXN1 (kanamycin resistant and sucrose [5% sucrose] sensitive). Strain 26695luxSXN1 was then transformed with either plasmid pGEMTluxSXN (encoding LuxSΔ75-156) or with pGEMT-luxS2 (encoding wild-type LuxS), and transformants in which sacB-kan had been replaced with the introduced luxS sequences were selected based on sucrose resistance and kanamycin sensitivity. These transformations yielded strains 26695luxSC-1 (wild-type luxS restored in its original locus) and 26695luxSXN (encoding LuxSΔ75-156). To introduce a functional luxS gene into a luxS mutant strain while leaving the nonfunctional truncated luxS allele intact (complementation), strain 26695luxSXN was transformed with plasmid pADluxS (13), a plasmid designed to introduce an intact luxS gene in the ureA locus of H. pylori, yielding strain 26695luxSPAD-1.
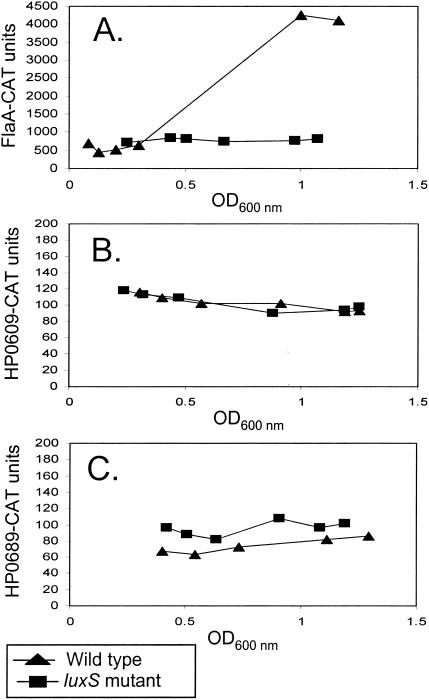
For our initial studies of growth-phase-dependent transcription in H. pylori, broth cultures were inoculated with each of the reporter strains and aliquots were removed at serial time points to measure optical density and to quantify the levels of reporter activity. As shown in Fig. 1A, the expression of the flaA-cat transcriptional fusion progressively increased as the culture density increased. In contrast, the expression of HP0609 and HP0689 transcriptional fusions remained essentially unchanged (Fig. 1B and C). Growth-phase-dependent changes in flaA were also detected in the lacZ transcriptional reporter strain (Fig. 2) and were similar to those detected in strain J99 by the cat transcriptional fusions. No growth-phase-dependent changes in expression of HP0609 or HP0689 were detected in the lacZ reporter strains (Fig. 2).
FIG. 1.
Growth-phase-dependent expression of flaA in H. pylori J99. H. pylori J99 or J99luxSKm (luxS mutant) harboring flaA-cat (A), HP0609-cat (B), or HP0689-cat (C) transcriptional fusions were grown in sulfite-free Brucella broth supplemented with 5% fetal bovine serum at 37°C in ambient air containing 5% CO2. At various time points, cultures were assayed for chloramphenicol acetyltransferase (CAT) activity by using the CAT-enzyme-linked immunosorbent assay (ELISA) kit (Roche Diagnostics). Briefly, 5-ml aliquots of H. pylori cells were lysed with the ELISA kit lysis buffer, and protein concentrations of lysates were determined with the bicinchoninic acid reagent kit (Pierce). The amount of cell protein added to each ELISA plate well ranged from 0.25 μg (for flaA-cat) to 2 μg (for HP0609-cat and HP689-cat). The levels of CAT bound per well were quantified by using the protocols detailed by the manufacturer. Absorbance data were obtained with an ELISA plate reader (Dynatech). CAT units represent picograms of CAT protein per microgram of cell extract. Each panel represents results of a single experiment. Experiments were repeated three times, with similar results.
FIG. 2.
Expression of flaA in H. pylori 1061. H. pylori 1061 or 1061luxSKm containing flaA-lacZ, HP0609-lacZ, or HP0689-lacZ transcriptional fusions were cultured in Brucella broth containing 5% fetal bovine serum. At various time points, 500-μl aliquots were removed and added to 500 μl of Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.01 M MgSO4, 0.05 M β-mercaptoethanol, pH 7.0). The cell suspensions were then lysed by the addition of 40 μl each of chloroform and 0.1% sodium dodecyl sulfate. β-Galactosidase expression was measured at OD570 with 4 mg of chlorophenol-red-galactopyranoside/ml as a substrate (2). Results are standardized for cell density (OD600) and are presented as β-galactosidase activity units (2). Each panel represents results of a single experiment. Experiments were repeated three times (for flaA) or two times (for HP0609 and HP0689), with similar results.
To determine if growth phase regulation of flaA in H. pylori is dependent on the AI-2 quorum-sensing system, the flaA-cat and flaA-lacZ fusions were introduced into luxS mutants of H. pylori J99 (i.e., J99luxSKm) and H. pylori 1061 (i.e., 1061luxSCAT), respectively. In contrast to the growth-phase-dependent expression of flaA observed in the wild-type strains, expression of flaA in the luxS mutant strains was not growth phase dependent (Fig. 1A and 2). Expression of HP0689 and HP0609 was not altered by mutation of the luxS gene (Fig. 1B and C). These data provide evidence that growth phase regulation of flaA expression is dependent on luxS.
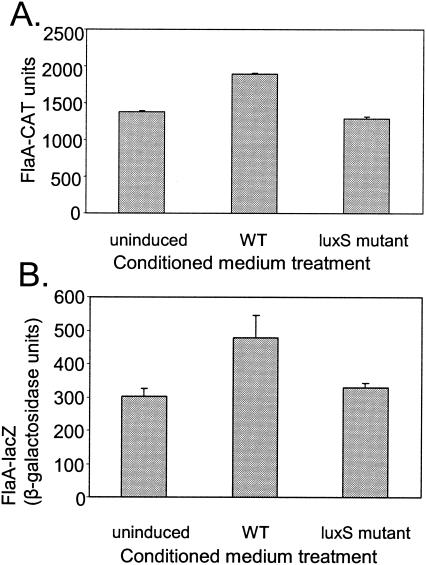
To further investigate a potential role of luxS in regulating flaA expression, luxS mutant strains containing either the flaA-cat or flaA-lacZ reporter were cultured in the presence of conditioned medium derived from either wild-type H. pylori J99 or its luxS mutant derivative (i.e., J99luxSKm). Conditioned medium from J99 induced 1,000-fold greater luminescence in V. harveyi BB170 (3, 29) than did conditioned medium from J99luxSKm (data not shown). However, the levels of flaA expression were only slightly higher when the flaA reporter strains were grown in wild-type conditioned medium than when grown in conditioned medium lacking AI-2 (P < 0.05, paired Student's t test) (Fig. 3). In these experiments, flaA expression was analyzed following a 15-h induction period, when culture optical densities at 600 nm (OD600) were in the range (0.8 to 1.0) where maximal flaA expression is observed (see Fig. 1). No stimulatory effect of wild-type conditioned medium on flaA reporter expression was observed when the reporter strains were incubated with conditioned medium for shorter time periods. While the lack of significant induction at early time points was surprising, this result was, nonetheless, consistently reproduced in experiments with both H. pylori J99 and H. pylori 1061 reporter strains. Conditioned medium had no effect on expression of either HP0609 or HP0689 following a 15-h induction period (data not shown).
FIG. 3.
Effect of conditioned medium on expression of flaA. (A) H. pylori J99luxSKm, containing a flaA-cat transcriptional fusion, was grown in sulfite-free Brucella broth containing 5% fetal bovine serum to an OD600 of 0.6. Bacterial cells were then pelleted by centrifugation and transferred to fresh Brucella broth containing conditioned medium (20%, vol/vol) derived from either wild-type (WT) H. pylori strain J99 or a luxS mutant derivative (J99luxSKm). The reporter strain was grown in these media for 15 h, resulting in a change in culture density from OD600 = 0.1 to OD600 = 0.8 to 1.0. The bacteria were then assayed for chloramphenicol acetyltransferase (CAT) activity. (B) H. pylori 1061luxSCAT, containing a flaA-lacZ transcriptional fusion, was analyzed as described for panel A. The bacteria were then assayed for β-galactosidase activity. All data represent analyses of triplicate samples. The means and standard deviations are indicated. Expression of flaA was slightly higher in cultures incubated in 20% wild-type-conditioned medium than in cultures incubated in 20% luxS mutant-conditioned medium (P < 0.05, paired Student's t test).
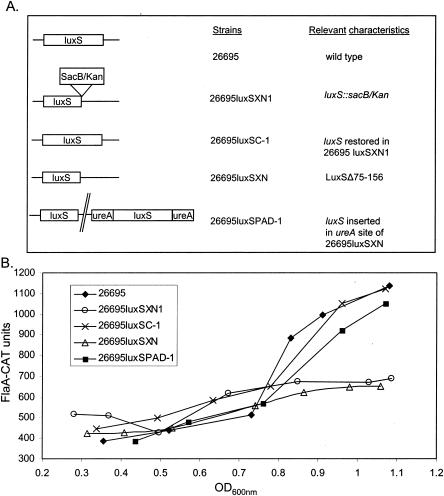
To more rigorously test the role of luxS in growth phase regulation of flaA, we analyzed expression of flaA in a wild-type H. pylori (26695) strain, two isogenic luxS mutant strains, and derivatives of these luxS mutant strains into which a functional copy of luxS was introduced. Schematic illustrations of these strains are shown in Fig. 4A. Conditioned media from 26695luxSXN1 (i.e., sacB/kan luxS mutant) and 26695luxSXN (i.e., LuxSΔ75-156) failed to induce luminescence in V. harveyi BB170 (data not shown). In contrast, conditioned media from derivatives of these luxS mutant strains, in which luxS was restored in its original locus (i.e., 26695luxSC-1) or inserted in the ureA locus (i.e., 26695luxSPAD-1), induced luminescence in V. harveyi in a manner similar to the effects of conditioned media from wild-type H. pylori 26695 (data not shown). Only strains 26695luxSC-1 and 26695luxSPAD-1 showed growth-phase-dependent expression of flaA similar to that of the 26695 wild-type strain (Fig. 4B). No growth-phase-dependent regulation of flaA was found in the luxS mutants 26695luxSXN1 and 26695luxSXN (Fig. 4B).
FIG. 4.
Growth-dependent expression of flaA in H. pylori 26695 requires luxS. (A) Schematic illustration of strains used for analysis of flaA-cat expression. Strain 26695luxSPAD-1 is a complemented luxS mutant strain. (B) Cultures were grown to various OD600s, and the cells were processed and analyzed for chloramphenicol acetyltransferase (CAT) expression. Data represent analyses of triplicate samples. The experiment was repeated twice, with similar results.
Taken together, our present work clearly indicates that luxS plays a role in regulating the growth-phase-dependent expression of flaA. A previous study demonstrated that luxS also upregulates expression of flagellar genes in EHEC and E. coli K-12 (8, 27). Notably, there are some features of luxS-dependent regulation of H. pylori flaA expression that are atypical compared to the features of AI-2-mediated regulation of gene expression in several other bacterial species. In particular, H. pylori-conditioned medium (containing extracellular AI-2) produced only a small effect on flaA transcription.
One possible explanation for the weak effects of H. pylori-conditioned medium on flaA expression is that specific proteins required for AI-2 signal transduction may not be present in H. pylori. This possibility is supported by results of BLAST searches of the H. pylori genome (34) which fail to identify homologs of various AI-2 signaling components found in V. harveyi (e.g., LuxQ, LuxU, LuxO, LuxP [4]), EHEC (QseBC [28]), and S. enterica serovar Typhimurium (Lsr transporter [32]). Alternatively, in H. pylori an intact luxS gene may be required for the synthesis of a putative AI-2 signal receptor. A third possibility would be that, rather than function as an extracellular signaling molecule in H. pylori, AI-2 may function primarily as an intracellular signal. In this case, the minimal induction of flaA expression by conditioned medium could be a result of AI-2 diffusing inefficiently back into the cell. Winzer et al. (36) proposed that besides contributing to quorum signaling, LuxS also has an important metabolic function. In support of this, the production of AI-2 has been linked to intracellular levels of S-adenosylmethionine, a molecule closely tied to the metabolic state of the cell. In this model, AI-2 signals the metabolic status of a bacterial population, leading to the appropriate up- or downregulation of target genes. In support of this notion, AI-2 is a global regulator in EHEC and regulates genes with diverse functions, including type III secretion, production of flagellar components, cell division and growth, and the SOS response (27). In H. pylori, Thompson et al. (33) found, using microarray studies, that the transcription of about 20% of H. pylori genes varied in association with changes in growth phase. A large proportion of these genes are known to be involved in cell maintenance and growth. Based on the model proposed by Winzer et al. (36), we speculate that AI-2 may serve to identify the metabolic status of an H. pylori population, leading to appropriate expression of target genes. Using AI-2, H. pylori may thus be able to modulate expression of various genes, including flaA, in response to different growth environments it would encounter within its host.
In conclusion, we have demonstrated that growth phase regulation of flaA expression in H. pylori is dependent on luxS. Future studies will seek to identify additional genes that are regulated by a luxS-dependent pathway as well as components of AI-2-dependent signaling pathways in H. pylori.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs.
We thank J. G. Kusters, Arnoud van Vliet, and Nicolette de Vries for generously providing the pBW reporter plasmid.
Editor: V. J. DiRita
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Banfalvi, Z., A. Nieuwkoop, M. Schell, L. Besl, and G. Stacey. 1988. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol. Gen. Genet. 214:420-424. [DOI] [PubMed] [Google Scholar]
- 3.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 5.Chung, W. O., Y. Park, R. J. Lamont, R. McNab, B. Barbieri, and D. R. Demuth. 2001. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 183:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Close, T. J., and R. L. Rodriguez. 1982. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene 20:305-316. [DOI] [PubMed] [Google Scholar]
- 7.Copass, M., G. Grandi, and R. Rappuoli. 1997. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect. Immun. 65:1949-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLisa, M. P., C. F. Wu, L. Wang, J. J. Valdes, and W. E. Bentley. 2001. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J. Bacteriol. 183:5239-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries, N., E. J. Kuipers, N. E. Kramer, A. H. van Vliet, J. J. Bijlsma, M. Kist, S. Bereswill, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 2001. Identification of environmental stress-regulated genes in Helicobacter pylori by a lacZ reporter gene fusion system. Helicobacter 6:300-309. [DOI] [PubMed] [Google Scholar]
- 10.Elvers, K. T., and S. F. Park. 2002. Quorum sensing in Campylobacter jejuni: detection of a luxS encoded signalling molecule. Microbiology 148:1475-1481. [DOI] [PubMed] [Google Scholar]
- 11.Fong, K. P., W. O. Chung, R. J. Lamont, and D. R. Demuth. 2001. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect. Immun. 69:7625-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsyth, M. H., J. C. Atherton, M. J. Blaser, and T. L. Cover. 1998. Heterogeneity in levels of vacuolating cytotoxin gene (vacA) transcription among Helicobacter pylori strains. Infect. Immun. 66:3088-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsyth, M. H., and T. L. Cover. 2000. Intercellular communication in Helicobacter pylori: luxS is essential for the production of an extracellular signaling molecule. Infect. Immun. 68:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman, J. A., and B. L. Bassler. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31:665-677. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, J. A., and B. L. Bassler. 1999. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 181:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 18.Hardie, K. R., C. Cooksley, A. D. Green, and K. Winzer. 2003. Autoinducer 2 activity in Escherichia coli culture supernatants can be actively reduced despite maintenance of an active synthase, LuxS. Microbiology 149:715-728. [DOI] [PubMed] [Google Scholar]
- 19.Jones, M. B., and M. J. Blaser. 2003. Detection of a luxS-signaling molecule in Bacillus anthracis. Infect. Immun. 71:3914-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce, E. A., B. L. Bassler, and A. Wright. 2000. Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded autoinducer. J. Bacteriol. 182:3638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 22.Niehus, E., F. Ye, S. Suerbaum, and C. Josenhans. 2002. Growth phase-dependent and differential transcriptional control of flagellar genes in Helicobacter pylori. Microbiology 148:3827-3837. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani, K., H. Hayashi, and T. Shimizu. 2002. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol. Microbiol. 44:171-179. [DOI] [PubMed] [Google Scholar]
- 24.Schauder, S., and B. L. Bassler. 2001. The languages of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 25.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 26.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 29.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surette, M. G., and B. L. Bassler. 1999. Regulation of autoinducer production in Salmonella typhimurium. Mol. Microbiol. 31:585-595. [DOI] [PubMed] [Google Scholar]
- 31.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taga, M. E., J. L. Semmelhack, and B. L. Bassler. 2001. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol. Microbiol. 42:777-793. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, L. J., D. S. Merrell, B. A. Neilan, H. Mitchell, A. Lee, and S. Falkow. 2003. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect. Immun. 71:2643-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. (Erratum, 389:412.) [DOI] [PubMed] [Google Scholar]
- 35.van Vliet, A. H., E. J. Kuipers, B. Waidner, B. J. Davies, N. de Vries, C. W. Penn, C. M. Vandenbroucke-Grauls, M. Kist, S. Bereswill, and J. G. Kusters. 2001. Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional level. Infect. Immun. 69:4891-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. Holden, R. Linforth, K. A. Cornell, A. J. Taylor, P. J. Hill, and P. Williams. 2002. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909-922. [DOI] [PubMed] [Google Scholar]