Abstract
An epidural abscess represents a rare acute medical emergency, with a reported incidence of 2.5/10 000 hospital admissions annually. The clinical features include fever, spinal pain, radiating nerve root pain and leg weakness. When sepsis is present, prompt recognition is required to initiate appropriate antimicrobial therapy and surgical decompression. We present the case of a man aged 68 years presenting to the emergency department with a 3-day history of fever, low back, right hip and leg pain. He was hypoxic, tachycardic and hypotensive. He required intubation and ventilation. An MRI spine confirmed a posterior epidural abscess from T12 to L4. Blood cultures revealed Staphylococcus aureus. He started treatment with linezolid and underwent incision and drainage. He remained septic and 8 days later, a repeat MRI spine showed a peripherally enhancing posterior epidural collection from L2/L3 to L4/L5, consistent with a recurrent epidural abscess. Further drainage was performed. He developed bilateral knee pain requiring washout. His right knee synovial biopsy cultured S. aureus. He continued treatment with linezolid for 6 weeks until his C reactive protein was 0.8 ng/L. He started neurorehabilitation. 10 weeks later, he became feverish with lumbar spine tenderness. An MRI spine showed discitis of the L5/S1 endplate. A CT-guided biopsy confirmed discitis and osteomyelitis. Histology was positive for S. aureus and he started treatment with oral linezolid. After 19 days, he was discharged with 1 week of oral linezolid 600 mg 2 times per day, followed by 1 further week of oral clindamycin 600 mg 4 times daily. This case report reinforces the importance of maintaining a high clinical suspicion, with a prompt diagnosis and combined medical and surgical treatment to prevent adverse outcomes in this patient cohort. With spinal surgical services centralised, physicians may not encounter this clinical diagnosis more often in day-to-day hospital medical practice. The unique aspect of this case is the persistence and then the recurrence (despite 6 weeks of antimicrobial therapy and a second debridement) of S. aureus infection. Furthermore, the paucity of clinical recommendations and the controversy regarding the adequate duration of antimicrobial therapy are notable features of this case.
Case presentation
A man aged 68 years, born and resident in the UK presented to the emergency department with a 3-day history of bilateral leg weakness, fatigue, fever, lower back and right hip pain. In addition, he reported decreased urinary frequency and acute-onset confusion. He was a non-smoker and consumed 8 units of alcohol weekly. His medical history included hypercholesterolaemia. He was taking simvastatin 20 mg daily. Observations were as follows: RR 14, SpO2 96% on 12 L FiO2, BP 90/57 mm Hg, HR 110 bpm and temperature 38.6°C. Respiratory examination confirmed good air entry bilaterally and breath sounds were vesicular. His GCS was 14/15 (E4M6V4). Upper limb examination confirmed normal power, tone and reflexes, with intact sensation and proprioception. Lower limb examination confirmed normal tone, reduced power of hip flexors (3/5) and reduced sensation from L2 to L4. Rectal examination demonstrated normal anal tone and sensation. Mild nuchal rigidity was elicited.
Investigations
Haematological investigations confirmed an elevated C reactive protein (CRP) (311.3 mg/L) and an acute kidney injury. His INR was 2.7 mmol/L, corrected with 10 mg of vitamin K intravenously (table 1).
Table 1.
Haematological investigations showed an elevated C reactive protein, deranged liver function tests and an acute kidney injury
| Hb 160 g/L | Magnesium 0.78 mmol/L | Urea 14.0 mmol/L |
| WCC 10.70×109/L | Albumin 39 g/L | Creatinine 150 umol/L |
| Neutrophils 9.86×109/L | Alkaline phosphatase 87 IU/L | eGFR 40 mL/min/L |
| Platelets 154×109/L | ALT 315 IU/L | Na 134 mmol/L |
| INR 2.7 mmol/L | CRP 311.3 mg/L | K 4.8 mmol/L |
A venous blood gas revealed: pH 7.341, base excess −3.3 mmol/L, HCO3 −21.4 mmol/L and lactate 2.7 mmol/L. A chest radiograph showed atelectasis bilaterally within the lung bases (figure 1). An unenhanced CT head showed no evidence of intracranial bleed, extracerebral collection or focal mass lesion (figure 2). He started treatment with acyclovir and ceftriaxone. However, an MRI head with contrast displayed no evidence of leptomeningeal disease (figure 3). He received intravenous teicoplanin and gentamicin for sepsis of unknown origin. HIV and hepatitis serology were negative. He remained feverish, tachycardic, hypotensive and hypoxic. He was admitted to the Department of Intensive Care Medicine requiring intubation, ventilation and inotropic support. An MRI spine demonstrated a posterior epidural collection extending from T12 to L4 (figure 4), with mixed signal intensity on STIR and T2-weighted images, as well as low to intermediate signal intensity on T1-weighted imaging. The lesion favoured the right posterolateral aspect of the epidural space cranially and more caudally the left posterolateral epidural space. Moulding of the adjacent posterolateral margin of the thecal sac, most pronounced at the L2–L3 level, measuring 17×7 mm in the axial section, with subtle rim enhancement was noted. He was reviewed urgently by the Spinal Team. On microbiology advise, he stopped treatment with gentamicin and teicoplanin and started treatment with meropenem (12 days), clarithromycin (2 days) and clindamycin (8 days). His methicillin-resistant Staphylococcus aureus (MRSA) screening swabs were negative.
Figure 1.
Chest radiograph showed atelectasis within the lung bases bilaterally.
Figure 2.
Unenhanced CT head. An unenhanced CT head showed no evidence of intracranial bleed, extracerebral collection or focal mass lesion.
Figure 3.
MRI head with contrast. An MRI head with contrast displayed no evidence of leptomeningeal disease.
Figure 4.
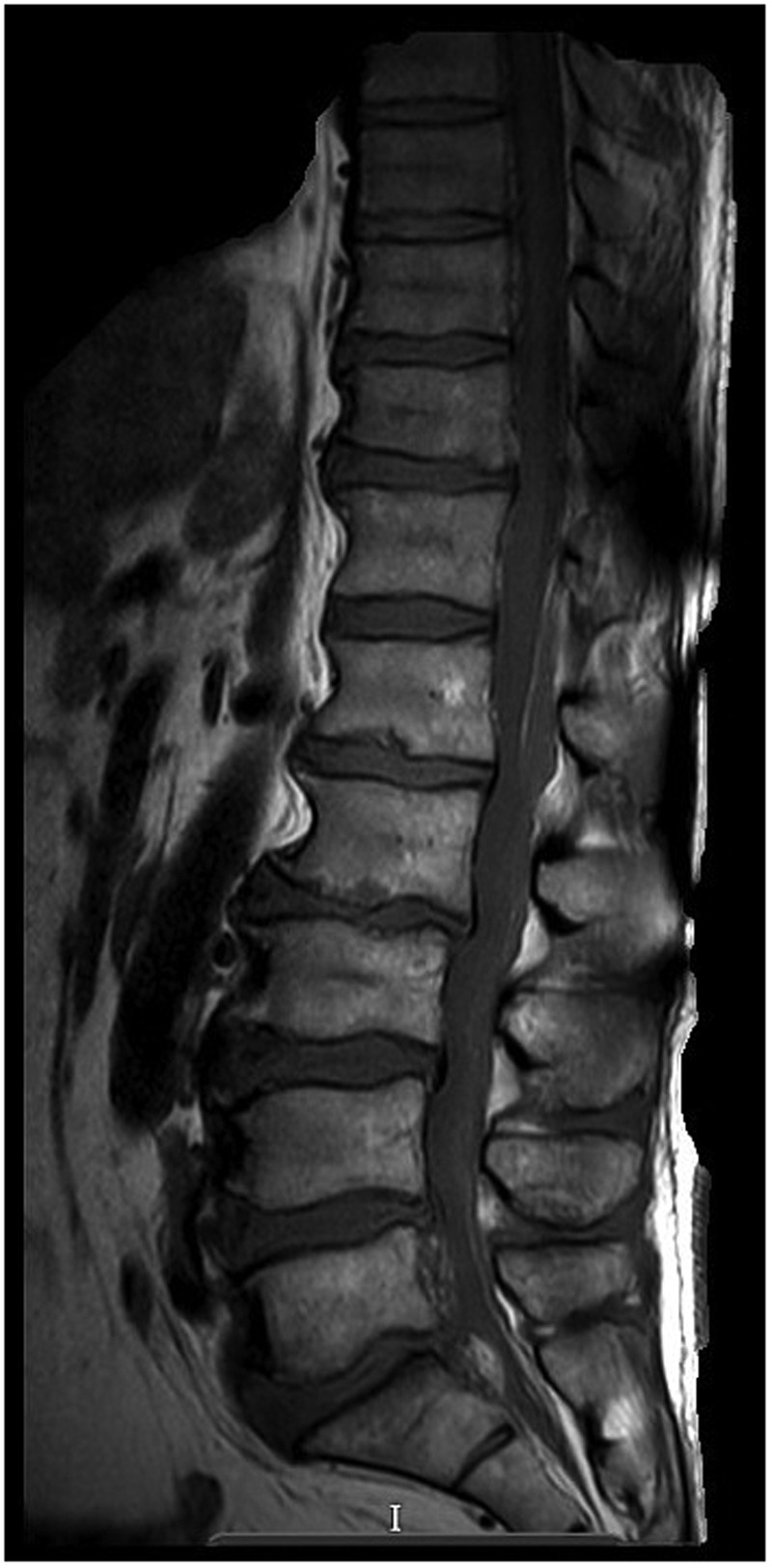
MRI whole spine with contrast. An MRI spine demonstrated normal alignment and vertebral body height. A posterior epidural collection extending from T12 to L4 was observed.
Treatment
Two days postadmission, he underwent emergency washout of his epidural abscess via a midline incision to enter the spinal canal at the point of most marked stenosis, L2–L3 (figure 5). A partial laminectomy with flavectomy to decompress the dura was performed. Blood-stained pus was evacuated and copious saline lavage carried out. Staphylococcus aureus was isolated from the aerobic and anaerobic culture bottles. Pus and wound swabs cultured S. aureus as well as tissue from the ligamentum flavum. Postoperatively, he remained septic, with no reduction in his inflammatory markers and an elevated white cell count (18.9×109/L) with a neutrophilia (14×109/L). On microbiology advise, he started treatment with intravenous linezolid. His urinary Legionella pneumophila and Pneumococcal antigens were negative. He developed transaminitis and thrombocytopenia. An ejection systolic murmur was audible on auscultation, but a transoesophageal echocardiogram showed no evidence of infective endocarditis. His CRP remained elevated at 319.4 g/L, with a white cell count of 12.7×109/L. Repeat blood cultures 48 and 72 hours after starting antimicrobial therapy showed no growth. A repeat MRI spine 6 days later showed a discrete peripherally enhancing posterior epidural collection from L2/L3 to L4/L5, consistent with a recurrent epidural abscess, larger than the preoperative MRI. Severe distortion and compression of the cauda equine was noted (figure 6). Furthermore, there was evidence of discitis in the L2/L3 and L5/S1 discs, with increasing endplate oedema at these levels, particularly at L5/S1 with extension of the abscess into the paraspinal soft tissues. Eight days postadmission, he returned to theatre, the old wound was opened and pus was evident superficially. The laminectomy site was reopened and pus was visualised adjacent to the dura. A further midline incision was made at L3–L4. A right-sided L5–S1 laminectomy was performed and the dura was unremarkable. Wound and pus swabs cultured S. aureus. Macroscopically, the ligament adjacent to the spinal abscess, comprised a brown fibrous piece of tissue, measuring 20×10×7 mm. Histological examination identified microscopic bony fragments with marrow, fibro-fatty tissue and skeletal muscle, with no evidence of significant inflammation. His spinal tissue revealed a scanty growth of S. aureus, sensitive to clindamycin, linezolid and flucloxacillin. He continued treatment with linezolid intravenously.
Figure 5.
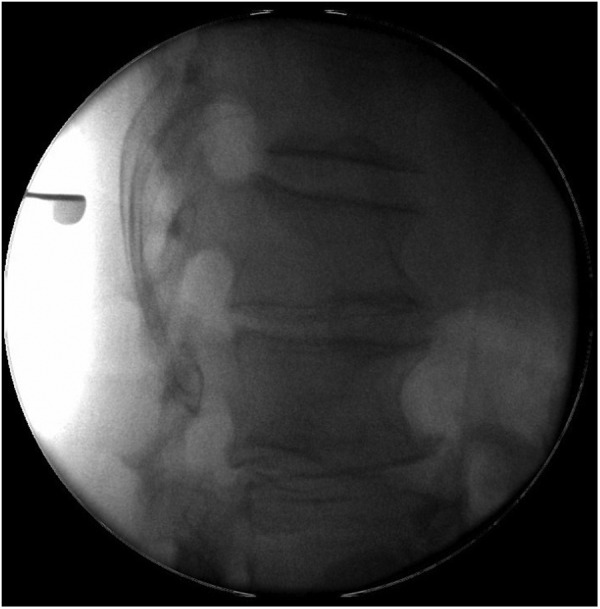
Mobile image intensifer lumbar spine demonstrated the epidural abscess intraoperatively.
Figure 6.
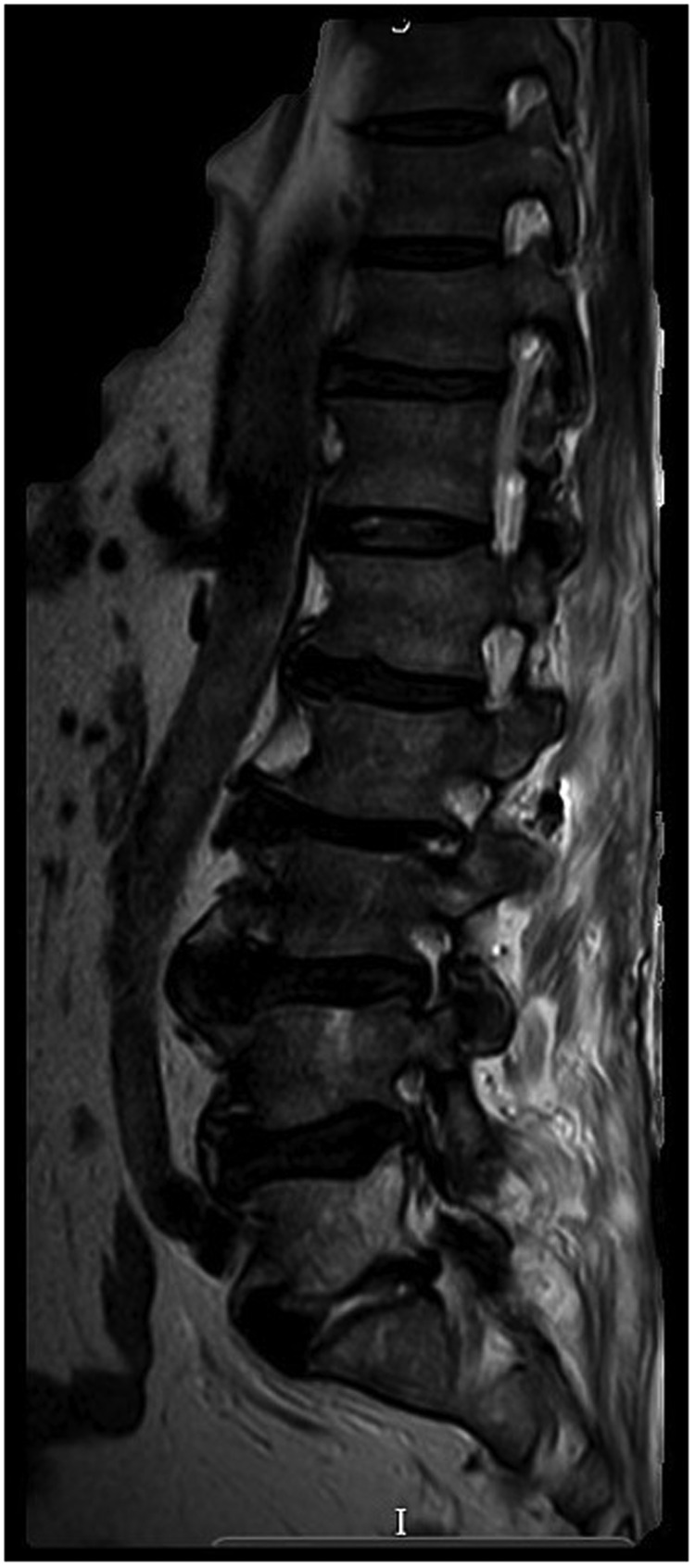
MRI whole spine with contrast. An epidural abscess from L2/L3 to L4/L5 causing significant central canal stenosis and distortion of the cauda equine was observed as well as progressive discitis at L2/L3 and L5/S1.
Outcome and follow-up
He failed to wean off the ventilator requiring a percutaneous tracheostomy. Two weeks later, he was decannulated successfully. He remained feverish (temperature 38.7°C), and clinical examination confirmed septic arthritis of his knees. He underwent bilateral arthroscopic wash out of both his knees. Straw-coloured fluid was aspirated from his right knee. A synovectomy and a chondroplasty was performed. His left knee revealed a serous effusion with no evidence of infection. His right knee synovial fluid showed chronic inflammation, with acute inflammatory cells. No crystals were identified. His knee fluid cultured Gram-positive cocci in clumps, further identified as S. aureus. After 29 days in intensive care unit, he was stepped down to the Orthopaedic Ward, requiring neurorehabilitation for his critical illness polyneuropathy. He required a second arthroscopic washout of his right knee. The synovial biopsy comprised fibroconnective tissue with fibropurulent exudate on its surface. The infiltrate included numerous polymorph neutrophils in keeping with a bacterial infection. He continued treatment with linezolid for 6 weeks until his CRP was 0.8 mg/L (table 2).
Table 2.
The patient's linezolid was stopped after 6 weeks as his C reactive protein was 0.8 mg/L and his observations were stable
| Hb 85 g/L | CRP 0.8 mg/L | Na 138 mmol/L |
| WCC 50×109/L | Albumin 30 g/L | K 5.2 mmol/L |
| Neutrophils 6.98×109/L | ALP 136 IU/L | Urea 5.2 mmol/L |
| Platelets 437×109/L | ALT 23 IU/L | Creatinine 58 µmol/L |
He was subsequently discharged to the Neurorehabilitation Department. Four weeks later, he was readmitted with non-specific symptoms of general malaise, fatigue and lower back pain. Neurological examination demonstrated tenderness on palpation of L5–S1. Haematological investigations revealed a leucocytosis (14.20×109/L) with a neutrophilia (8.53×109/L) and a CRP of 252 g/L. A chest radiograph demonstrated some linear atelectasis on the left side at the lung base. A CT thorax, abdomen and pelvis revealed normal lungs and pleura, with no hilar or mediastinal lymphadenopathy. His liver, gallbladder, pancreas, spleen, adrenals, kidneys, small and large bowels were unremarkable. There was no evidence of intra-abdominal or pelvic lymphadenopathy. Destruction of the L5/S1 endplate consistent with discitis (figure 7) as well as inflammatory paravertebral changes were shown. An MRI lumbar and sacral spine with contrast showed increased bone oedema within the inferior endplate of L2 (figure 8). Signal change within the L2–L3 and L5–S1 intervertebral discs persisted in keeping with ongoing infective discitis. The previously demonstrated SEA lying between L3 and L5 appeared to have resolved. Extensive areas of pathological enhancement were demonstrated within the interspinous ligaments between L3 and L5, the inferior vertebral endplates of L2, L5 and S1 vertebral bodies, the sacral canal and the perivertebral soft tissues mainly at L5 and S1 levels, in keeping with an ongoing infectious process. He underwent a CT-guided biopsy, using an 11 G core biopsy of his right-sided L5–S1 disc (figure 9). A second needle was inserted and multiple 18 G core biopsies were obtained of the disc itself. Macroscopically, the biopsy specimen comprised pieces of fibrocartilage from an intervertebral disc, focally infiltrated by polymorph neutrophils with some vascularisation. Fragments of necrotic material and collections of polymorph neutrophils admixed with macrophages were noted. The histological features confirmed an acute discitis. The L5/S1 biopsy consisted of a core of largely necrotic lamellar bone with fibrosis in the marrow space, suggestive of osteomyelitis. The specimens cultured a scanty growth of S. aureus, sensitive to flucloxacillin, erythromycin, clindamycin, linezolid and tetracycline. On microbiology advise, he started treatment with oral linezolid, 600 mg two times per day. After 19 days, he was discharged with 1 week of oral linezolid, followed by 1 week of oral clindamycin 600 mg four times daily. He remains committed to his neurorehabilitation.
Figure 7.
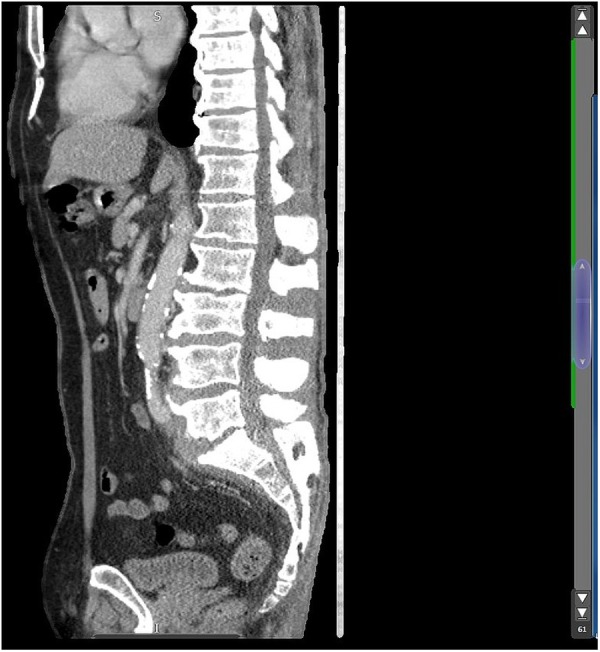
CT thorax, abdomen and pelvis showed destruction of the L5/S1 endplate consistent with known discitis.
Figure 8.
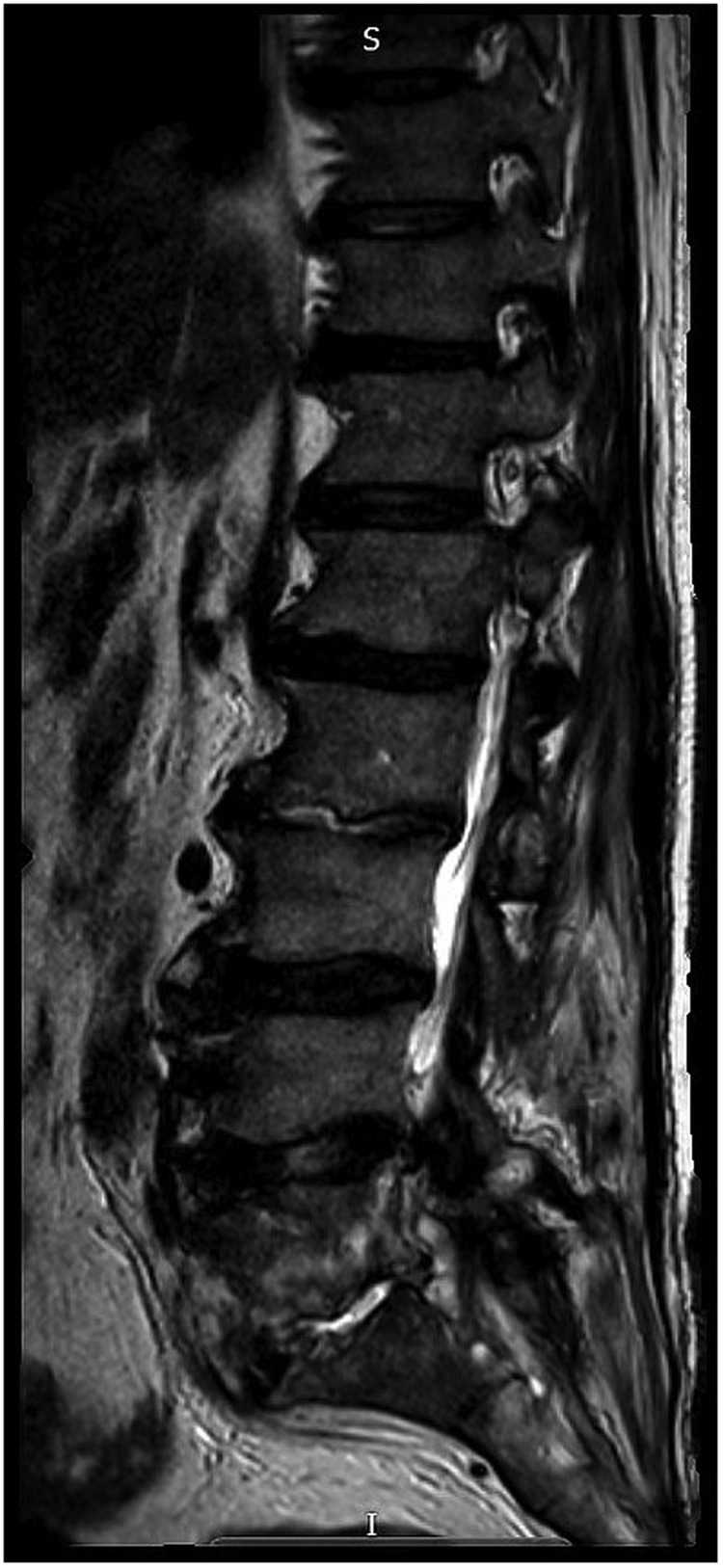
MRI whole spine with contrast demonstrated extensive areas of pathological enhancement within the interspinous ligaments between L3 and L5, the inferior vertebral endplates of L2, L5 and S1 vertebral bodies, the sacral canal and the perivertebral soft tissues mainly at L5 and S1 levels, in keeping with an ongoing infectious/inflammatory process.
Figure 9.
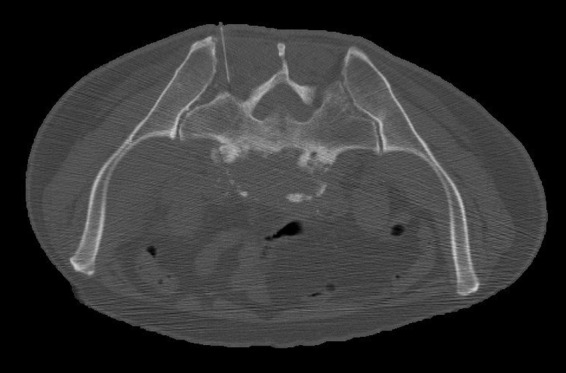
CT-guided biopsy of L5–S1. He underwent a CT-guided biopsy, using an 11 G core biopsy of his right-sided L5–S1 disc.
Discussion
An epidural abscess is a rare suppurative infection of the central nervous system, first reported in 1761 by Sir Percival Pott, an English surgeon, who also described tuberculosis of the spine (Pott's disease).1 Until the 1980s, it accounted for 0.2–1.2/10 000 hospital admissions annually, with the current annual incidence estimated to be 2.5–3/10 000 hospital admissions.2 3 This increase can partly be explained by an ageing population with multiple comorbidities, spinal abnormalities, anaesthetic interventions and enhanced imaging modalities. It has a male predilection and a peak incidence in the fifth to seventh decade, with a reported death of 2–20%. Risk factors include immunosuppression, steroids, epidural catheter placement, paraspinal injections, intravenous drugs, HIV, cancer and chronic renal failure. It is postulated that trauma may result in vertebral haematoma formation, thus providing a nidus for infection. Studies have reported diabetes as a risk factor in 18–54% of cases, with a preponderance of lower thoracic and lumbar abscesses.4 5 The initial manifestations of SEA are non-specific, with the classical diagnostic triad of fever in 50%, spinal pain and neurological deficits, including motor weakness, sensory change, bladder or bowel dysfunction and paralysis, present in a small proportion.6 Admittedly, in the emergency department, SEA is not always considered, as neurological symptoms may not always be apparent in the early stages.6 With cervical lesions, a history of neck stiffness might be reported and patients may present with symptoms mimicking other pathologies such as pancreatitis or heart disease. When sepsis dominates the clinical presentation, a high index of clinical suspicion is required to discern the neurological symptoms. In 1948, Heusner7 summarised the clinical features into four stages, ranging from non-specific symptoms in Stage I to paralysis in Stage IV (box 1).
Box 1. Stages according to the clinical progression of spinal epidural abscess.
Stage clinical signs
Back pain, fever and tenderness
Radicular pain, nuchal rigidity, neck stiffness, reflex changes
Sensory abnormalities, motor weakness, bowel and bladder dysfunction
Paralysis
Haematological investigations demonstrate leucocytosis with polymorphonuclear predominance, an elevated erythrocyte sedimentation rate and hyponatraemia.8 Blood cultures may identify the infecting organism, although they are negative in 40% of cases. Lumbar puncture should not be routinely performed. A high index of clinical suspicion is required in those individuals intoxicated with alcohol, as symptoms might be misinterpreted as sequelae of alcohol. Sendi et al6 describe a SEA as a collection of pus or inflammatory granulation tissue between the dura mater and the overlying vertebral column. Its pathophysiology includes haematogenous spread of bacteria from a cutaneous (vertebral body, psoas muscle) or mucosal source (dental abscess, furuncle, pharyngitis). Skin, soft tissue, urinary and respiratory tract infections are frequent primary sources of haematogenous seeding. The direct spread of infection into the epidural space from discitis and vertebral osteomyelitis have also been described.9 10 The majority of SEA are primarily located in the posterior aspect of the spinal cord, with anterior SEAs usually occurring below L1.6 It is important to consider iatrogenic causes such as spinal surgery, epidural catheter placement and nerve block injections. MRI is the imaging modality of choice, with abscesses demonstrating fluid equivalent signal intensity on T2-weighted images with rim enhancement and a hypointense centre. MRI is the most sensitive and specific test for the detection of vertebral osteomyelitis.10 MRI findings may also correlate with outcome; in a study of 18 patients, central stenosis of >50% and an abscess length of >3 cm were associated with a worse outcome.11 A large variety of pathogens are causative, including mycobacteria, fungi and parasites, but S. aureus is the most frequently encountered organism, occurring in 57–93%, followed by Streptococcus (18%) and Gram-negative bacilli (Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa).12 In the UK, around 12 500 cases of S. aureus bacteraemia (SAB) are reported annually, associated with significant mortality and morbidity, including vertebral osteomyelitis, with infection resulting from haematogenous seeding of the vertebral bodies by arterial or venous vessels.13 Fever is common with tenderness over the involved vertebrae and neurological deficits in 15–20%. The Waldvogel classification system divides osteomyelitis into haematogenous, contiguous and chronic complex disease states14(box 2). Haemophilus parainfluenzae, Brucella and Actinomyces israeli are among other isolates described.15 It is important to consider other pathologies presenting with back pain, fever and spinal tenderness, including tuberculosis, degenerative disease, metastatic tumours, vertebral discitis, osteomyelitis, meningitis, neurological disease or herpes zoster. Early surgical decompression and drainage, with prolonged antibiotic therapy, is the mainstay of treatment. However, indications for percutaneous drainage of abscesses and for surgical intervention in patients with S. aureus haemolytic vertebral osteomyelitis have not been standardised.16 Antimicrobial therapy should be guided by culture and sensitivity (positive in 60%) as well as empirical therapy, with several authors advocating CT-guided biopsy to further delineate treatment. Rapid surgical intervention is not only needed to reduce neurological damage, but also for controlling sepsis. Conservative therapy with antibiotics alone might be required in those individuals posing a high anaesthetic risk. There is a paucity of uniform recommendations about antimicrobial treatment duration and it remains controversial with no prospective, randomised, double-blind clinical trials to date. Several studies suggest a total duration of therapy of between 4 and 16 weeks with resolution of the SEA generally achieved after 4–6 weeks. In the case of concomitant vertebral osteomyelitis, parenteral antibiotics are given for 6–8 weeks.3 In 2015, Professor Bernard et al17 performed an open label, non-inferiority, randomised, controlled trial and suggested that the standard antibiotic treatment duration could be 6 weeks. The nucleus pulposus and the inner two-thirds of the annulus fibrosis of normal intervertebral discs are avascular; therefore, the penetration of intravenous antibiotics into intervertebral discs depends on passive diffusion.18 Clindamycin, vancomycin and teicoplanin have been shown to penetrate into rabbit nucleus pulposus, the anatomy and biochemical characteristics of the aforementioned is similar to that of humans.19–21 Clindamycin has particularly good bone penetration, attaining a high bone-to-serum ratio. Vancomycin has excellent penetration into the bones of experimental animals.22 Tai et al and Vaverka and Petrzelova have shown that gentamicin can penetrate diseased intervertebral discs.23 24 Pharmacokinetic studies have shown that non-β lactams such as clindamycin, aminoglycosides and glycopeptides achieve therapeutic concentrations in discs, but β lactams such as penicillin and cephalosporins do not.24 The British Society for Antimicrobial Chemotherapy concurred that most of the published studies on the use of antibiotics in spinal surgery are retrospective and that the only absolute requirement of a drug selected for use in patients undergoing procedures involving disc penetration is that it possesses in vitro activity against Staphylococci. Linezolid has in vitro activity against methicillin-susceptible S. aureus (MSSA) and MRSA, with clinical activity confirmed in nosocomial pneumonia, ventilator-associated pneumonia, complicated skin and soft tissue infections and MRSA infections, such as staphylococcal and vancomycin-resistant enterococcal osteomyelitis.25 Other surgeons choose an antibiotic based on their own favourable experience.26 Antibiotics that can be used for the treatment of MSSA bacteraemia include the penicillinase-resistant semisynthetic penicillins, such as flucloxacillin, first-generation cephalosporins, such as cefazolin and the cyclic lipopeptide daptomycin.27 28 Leder et al demonstrated the clinical efficacy of continuous-infusion flucloxacillin in serious Staphylococcal sepsis in 20 patients, with a clinical and microbiological cure achieved for 82%; Mehtar et al demonstrated clinical success rates of 89%.29 30 The aforementioned may prompt the question, ‘why then was flucloxacillin not used in our case? Was linezolid causative in his recurrent epidural abscess?’. In our case, S. aureus sensitivities to clindamycin, linezolid and flucloxacillin were reported. According to Gibson et al,31 flucloxacillin does not penetrate the avascular normal human vertebral discs, therefore it was not the antimicrobial of choice in our case. Our microbiologist, experienced in orthopaedic pathologies, deemed linezolid the antimicrobial of choice, demonstrating excellent tissue penetration and equivalent bioavailability between oral and intravenous therapy.25 Linezolid, a member of the oxazolidinone class of antibiotics, is indicated for the treatment of skin and soft tissue infections caused by MSSA, MRSA or vancomycin-resistant enterococci and other susceptible microorganisms. Linezolid blocks the 50S ribosomal subunit and has bacteriocidal activity against Gram-positive organisms such as enterococci, staphylococci, streptococci and Mycobacterium tuberculosis. Linezolid has been suggested as an alternative to vancomycin in patients with SAB, but data are lacking; hence, clinicians may have concerns about the efficacy of linezolid when the blood culture is positive for S. aureus. Shorr's pooled analysis of five prospective, randomised, controlled studies showed that linezolid appeared to be well tolerated and associated with clinical, microbiological and survival outcomes that were not inferior to those of vancomycin in patients with secondary SAB.32 33 Two recent meta-analyses have demonstrated the superior efficacy of linezolid in the treatment of bone and joint infections as well as skin and soft tissue infections.34 35 A meta-analysis by Fu et al36 showed that linezolid is associated with better clinical and microbiological outcomes than glycopeptides for the treatment of S. aureus infections. Caution is advised due to side effects of anaemia, neutropenia, thrombocytopenia, leucopenia, pancytopenia and raised serum transaminase levels. It requires initiation under the supervision of a microbiologist. It is contraindicated with the concomitant use of serotenergic agents, tricyclic antidepressants and serotonin agonists because of the risk of serotonin syndrome. The main determinant of outcome is the neurological status at the time of diagnosis. Other predictors include age <60 years, <50% degree of thecal compression, <72 hours of cord symptoms and no comorbidities. In 1926, a mortality of 81% was reported, but with the advent of antimicrobial therapy, enhanced imaging techniques and prompt surgical decompression, the mortality rate now ranges from 2% to 20%.37 Death may be due to sepsis or prolonged immobilisation, with subsequent pneumonia.38 39 This case report reinforces the importance of considering a SEA in the differential diagnosis in patients presenting with sepsis, back pain, nerve root pain, motor weakness, sensory change and bladder or bowel dysfunction. Prompt recognition and a multidisciplinary approach with early initiation of antimicrobial therapy and surgical decompression is required to reduce morbidity and mortality. It also demonstrates the paucity of guidelines on antimicrobial therapy and its duration. The authors are not advocating linezolid as first-line antimicrobial therapy for S. aureus vertebral osteomyelitis, just reporting our experience. Perhaps our patients relapse was due to the use of linezolid, albeit a reduction in inflammatory markers was observed (CRP 0.8 mg/L). Prospective, randomised, double-blind clinical trials or large prospective observational studies are required to further delineate the efficacy of linezolid in the treatment of methicillin-sensitive S. aureus and to determine its ability to penetrate the avascular vertebral disc. This case also demonstrates the challenges posed in the treatment of S. aureus vertebral osteomyelitis and discitis with linezolid, the importance of initiation under the guidance of a microbiologist with close monitoring for signs of immune suppression.
Box 2. The Waldvogel classification of osteomyelitis.
Waldvogel classification system for osteomyelitis
Haematogenous osteomyelitis
Osteomyelitis secondary to contiguous focus of infection
No generalised vascular disease
Generalised vascular disease
Chronic osteomyelitis (necrotic bone)
Learning points.
Spinal epidural abscess is a rare but severe infection requiring prompt recognition. It poses a diagnostic challenge to physicians as prior to the development of neurological signs, its presentation can mimic a broad spectrum of clinical pathologies.
It has a male predominance, with a predilection for the fifth to seventh decade of life.
Predisposing conditions include diabetes mellitus, trauma to the spine, intravenous drug use, immunosuppressive therapy, cancer, HIV/AIDs, alcoholism and chronic renal failure.
Symptoms and signs at diagnosis include back pain, tenderness, motor weakness, radicular pain, sensory abnormalities, fever, bladder and bowel dysfunction, paralysis, neck stiffness, confusion, headache, nausea and vomiting.
Inflammatory markers such as white cell count, C reactive protein and erythrocyte sedimentation rate (ESR) are generally elevated. Leucocytosis is found in 60–80%, and an ESR >20 mm/hour in up to 95% of reported cases.
MRI with gadolinium has a specificity and sensitivity >90% to detect SEA and is therefore the diagnostic method of choice. In most studies, SEA is predominantly located in the thoracic and lumbosacral region.
In addition to blood cultures, which remain negative in 40% of the cases, CT-guided needle aspiration of the abscess should be attempted because it has a higher sensitivity in identifying the causative microorganism.
The management of SEA should always be multidisciplinary, involving spinal surgeons, radiologists and microbiologists. Drainage of the abscess and antimicrobial therapy are the basic principles of treatment. This case also emphasises the paucity of recommendations on antimicrobial treatment duration. Staphylococcus aureus infection can actually be very heterogenous and management needs to be tailored to the severity and characteristics of the individual case. The main determinant of outcome is the neurological status at the time of diagnosis.
Footnotes
Contributors: All authors contributed to the writing of this manuscript. LD is responsible for writing the case report and literature review. SI is responsible for microbiology advise and management, literature review. CB is responsible for operation notes.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000;23:175–204. 10.1007/PL00011954 [DOI] [PubMed] [Google Scholar]
- 2.Grewal S, Hocking G, Wildsmith JAW. Review article: epidural abscess. Br J Anaesth 2006;96:292–302. 10.1093/bja/ael006 [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO. Spinal epidural abscess. N Engl J Med 2006;355:2012–20. 10.1056/NEJMra055111 [DOI] [PubMed] [Google Scholar]
- 4.Hlavin ML, Kaminski HJ, Ross JS et al. . Spinal epidural abscess: a ten year perspective. Neurosurgery 1990;27:177–84. 10.1227/00006123-199008000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Lanfermann H, Heindel W, Gierenz M et al. . [The MR tomographic diagnosis of intra and paraspinal abscesses]. Rofo 1996;165:36–42. 10.1055/s-2007-1015711 [DOI] [PubMed] [Google Scholar]
- 6.Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. Q J Med 2008;101:1–12. 10.1093/qjmed/hcm100 [DOI] [PubMed] [Google Scholar]
- 7.Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med 1948;239:845–54. 10.1056/NEJM194812022392301 [DOI] [PubMed] [Google Scholar]
- 8.Darouiche RO, Hamill RJ, Greenberg SB et al. . Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine [Baltimore] 1992;71:369–85. 10.1097/00005792-199211000-00004 [DOI] [PubMed] [Google Scholar]
- 9.Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Ortho Clin North Am 1996;27:87–94. [PubMed] [Google Scholar]
- 10.MacKenzie AR, Laing RBS, Smith CC et al. . Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurosurg Psychiatry 1998,85:209–12. 10.1136/jnnp.65.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586–97. 10.7326/0003-4819-137-7-200210010-00010 [DOI] [PubMed] [Google Scholar]
- 12.Tung GA, Yim JW, Mermel LA et al. . Spinal epidural abscess: correlation between MRI findings and outcome. Neuroradiology 1999;41:904–9. 10.1007/s002340050865 [DOI] [PubMed] [Google Scholar]
- 13.Khanna RK, Malik GM, Rock GM et al. . Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery 1996;39:958–64. [DOI] [PubMed] [Google Scholar]
- 14.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med 1970;282:198–206. 10.1056/NEJM197001222820406 [DOI] [PubMed] [Google Scholar]
- 15.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E et al. . Clinical management of Staphylococcus bacteraemia. Lancet Infect Dis 2011;11:208–22. 10.1016/S1473-3099(10)70285-1 [DOI] [PubMed] [Google Scholar]
- 16.Priest DH, Peacock JE Jr. Haematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J 2005;98:854–62. 10.1097/01.smj.0000168666.98129.33 [DOI] [PubMed] [Google Scholar]
- 17.Bernard L, Dinh A, Ghout I et al. . Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic Vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875–82. 10.1016/S0140-6736(14)61233-2 [DOI] [PubMed] [Google Scholar]
- 18.Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br 1987;69:26–35. [DOI] [PubMed] [Google Scholar]
- 19.Eismont FJ, Wiesel SW, Brighton CTA et al. . Antibiotic penetration into rabbit nucleus pulposus. Spine 1987;12:254–6. 10.1097/00007632-198704000-00011 [DOI] [PubMed] [Google Scholar]
- 20.Brodin H. Paths of nutrition in articular cartilage and intervertebral discs. Acta Orthop Scand 1954;24:177–83. 10.3109/17453675408988561 [DOI] [PubMed] [Google Scholar]
- 21.Souderi GJ, Greenberg SS, Banovac K et al. . Penetration of glycopeptide antibiotics in nucleus pulposus. Spine 1993;18:2039–42. 10.1097/00007632-199310001-00019 [DOI] [PubMed] [Google Scholar]
- 22.Mader JT, Landon GC, Calhoun J. Antimicrobial treatment of osteomyelitis. Clin Orthop 1993;295:87. [PubMed] [Google Scholar]
- 23.Tai CC, Want S, Quraishi NA et al. . The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br 1987;69:784. [DOI] [PubMed] [Google Scholar]
- 24.Vaverka M, Petrzelova J. Penetration of antibiotics into the intervertebral disk. Acta Chir Orthop Traumatol Cech 1991;58:98–103. [PubMed] [Google Scholar]
- 25.Gee T, Ellis R, Marshall G, et al Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother 2001;45:1843–6. 10.1128/AAC.45.6.1843-1846.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown EM, Pople IK, De Louvois J et al. . Spine update. Prevention of postoperative infection in patients undergoing spinal surgery. Spine 2004;29:938–45. 10.1097/00007632-200404150-00023 [DOI] [PubMed] [Google Scholar]
- 27.Joint Formulary Committee. British national formulary. 53rd edn London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2007. [Google Scholar]
- 28.Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis 2009:48(Suppl 4):S254–9. 10.1086/598186 [DOI] [PubMed] [Google Scholar]
- 29.Leder K, Turnidge JD, Korman TM et al. . The clinical efficacy of continuous-infusion flucloxacillin in serious staphylococcal sepsis. J Antimicrob Chemother 1999;43:113–18. 10.1093/jac/43.1.113 [DOI] [PubMed] [Google Scholar]
- 30.Mehtar S, Drabu Y, Wilson AP et al. . A comparative study between teicoplanin alone and flucloxacillin, plus or minus fusidic acid, in the treatment of serious infections caused by methicillin-susceptible gram-positive bacteria. Chemotherapy 1995;41:412–19. 10.1159/000239374 [DOI] [PubMed] [Google Scholar]
- 31.Gibson MJ, Karpinski MR, Slack RC et al. . The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br 1987;69:784–6. [DOI] [PubMed] [Google Scholar]
- 32.Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005;56:923–9. 10.1093/jac/dki355 [DOI] [PubMed] [Google Scholar]
- 33.Melzer M, Goldsmith D, Gransden W. Successful treatment of vertebral osteomyelitis with linezolid in a patient receiving hemodialysis and with persistent methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus bacteremias. Clin Infect Dis 2000;31:208–9. 10.1086/313897 [DOI] [PubMed] [Google Scholar]
- 34.Falagas ME, Siempos II, Vardakas KZ. Linezolid versus glycopeptide or beta-lactam for treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis 2008;8:53–66. 10.1016/S1473-3099(07)70312-2 [DOI] [PubMed] [Google Scholar]
- 35.Beibei L, Yun C, Mengli C et al. . Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2010;35:3–12. 10.1016/j.ijantimicag.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 36.Fu J, Ye K, Chen C et al. . The efficacy and safety of linezolid and glycopeptides in the treatment of Staphylococcus infections. PLoS ONE 2013;8:e58240 10.1371/journal.pone.0058240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paz JF, Alvarez FJ, Roda JM. Spinal epidural abscess caused by Brucella: case report. J Neurosurg Sci 1994;38:245–9. [PubMed] [Google Scholar]
- 38.Dandy WE. Abscesses and tumours in the spinal epidural space. Arch Surg 1926;13:477–94. 10.1001/archsurg.1926.01130100021002 [DOI] [Google Scholar]
- 39.Park KH, Cho OH, Jung M et al. . Clinical characteristics and outcomes of haematogenous vertebral osteomyelitis caused by gram negative bacteria. J Infect 2014;69:42 10.1016/j.jinf.2014.02.009 [DOI] [PubMed] [Google Scholar]


