Abstract
Using a DNA microarray, we determined changes in enterohemorrhagic Escherichia coli O157:H7 gene expression during binding to plasma membranes. Analysis of the complete transcriptomes of the bound bacteria revealed increased levels of stress-associated mRNAs and decreased levels of mRNA encoding proteins involved in translation and type III secretion.
Enterohemorrhagic Escherichia coli (EHEC) is an important cause of acute gastroenteritis in humans (26). EHEC causes a wide spectrum of illnesses ranging from mild diarrhea to severe diseases, such as hemorrhagic colitis and hemolytic-uremic syndrome; hemolytic-uremic syndrome is the leading cause of acute renal failure in children and is associated with the production of potent Shiga toxins (Stx) (26). Strains of EHEC belonging to serogroup O157 are most commonly associated with severe human disease.
Adhesion of EHEC to intestinal epithelial cell results in induction of a histopathological feature known as attaching and effacing lesions (8), which are characterized by localized destruction of brush border microvilli and intimate attachment of the bacteria to actin-rich pedestal-like structures that are formed on the apical membrane underneath attached bacteria. The capacity to form attaching and effacing lesions is encoded on a pathogenicity island termed the locus of enterocyte effacement (LEE) (21). The LEE can be divided into three functional regions (6); the 5′ end consists of three operons (LEE1 to LEE3) encoding a positive regulator, Ler (23), and the main structural components of the bacterial type III secretion system (TTSS). The central part of the LEE harbors an operon (LEE5) encoding the outer membrane adhesin intimin (15) and the translocated intimin receptor (Tir) (17). The 3′ end of the LEE (LEE4) encodes additional TTSS structural proteins, translocators, and effectors.
TTSSs are commonly found in pathogenic, gram-negative bacteria (13) and are used to transfer effector proteins directly into eukaryotic cells, where the normal cellular functions are subverted for the benefit of the pathogen. Both the organization and the composition of TTSSs are broadly conserved in different bacteria, and TTSSs contain homologues of many flagellar components (1). However, the TTSS of EHEC is unique in that, in addition to the conserved cell wall-associated needle complex, it contains a filamentous extension to the needle formed by one of the secreted proteins, EspA (20, 27). The three-dimensional structure of the EspA filament shows that it consists of a helical tube with an outer diameter of ca. 120 Å containing a hollow central channel that is ca. 25 Å in diameter and has a continuous wall, which is likely to contain the proteins within the channel during transfer (2). Evidence suggests that EspA is the major component of this filamentous structure, while EspD, in complex with a second translocator protein, EspB, is predicted to form a translocation pore in the host cell membrane that facilitates subsequent entry of effector proteins (Tir, EspF, Map, EspG, EspH, Cif, and EspI/NleA) (5, 9, 17, 18, 22, 25, 36).
Recent reports showed that EHEC can induce hemolysis of red blood cells (RBCs) (31), that EspA filaments are responsible for binding of EHEC to the RBC membrane (27, 31), and that binding is followed by protein translocation, as Tir (32) and EspD (31) were both detected in the plasma membrane.
The genomic sequences of two clinical EHEC O157 isolates, EDL933 (28) and Sakai (10), were recently determined. The aim of this study was to use a whole-genome microarray to screen for alterations in EHEC O157:H7 strain RIMD 0509952 (= Sakai; Stx− Kanr) gene expression at a time when bacteria are attached to the RBC plasma membranes and the TTSS is actively engaged in protein translocation. The Sakai strain was isolated from the Sakai city outbreak in Japan in 1996 (10, 37) and was kindly provided by Chihiro Sasakawa, University of Tokyo, Tokyo, Japan. The wild-type strain possesses a double knockout; a kanamycin cassette was inserted into the SmaI site in the stx2A gene, and a 0.6-kb BsiWI fragment, which contained the stx1A gene and the upstream region, was deleted.
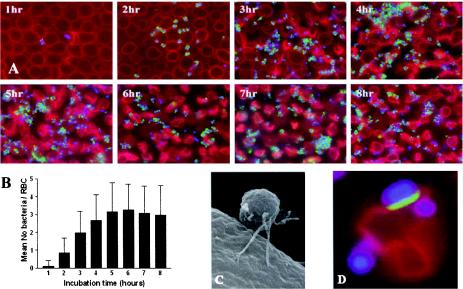
In order to determine the time postinfection at which to perform the DNA microarray analysis, we quantified the level of EHEC adhesion over time. Monolayers of rabbit RBCs (Harlan serum lab) were prepared, infected with EHEC, fixed, and stained with Tir or EspA antisera as previously described (31, 32). Fluorescence images were taken at hourly intervals up to 8 h postinoculation, random squares were selected, and the number of adherent bacteria on 100 RBCs was counted within each square. The average number of bacteria adhering to a single RBC was then determined.
Small numbers of adhering bacteria were detected at 1 h postinoculation (Fig. 1A and B). By 2 h postinfection, the mean number of EHEC adhering to a single RBC was determined to be 0.8. The mean number increased to 2 bacteria per RBC at 3 h postinfection, peaked at ∼3 bacteria per RBC after 5 to 6 h, and then declined somewhat at 7 and 8 h postinfection (Fig. 1A and B). At the peak (5 to 6 h), bacteria were bound to the RBCs via EspA filaments (Fig. 1C), and Tir was translocated to the RBC membrane (Fig. 1D). Based on these results, the transcriptome of RBC-associated EHEC was determined 5 h postinoculation.
FIG. 1.
Binding of EHEC Sakai to RBCs. Immunofluorescent staining (A) and counting of attached bacteria (B) showed that there was increased adhesion from 1 to 5 or 6 h postinoculation but the level of binding decreased after this. EHEC adhered to and translocated Tir (D) (arrow) into the membrane of RBCs via EspA filaments, as visualized by immunofluorescence (A) and scanning electron microscopy (C).
For DNA microarray experiments, overnight cultures were subcultured at a 1:100 dilution in HEPES-buffered Dulbecco's modified Eagle's medium (DMEM) and grown at 37°C for 5 h; the pH of the medium at the end of the incubation was 6.9. Following incubation, 2 volumes of RNA Protect bacterial reagent (QIAGEN) was added to each culture, and the bacteria were sedimented by centrifugation. The supernatant was removed, and the pellet was stored at −80°C for subsequent purification of RNA.
In parallel, overnight cultures were diluted 1:25 in HEPES-buffered DMEM, added to RBC monolayers, and incubated for 5 h at 37°C; the pH of the medium at the end of the incubation was 7.1 Nonattached bacteria were removed, and the infected monolayers were washed three times with phosphate-buffered saline. To isolate adherent bacteria, 1 ml of phosphate-buffered saline and 2 ml of RNA Protect bacterial reagent were added to the monolayers, and then the cells were scraped and sedimented by centrifugation, the supernatant was removed, and the pellet was kept at −80°C for RNA purification.
Bacterial total RNA was extracted with an RNeasy kit (QIAGEN) by following the manufacturer's instructions. DNase treatment was applied to all samples (QIAGEN). The purified RNA was stored at −20°C. Quantities of RNA were determined by measuring absorbance at 260 and 280 nm (A260/A280), and integrity was verified by visualization on 1% agarose gels.
An MWG E. coli O157 array (MWG-Biotech AG) was used to examine differential expression of EHEC O157:H7 strain Sakai following infection of the RBC monolayer. The array was composed of 5,318 50-mer oligonucleotides specific for EHEC strain Sakai. Oligonucleotides were spotted on Pan Epoxy slides (72 by 22 mm; MWG-Biotech AG) by using a robotic spotting device equipped with stainless steel printing tips (GENEMECHINES OMMIGRID). Following printing, slides were kept in an incubator at 42°C and 50% humidity for 8 h, washed with 0.2% sodium dodecyl sulfate (SDS) for 2 min, and then washed three times with double-distilled H2O for 1 min with shaking at room temperature. The slides were then incubated for 20 min in double-distilled H2O at 50°C, dried with compressed air, and stored in the dark at room temperature.
cDNA probes were generated by standard cDNA synthesis. One microliter of random primers (3 μg/μl; Invitrogen) was added to 5 μg of RNA to obtain a final volume of 10 μl (adjusted with RNase-free H2O [Ambion]). The samples were incubated for 5 min at 70°C, and the reaction contents were collected by brief centrifugation, followed by incubation on ice. The RNA was reverse transcribed with 4 μl of SuperScript II strand synthesis buffer (Invitrogen), 2 μl of 0.1 M dithiothreitol, 2 μl of either Cy3-deoxynucleoside triphosphate (bacteria attached to RBCs) or Cy5-deoxynucleoside triphosphate (DMEM-grown EHEC) (Amersham Pharmacia Biotech), 0.4 μl of a dexoyribosylthymine-nucleoside triphosphate mixture (25 mM deoxyribosyladenine, 25 mM deoxyribosylcytosine, 25 mM deoxyribosylguanine, 10 mM dexoyribosylthymine), and 1.5 μl of SuperScript II (Invitrogen). The samples were incubated for 2 h at 42°C. The reaction was stopped by adding 1 μl of 20 mM EDTA, followed by incubation for 5 min at 70°C. Sixty microliters of RNase-free H2O was added to the Cy3 reaction mixture, which was combined with the Cy5 reaction mixture, and 10 μl of 3 M sodium acetate (pH 5.0) was added.
The fluorescent probe was purified with a QIAquick PCR purification kit (QIAGEN) by following the manufacturer's instructions. The probe was eluted twice with 60 μl of Tris-EDTA buffer, 2 μl of a 5-μg/μl solution of the oligonucleotide poly(A) was added, and the probe was dried in a Speed-Vac. Spotted slides were incubated in prehybridization solution (6× SSC, 0.5% SDS, and 1% bovine serum albumin [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) for 1.5 h at 42°C. The slides were washed extensively with H2O and dried with compressed air. The labeled cDNA probe was resuspended in 20 μl of hybridization buffer (50% deionized formamide, 6× SSC, 0.5% SDS, and 5× Denhardt's reagent consisting of 0.5 g of Ficoll, 0.5 g of polyvinylpyrrolidone, and 0.5 g of bovine serum albumin), denatured for 2 min at 95°C, and cooled at room temperature for 2 min before it was applied to dust-free coverslips (BDH), which were precut in order to fit to the array surface. The microarray slides (with the spots facing down) were placed over the coverslips, transferred into a hybridization chamber containing 3× SSC, and incubated for 16 h at 42°C. The slides were washed with 0.1× SSC-0.1% SDS for 15 min (with one solution change after 10 min) and then with 0.1× SSC for 5 min with gentle agitation at room temperature. The microarrays were subsequently dried with compressed air and read by using a GenePix 4000B dual-channel confocal laser scanner (Axon Instruments), which scanned the arrays twice to obtain images of both Cy3 and Cy5 signals.
The GeneSpring software package from Silicon Genetics (Redwood City, Calif.) was used for analysis of microarray data, which were normalized by using a per-spot and per-chip intensity-dependent (Lowess) normalization method. The cross-gene error model was applied and based on sample replicates. To ensure reliability, seven replicates from three independent RNA preparations were used. Additionally, genes were spotted as duplicates, generating 14 independent spots for each gene. Only genes with at least three good replicates (spots that were flagged as present) were selected for statistical analysis by the t test (GeneSpring). Only genes with a P value of ≤0.05 were analyzed further. Genes showing a twofold or greater difference in the expression ratio between the two conditions were classified as regulated genes (up- or down-regulated twofold or more). Genes showing expression ratios between 0.5- and 2-fold were classified as unchanged.
Expression profiles.
In general, expression of 404 genes was different in RBC-associated EHEC and DMEM-grown EHEC; the expression of 299 genes was lower and the expression of 105 genes was higher in the RBC-associated bacteria (Table 1) (also see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). Of the 404 proteins, 88 were hypothetical proteins with unknown functions. Interestingly, no changes in mRNA levels of fimbrial or flagellar genes were detected. Similar results were obtained when the transcriptome of RBC-associated EHEC was compared with that of bacteria that did not attach to the RBCs and were collected from the supernatant (data not shown), suggesting that the differences in expression were specifically due to EHEC-RBC binding.
TABLE 1.
Ratios of the mRNAs for LEE genes and the mRNAs for the transcriptional activators of the LEE genes in DMEM-grown and RBC-associated EHEC
| Ecs no. | Gene | Description or function | Relative mRNA expressiona |
|---|---|---|---|
| LEE genes | |||
| ecs4550 | espF | Effector protein | 16.19 |
| ecs4551 | orf29 | Unknown function | 5.62 |
| ecs4552 | escF | Type III secretion needle protein | 136.86 |
| ecs4553 | cesD2 | Chaperone for EspD | 30.11 |
| ecs4554 | espB | Translocator protein | 23.21 |
| ecs4555 | espD | Translocator protein | 20.82 |
| ecs4556 | espA | Translocator protein | 26.89 |
| ecs4557 | sepL | TTSS | 19.68 |
| ecs4559 | eae | Intimin adherence protein | 69.02 |
| ecs4560 | cesT | Chaperone for Tir | 39.96 |
| ecs4561 | tir | Translocated intimin receptor | 7.91 |
| ecs4562 | map | Effector protein | 29.58 |
| ecs4563 | cesF | Chaperone for EspF | 6.23 |
| ecs4564 | espH | Effector protein | 5.97 |
| ecs4565 | sepQ | TTSS | 8.77 |
| ecs4566 | orf16 | Unknown function | 3.08 |
| ecs4569 | escV | TTSS | 10.32 |
| ecs4570 | orf12 | Unknown function | 6.75 |
| ecs4571 | sepZ | Effector protein | 28.22 |
| ecs4572 | rorf8 | Unknown function | 10.37 |
| ecs4573 | escJ | TTSS | 12.15 |
| ecs4574 | sepD | TTSS | 14.16 |
| ecs4575 | escC | Secretin (TTSS) | 13.02 |
| ecs4576 | cesD | Chaperone for EspD | 22.87 |
| ecs4577 | grlA | Global regulator of LEE activator | 13.07 |
| ecs4578 | grlR | Global regulator of LEE repressor | 4.22 |
| ecs4580 | escUb | TTSS | 2.5 |
| ecs4581 | escT | TTSS | 2.33 |
| ecs4582 | escSb | TTSS | 2.8 |
| ecs4583 | escR | TTSS | 5.13 |
| ecs4584 | orf5 | Unknown function | 3.68 |
| ecs4585 | orf4 | Unknown function | 5.29 |
| ecs4586 | cesAB | Chaperone for EspA and EspB | 7.21 |
| ecs4587 | orf2 | Unknown function | 3.64 |
| ecs4588 | ler | Transcriptional regulator | 5.62 |
| Transcriptional regulators of LEE genes | |||
| ecs4378 | yhiF | 0.40 | |
| ecs4392 | yhiE | 0.31 | |
| ecs2654 | sdiA | 0.49 | |
| ecs4396 | yhiX | 0.31 |
Ratio of mRNA expression in DMEM-grown EHEC to mRNA expression in attached EHEC.
P > 0.05.
Stress responses.
Binding of EHEC to RBC was associated with changes in expression of a number of genes associated with metabolism and stress. Alternative sigma factors control gene expression in response to environmental stress. The rpoE and rseB mRNA levels, but not the rseA or rseC mRNA levels, were greater in RBC-associated EHEC (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). RpoE controls the cell envelope stress response and is transcribed when misfolded or unfolded proteins accumulate in the periplasm or outer membrane or when cells enter the stationary growth phase. ResB is a regulator of RpoE, and the higher level of expression in attached bacteria is therefore consistent with the elevated rpoE mRNA level. However, the levels of mRNA for other RpoE-controled genes (e.g., htrA) were not statistically different in the two conditions. In Salmonella RpoE is essential for survival in vivo, and expression of the RpoE regulon is strongly induced upon entry into the stationary phase and in the intracellular environment (14). The RpoE regulon is not activated in adherent EHEC, and therefore the physiological significance of the elevated rpoE mRNA levels is not yet clear. However, unidentified members of the RpoE regulon, which constantly grow (29), might be involved.
While rpoE is induced under stress conditions in the membrane and periplasm, groES and groEL are expressed in response to a variety of stress conditions in the cytoplasm (12). Expression of groES and groEL is controlled by RpoH. Although no difference in the level of rpoH mRNA was recorded, elevated levels of groES and groEL mRNA, as well as sugE mRNA (sugE is located four open reading frames downstream of groEL), a suppressor of groEL, were found in RBC-associated EHEC (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). Moreover, the mRNA levels for heat shock chaperone proteins DnaK and DnaJ (12) were also elevated in the RBC-bound bacteria, although the P value was not as significant (P = 0.15). The mechanism controlling expression of these chaperones under the experimental conditions tested has not been defined yet.
EHEC normally encounters plasma membranes of eukaryotic cells only during infection. It is therefore possible that contact with eukaryotic plasma membranes induces membrane stress responses (i.e., activation of rpoE and groEL/groES) in EHEC in order to alert the bacteria to possible stress conditions in vivo and to induce specific changes in gene expression that would be beneficial in this environment. A good example that supports this hypothesis is the fact that the level of sodC mRNA, encoding a periplasmic CuZn superoxide dismutase which protects gram-negative bacteria from exogenous oxidative damage, was higher in attached bacteria (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm) even though RBCs are not a source of superoxide. In E. coli expression of sodC was reported to be RpoS dependent but independent of Fur or an iron supplement. Interestingly, the sodC mRNA level was also elevated in intracellular Salmonella enterica serovar Typhimurium (7).
Transport.
Expression of outer membrane proteins plays an important role in adaptation to new environments as these proteins control the permeability of the membrane. Indeed, E. coli responds to misfolded or unfolded outer membrane proteins by inducing transcription of stress genes in the cytoplasm. Interestingly, the mRNA levels of both ompC and ompF were lower in attached bacteria (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). Expression of ompC/ompF is regulated by the two-component regulatory system OmpR/EnvZ, but the levels of their mRNAs in attached EHEC were unchanged. The mechanism responsible for the reduced levels of both porins following attachment is not known. Of note is the fact that the levels of mRNAs encoding proteins involved in different transport pathways were different in attached EHEC and DMEM-grown EHEC (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). The significance of this is not clear, although down-regulation of some transport systems might increase resistance to antimicrobial peptides, bile salts, etc., once EHEC infection is established in the intestine. Importantly, the level of mRNA for the feoAB operon, encoding an iron transport system, was lower in RBC-attached EHEC (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm), possibly because of the direct acquisition of iron from the RBCs.
mRNAs for genes involved in translation and secretion.
With the exception of rpsV encoding the 30S ribosomal subunit protein S22 (also known as stationary-phase-induced ribosome-associated protein [SRA]), the levels of the majority of mRNAs encoding ribosomal proteins were lower in the attached bacteria. Transcription of rpsV was reported to increase after entry into the stationary phase and to be partially dependent on the alternative sigma factor RpoS. The levels of rpoS mRNA were not statistically different between RBC-associated EHEC and DMEM-grown EHEC, indicating that binding to the plasma membrane does not signal entry into the stationary phase. However, these results suggest that there is a reduction in de novo protein synthesis in attached bacteria. This hypothesis is supported by the fact that the level of tig mRNA, encoding the ribosome-bound trigger factor chaperone that maintains newly translated proteins in an open conformation (3), is also reduced in attached bacteria. Interestingly, no changes in the mRNA encoding ribosomal protein were detected in a recent screening designed to determine changes in Salmonella gene expression during intracellular bacterial growth (7).
In E. coli, inner membrane proteins are usually delivered through the Sec translocon, which can target proteins into either the periplasm or the lipid bilayer of the inner membrane (4). Lateral transport is mediated by the inner membrane protein YidC (30). Importantly, although there were no differences in the mRNA levels for secE, secG, and secY (encoding the core of the Sec translocon), as well as secA (encoding the ATPase), between RBC-associated bacteria and DMEM-grown bacteria, the mRNA level for yidC was reduced 4.5-fold in attached EHEC (data were derived from only two replicates). The biological significance of this observation is not yet known.
Nitrogen regulon.
An unexpected result was the remarkable decrease in mRNA levels for genes related to nitrogen metabolism, especially those regulated by the NtrC (glnG)/Nac(nac) regulon (see supplemental material at http://www.imperial.ac.uk/cmmi/research/frankel1.htm). The level of glnG mRNA was fivefold lower in attached EHEC, and the level of nac mRNA was 26-fold lower, although transcription activated by NtrC and Nac is dependent on σ54 and σ70, respectively (both of which remained unchanged). Importantly, recent mRNA expression profiles of E. coli ingested by human neutrophils showed that an identical set of genes within the Ntr regulon was suppressed (34). At present, there is no satisfactory explanation for this pattern of expression profiles; however, the fact that it was recorded in experimental systems involving extracellular and intracellular environments increases the likelihood that this response is biologically significant.
mRNA expression profiles of the LEE genes.
Of major interest was the expression profiles of mRNAs for the LEE genes (Table 1). In general, the levels of expression of most of the LEE mRNAs were significantly lower in RBC-associated bacteria, which is consistent with the observed down-regulation of Salmonella SP1 genes intracellularly (7). In particular, the levels of mRNAs encoding the translocator proteins EspA, EspB, and EspD were over 20-fold lower and the level of mRNA encoding intimin was 69-fold lower, which is consistent with observations that were previously reported showing that 6 h after infection of epithelial cells in vitro both EspA filaments and intimin disappeared from the bacterial cell surface (19, 20). It is therefore likely that in the same way that Salmonella might not need SP1 gene products after cell invasion, EHEC does not need LEE gene products following protein translocation and intimate attachment and expression of both gene clusters is down-regulated.
In contrast to the almost uniform lower abundance of LEE mRNAs in RBC-associated EHEC, the levels of mRNAs for four genes (espG, rorf3, orf15, and escN, encoding the effector EspG, two proteins with unknown functions, and the TTSS ATPase, respectively) were unchanged, although only the data for orf15 were also statistically significant. These results suggest that although many of the LEE genes are transcribed as long polycistronic operons, the long mRNA transcripts are processed or differentially protected from degradation.
Confirmation of the microarray results by RT-PCR.
The finding that there was variability in the mRNA levels for LEE2 genes was unexpected. In order to obtain independent confirmation of this result, we employed reverse transcription (RT)-PCR. PCR primers were designed for mRNAs of nine genes (espG, cesAB, escS, escC, escN, espH, map, cesT, and espA) scattered in the LEE region. RT-PCR was performed by using the same RNA batch that was used for the DNA microarrays. The RT and PCR steps were performed by using Omniscript (QIAGEN) and HotStartTaq (QIAGEN) kits, respectively, as recommended by the manufacturer. The specific primer pairs and the sizes of PCR products are shown in Table 2. To ensure that no DNA contamination occurred, PCRs were also performed without RT. O157:H7 strain Sakai genomic DNA was used as a positive control. The intensities of the RT-PCR products were analyzed by using the imageJ software (www.rsb.info.nih.gov/ij).
TABLE 2.
Primers used for RT-PCR analysis
| Gene | Sense primer | Antisense primer | Size of PCR product (bp) |
|---|---|---|---|
| espG | 5′CATTTCAAGAGTTGTTAACCG | 5′TCCACAACTTCAGCTTTCTG | ∼600 |
| cesA | 5′ATGAGTATTGTGAGCCAAAC | 5′TTCTATTCCGTTGATTCATTG | ∼300 |
| escS | 5′ATGGATACTGGATATTTTGTTC | 5′TTAGCCGTTCACCTTCGG | ∼250 |
| escC | 5′GATGTTGTTAAGGTCTTTAAAC | 5′GAATATCAAGTAACAAACGAAC | ∼700 |
| escN | 5′CTTGTATGCTGAACCACCAG | 5′CAAGTTCTCGGGTAAGTACG | ∼640 |
| espH | 5′ATGTCGTTATCAGGAGCGG | 5′TAAGAGGAAGCTCTTGTTGC | ∼460 |
| map | 5′ATGTTTAGTCCAATGACAA | 5′TCGCATTCTGAGTAATCGC | ∼500 |
| cesT | 5′TCATCAAGATCTGAACTTTTAT | 5′TTATCTTCCGGCGTAATAATG | ∼470 |
| espA | 5′ATGGATACATCAAATGCAACATC | 5′GTAGAGATTGCACATCAGAAC | ∼470 |
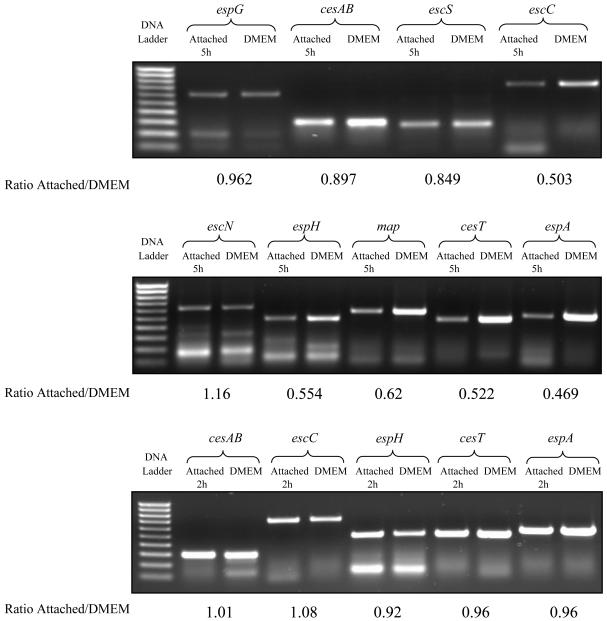
In accordance with the array data, no change in the level was noted for espG mRNA (ratio of RBC-associated mRNA to DMEM-grown mRNA, 0.96) and escN mRNA (ratio of RBC-associated mRNA to DMEM-grown mRNA, 1.16). In contrast, the levels of all other mRNAs were lower in RBC-associated EHEC than in DMEM-grown EHEC, and the ratios ranged from 0.90 to 0.47 (Fig. 2). This experiment validated the DNA microarray analysis and supported the hypothesis that the polycistronic type III mRNAs are not degraded at the same rate. The biological significance of our finding that the mRNA levels for the TTSS ATPase, orf15, and espG were unchanged is still not clear, although the data may suggest that EspG is translocated at a later stage of the infection.
FIG. 2.
The two gels at the top show the results of an RT-PCR analysis of the relative mRNA abundance in bacteria attached to RBCs 5 h postinoculation compared to the mRNA abundance in EHEC grown for 5 h in DMEM. RT-PCR was performed by using nine specific primers for selected EHEC genes. The results confirm the data obtained by DNA microarray analysis because in most cases higher levels of mRNA were found in DMEM-grown EHEC; the exceptions were the levels of mRNAs of escN and espG, which were similar under the two conditions. The ratios of RBC-associated EHEC to DMEM-grown EHEC are shown. The gel at the bottom shows the results for an RT-PCR analysis of relative mRNA abundance in RBC-bound EHEC at 2 h postinfection compared to the mRNA abundance in EHEC grown for 5 h in DMEM. cesAB, ecsC, espH, espA, and cesT represent the major LEE operons, LEE1 to LEE5. No difference in mRNA abundance was observed between the two conditions, and the ratios of RBC-associated EHEC to DMEM-grown EHEC are shown.
Dynamics of down-regulation of LEE mRNA.
In order to determine the levels of LEE mRNAs in adherent bacteria at earlier times postinfection, EHEC that was grown for 2 h in DMEM was used to inoculate RBC monolayers. Total RNA was extracted from RBC-bound EHEC at 2 h postinfection and from EHEC grown in DMEM for 5 h. Two micrograms of the RNA was then used for RT-PCR; cesAB, ecsC, espH, espA, and cesT were selected to represent operons LEE1 to LEE5, respectively. The RT-PCR was repeated three times, and a representative gel is shown in Fig. 2. Analysis of the PCR products of the three repeats by densitometry revealed no difference in the mRNA levels between DMEM-grown EHEC and RBC-associated EHEC. Subjecting the measurements for the five genes to a Student t test revealed that there was a significant difference between the levels of mRNAs in RBC-associated EHEC at 2 and 5 h after infection of RBC (P < 0.01). These results show that down-regulation of LEE genes occurs at later times, and this observation is consistent with previous data showing that both intimin and EspA filaments were present on the bacterial cell surface 2 and 3 h after infection of epithelial cells in culture but were eliminated by 6 h (19, 20).
Regulators.
yhiE and yhiF were recently reported to be negative regulators of LEE4 gene expression, and null function mutations in these genes resulted in enhanced binding of EHEC to Caco-2 cells (35). Moreover, overexpression of yhiX (gadX) reduced the production of virulence proteins in enteropathogenic E. coli (33). Consistent with this, the levels of yhiE, yhiF, and yhiX mRNAs were higher in RBC-associated EHEC than in DMEM-grown EHEC (Table 1). These transcriptional regulators influence expression of genes implicated in the maintenance of pH homeostasis (11). Therefore, yhiE (gadE) in EHEC (35; this study), like gadX in enteropathogenic E. coli (33), may regulate expression of genes required for acid resistance and virulence. SdiA was also reported to have a negative effect on LEE gene expression (16). Consistent with this, the level of sdiA mRNA was higher in RBC-associated EHEC. The level of mRNA for the gene encoding a two-component sensor protein, phoQ, which plays a major role in controlling virulence determinants in Salmonella (24), was also higher in adherent bacteria, but the biological significance of this observation in EHEC is not known yet.
Acknowledgments
We thank Chihiro Sasakawa, University of Tokyo, for the Stx-negative Sakai strain.
This project was supported by the European Union Fifth Framework Quality of Life Program (grant QLK2-2000-00600), by a DEFRA Senior Fellowship in Veterinary Microbiology, and by the Wellcome Trust.
Editor: J. B. Bliska
REFERENCES
- 1.Aizawa, S. I. 2001. Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett. 202:157-164. [DOI] [PubMed] [Google Scholar]
- 2.Daniell, S. J., E. Kocsis, E. Morris, S. Knutton, F. P. Booy, and G. Frankel. 2003. 3D structure of EspA filaments from enteropathogenic Escherichia coli. Mol. Microbiol. 49:301-308. [DOI] [PubMed] [Google Scholar]
- 3.Deuerling, E., A. Schulze-Specking, T. Tomoyasu, A. Mogk, and B. Bukau. 1999. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400:693-696. [DOI] [PubMed] [Google Scholar]
- 4.Driessen, A. J., E. H. Manting, and C. van der Does. 2001. The structural basis of protein targeting and translocation in bacteria. Nat. Struct. Biol. 8:492-498. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 8.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 9.Gruenheid, S., I. Sekirov, N. A. Thomas, W. Deng, P. O'Donnell, D. Goode, Y. Li, F. A. Frey, N. F. Brown, P. Metalnikov, T. Pawson, K. Ashman, and B. B. Finlay. 2004. Identification and characterization of NleA, a non-LEE encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51:1233-1249. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 28:11-22. [DOI] [PubMed] [Google Scholar]
- 11.Hommais, F., E. Krin, J. Y. Coppee, C. Lacroix, E. Yeramian, A. Danchin, and P. Bertin. 2004. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150:61-72. [DOI] [PubMed] [Google Scholar]
- 12.Houry, W. A. 2001. Chaperone-assisted protein folding in the cell cytoplasm. Curr. Protein Pept. Sci. 2:227-244. [DOI] [PubMed] [Google Scholar]
- 13.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys, S., A. Stevenson, A. Bacon, A. B. Weinhardt, and M. Roberts. 1999. The alternative sigma factor, σE, is critically important for the virulence of Salmonella typhimurium. Infect. Immun. 67:1560-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanamaru, K., K. Kanamaru, I. Tatsuno, T. Tobe, and C. Sasakawa. 2000. SdiA, an Escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 38:805-816. [DOI] [PubMed] [Google Scholar]
- 17.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 19.Knutton, S., J. Adu-Bobie, C. Bain, A. D. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 65:1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara, B. P., and M. S. Donnenberg. 1998. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett. 166:71-78. [DOI] [PubMed] [Google Scholar]
- 23.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 24.Miller, S. I., A. M., Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mundy, M., L. Petrovska, K. Smollett, N. Simpson, R. K. Wilson, J. Yu, X. Tu, I. Rosenshine, S. Clare, G. Dougan, and G. Frankel. 2004. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and the roles of this and other secreted proteins in infection. Infect. Immun. 72:2288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves, B. C., R. K. Shaw, G. Frankel, and S. Knutton 2003. Polymorphisms within EspA filaments of enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 71:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perna, N. T., G, Plunkett, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 29.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Scotti, P. A., M. L. Urbanus, J. Brunner, J.-W. L. de Gier, G. von Heijne, C. van der Does, A. J. M. Driessen, B. Oudega, and J. Luirink. 2000. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 19:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw, R. K., S. Daniell, F. Ebel, G. Frankel, and S. Knutton. 2001. EspA filament-mediated protein translocation into red blood cells. Cell. Microbiol. 3:213-222. [DOI] [PubMed] [Google Scholar]
- 32.Shaw, R. K., S. Daniell, G. Frankel, and S. Knutton. 2002. Enteropathogenic Escherichia coli translocate Tir and form an intimin-Tir intimate attachment to red blood cell membranes. Microbiology 148:1355-1365. [DOI] [PubMed] [Google Scholar]
- 33.Shin, S., M. P. Castanie-Cornet, J. W. Foster, J. A. Crawford, C. Brinkley, and J. B. Kaper. 2001. An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator, Per. Mol. Microbiol. 41:1133-1150. [DOI] [PubMed] [Google Scholar]
- 34.Staudinger, B. J., M. A. Oberdoerster, P. J. Lewis, and H. Rosen. 2002. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J. Clin. Investig. 110:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsuno, I., K. Nagano, K. Taguchi, L. Rong, H. Mori, and C. Sasakawa. 2003. Increased adherence to Caco-2 cells caused by disruption of the yhiE and yhiF genes in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 71:2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu, X., I. Nisan, C. Yona, E. Hanski, and I. Rosenshine. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595-606. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, H., A. Wada, Y. Inagaki, K. Ito, and K. Tamura. 1996. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotypes strains in Japan. Lancet 348:831-832. [DOI] [PubMed] [Google Scholar]