Abstract
Wartime toxin exposures have been implicated in the genesis of malignancy in war veterans. Agent Orange, one toxin among many, has been linked to malignancy and the subcomponent phenoxyacetic acid has been associated with soft tissue sarcomas (STSs). This case demonstrates the association between a wartime toxin exposure (Agent Orange) and subsequent cancer development. Ultimately, we aim to highlight the importance of simple, specific questions in the patient history to account for previous wartime toxin exposures.
Background
At the time of the Vietnam War, a task force was assembled by the US military to develop an easily deployable chemical that would deprive the Viet Cong of crops and vegetation cover. Operation Ranch Hand led to the deposition (via helicopter, boats and trucks) of 72 million litres of chemicals across the forested and rural areas of Vietnam for this sole purpose.1 2 The bulk of these chemicals was Agent Orange, a mixture of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a common contaminant of 2,4,5-T.1 TCDD is a toxic chemical with a reported carcinogenic potential. Approximately 45 million kg of Agent Orange and other herbicides were subsequently deposited in Vietnam without knowledge of the potential health and environmental consequences.1 3
Case presentation
A 69-year-old man presented to our clinic with a 1-year history of a painful, enlarging mass of the right lateral thigh. His pain was exacerbated by movement and there were no alleviating factors. He had a history of hypertension and hypercholesterolaemia and had no past surgical history. Family history included a father with basal cell carcinoma of the nose. Of note, the patient reported a history of exposure to Agent Orange while serving in the Vietnam War 50 years prior. From 1963 to 1965, for several hours at a time, he was in frequent contact with forested areas that were recently sprayed with Agent Orange. On examination, there was right thigh fullness and a mass palpable within the muscle. The overlying skin was normal and there was no palpable lymphadenopathy. Laboratory workup was within normal limits. Initial workup with an ultrasound revealed a heterogeneous mass that was subsequently confirmed on imaging (figure 1). CT with contrast of the right lower extremity revealed a 5.7×3.1×7.0 cm hypodense region of the vastus intermedius with multiple septations (figure 2). MRI of the right lower extremity revealed a significantly T2-hyperintense soft tissue mass juxtaposed to the anterior cortex of the right distal femur shaft measuring 5.4×2.9×6.7 cm (figure 3). This was located within the vastus intermedius muscle and there was no cortical elevation suspicious for bone involvement. CT scan of the chest revealed no evidence of metastatic disease within the lungs. Ultrasound-guided core biopsy of the right thigh mass revealed a myxofibrosarcoma. Management strategies were thoroughly discussed with the patient and he decided to undergo radical excision of the sarcoma with adjuvant radiotherapy. Histological examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient ultimately asked: “was it related to Agent Orange?”
Figure 1.
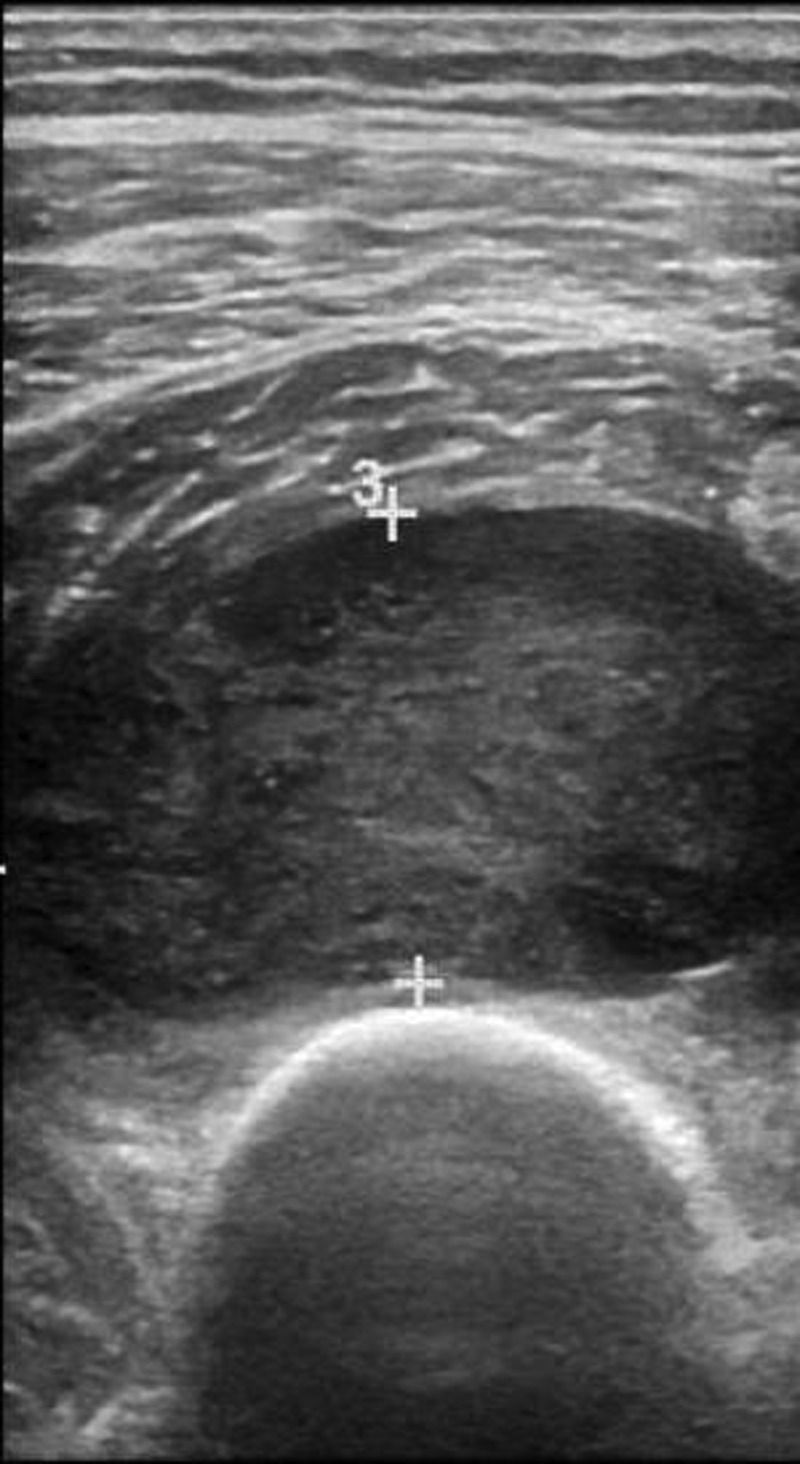
Ultrasound of the right midthigh: A hypoechoic, heterogeneous 4×2.5 cm mass.
Figure 2.
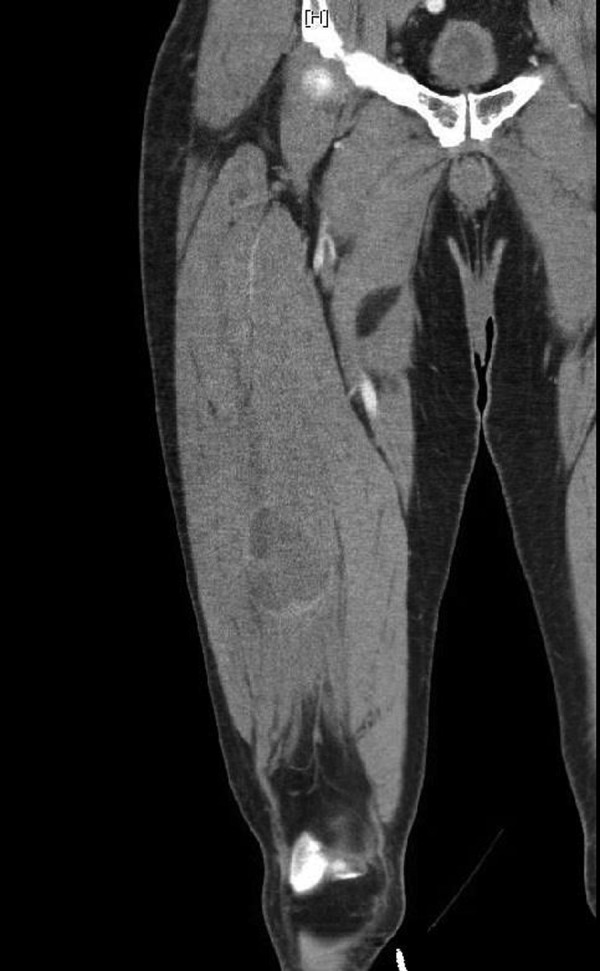
CT with contrast of the right lower extremity: a 5.7×3.1×7.0 cm hypodense region of the vastus intermedius with multiple septations.
Figure 3.
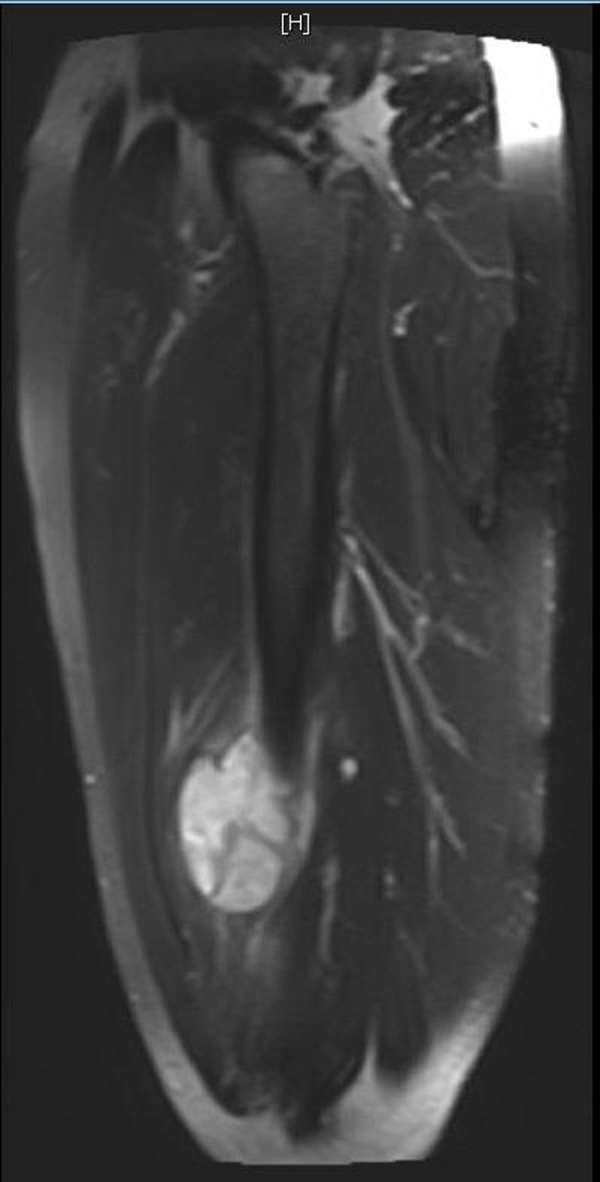
MRI of the right lower extremity: a significantly T2-hyperintense soft tissue mass juxtaposed to the anterior cortex of the right distal femur shaft measuring 5.4×2.9×6.7 cm.
Outcome and follow-up
The patient was educated about his diagnosis and was counselled about the unfortunate side effects with which Agent Orange has been associated. He continues to be monitored for disease recurrence and will continue to do so for years.
Discussion
TCDD can persist in the environment for decades. Fifteen years after spraying ceased, numerous studies revealed that dioxin levels had accumulated in higher amounts in the adipose tissue of individuals from Vietnam than in those from other countries.1 2 Particular areas of accumulation of Agent Orange include the liver and adipose tissue; therefore resulting in a slow metabolism and an estimated half-life of 11.3 years.1 3 Additionally, TCDD has been shown to cause indirect DNA damage through alterations of particular molecular compounds.2 Many reports have also shown that TCDD impacts lipid metabolism and have attributed hyperlipidaemia as a potential consequence of Agent Orange exposure.4 The pathogenesis of malignancy, however, is less clear.
The role of Agent Orange in sarcomagenesis is a topic of controversy and debate. This association remains unproven due to conflicting case-control studies and because large-scale clinical trials are not feasible. However, TCDD has been recognised as a carcinogen by some organisations, including a working group at the International Agency for Research on Cancer in Lyon, France.3 The carcinogenicity of TCDD has been established in many animal species to cause both common and rare tumours at various locations.1 TCDD has also been shown to suppress both innate and acquired immunity in humans. This is particularly interesting since the absence of Natural killer cells is associated with an increased risk of malignancy due to decreased immune surveillance.
When soldiers returned home from Vietnam in the 1970s, they began to report numerous pathologies over the years that were often labelled as stress induced. The USA Department of Veterans Affairs (VA) currently recognises certain cancers and health problems to be associated with past exposure to TCDD: AL amyloidosis, B-cell leukaemia's, chloracne, type II diabetes mellitus, Hodgkin's and non-Hodgkin's lymphoma, ischaemic heart disease, Parkinson's disease, early onset peripheral neuropathy, porphyria cutanea tarda, prostate cancer, respiratory cancers and soft tissue sarcoma (STS).2 4 5 Also, a recent cohort study of 479 Operation Rand Hand veterans found that this group had a significantly increased risk of monoclonal gammopathy of undetermined significance (MGUS), supporting an association between Agent Orange and multiple myeloma.6
The initial studies that attributed the link between Agent Orange and malignancy were case-control studies from Sweden.7 These reported an increased risk of STS in Swedish lumberjacks exposed to phenoxyacetic herbicides.7 8 These findings were supported by other studies in the 1980s associating exposure to phenoxyacetic acids and STS.9–13 The same group from Sweden performed a meta-analysis of their four case-control studies and consistently found an association between phenoxyacetic acids and STS.14 15 Subsequent case-control studies years later demonstrated no significant association between STS and Agent Orange exposure in Vietnam veterans.16–18 However, in one study subgroups of Vietnam veterans who had higher estimated exposure to Agent Orange appeared to be at greater risk for STS without statistical significance.18 Other studies demonstrated a slightly increased relative risk of STS for participants exposed to phenoxyherbicides and also described a link between thoracic STS and exposure to Agent Orange.1 3 19–23
In our patient, the link between pleomorphic liposarcoma and a wartime toxin exposure is possible. Pleomorphic liposarcoma occurs in ∼5% of liposarcomas and is therefore the rarest subtype of liposarcoma.24 It is a high-grade sarcoma with a high rate of local recurrence (28–34%) and metastasis (32–44%).25 26 There has been no well-established precipitating factor for liposarcomas. However, clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses, especially in patients with a previous military background. This should prompt the search for a possible toxin exposure. Our patient had a persistent and enlarging soft tissue mass over the course of 1 year that ultimately underwent a biopsy after he volunteered the information of a previous wartime toxin exposure. If a question was initially asked regarding wartime toxin exposures, the possible association between toxins and malignancy may have prompted an increasingly swift workup. This is particularly important in this case due to the high risk of metastasis and poor prognosis.
Agent Orange is one of many wartime toxin exposures that may lead to adverse outcomes decades later. When soldiers returned home from the Gulf War in 1991, veterans sought treatment for various medical problems, including nausea, headaches, dizziness, fatigue, muscle pain, memory loss and rashes.27 It is suspected that this ‘Gulf War Syndrome’ was attributed to exposure to pesticides, gases from burning oil wells, biological or chemical weapons or the antinerve gas drug pyridostigmine bromide.27 During the Iraq–Iran war in the 1980s, soldiers and civilians were exposed to sulfur mustard gas, a known carcinogen.28 Additionally, whether soldiers during the most recent war in Iraq were exposed to any toxins is undetermined and will be known with time.
We believe that the conduction of a careful history and implementation of simple questions, “Have you ever been in the military or had any previous wartime toxin exposure?”, will uncover causal relationships between risk factors and disease that may have been previously overlooked.
We recognise that further research is necessary to understand the risk factors, pathogenesis and link between Agent Orange and STS—hence inquiring about the possibility of previous wartime toxin exposures may go a long way in prompting an increasingly swift workup.
Learning points.
Wartime toxin exposures have been implicated in the genesis of various pathologies including malignancy in war veterans.
The conduction of a careful history and implementation of simple questions such as, “Have you ever been in the military or had any previous wartime toxin exposure?”, may help provide crucial details in diagnosing a malignancy or other associated risk factors that may have been previously overlooked.
Clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses, especially in patients with a previous military background. This should prompt further evaluation, including imaging and biopsy.
While these days we have access to a vast amount of diagnostic tests and imaging, one cannot underestimate the importance of understanding a patient's past history.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kramárová E, Kogevinas M, Anh CT et al. Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam. Environ Health Perspect 1998;106(Suppl 2):671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens MW, Kochuba AL, Carter ME et al. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg 2014;133:432–7. 10.1097/01.prs.0000436859.40151.cf [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri A, Harris MD. ‘Proximal-type’ epithelioid sarcoma: is Agent Orange still at large? Ann R Coll Surg Engl 2003;85:410–12. 10.1308/003588403322520816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi SW, Ohrr H, Hong JS et al. Agent orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health 2013;46:213–25. 10.3961/jpmph.2013.46.5.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang ET, Boffetta P, Adami HO et al. A critical review of the epidemiology of Agent Orange or 2,3,7,8-tetrachlorodibenzo-p-dioxin and lymphoid malignancies. Ann Epidemiol 2015;25:275–92. 10.1016/j.annepidem.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Shim YK, Michalek J et al. Agent orange exposure and monoclonal gammopathy of undetermined significance: an operation ranch hand veteran cohort study. JAMA Oncol 2015;1:1061–8. 10.1001/jamaoncol.2015.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardell L, Eriksson M, Axelson O. Agent Orange in war medicine: an aftermath myth. Int J Health Serv 1988;28:715–24. [DOI] [PubMed] [Google Scholar]
- 8.Sarma PR, Jacobs J. Thoracic soft-tissue sarcoma in Vietnam veterans exposed to Agent Orange. N Engl J Med 1982;306:1109. [DOI] [PubMed] [Google Scholar]
- 9.Honchar PA, Halperin WE. 2,4,5- T, trichlorophenol, and soft tissue sarcoma. Lancet 1981;1:268–9. [DOI] [PubMed] [Google Scholar]
- 10.Cook RR. Dioxin, chloracne, and soft tissue sarcoma. Lancet 1981;1(8220 Pt 1):618–19. [DOI] [PubMed] [Google Scholar]
- 11.Moses M, Selikoff IJ. Soft tissue sarcomas, phenoxy herbicides, and chlorinated phenols. Lancet 1981;1:1370. [DOI] [PubMed] [Google Scholar]
- 12.Johnson FE, Kugler MA, Brown SM. Soft tissue sarcomas and chlorinated phenols. Lancet 1981;2:40. [DOI] [PubMed] [Google Scholar]
- 13.Kogan MD, Clapp RW. Soft tissue sarcoma mortality among Vietnam veterans in Massachusetts, 1972 to 1983. Int J Epidemiol 1988;17:39–43. [DOI] [PubMed] [Google Scholar]
- 14.Hardell L, Eriksson M, Degerman A. Metaanalysis of 4 Swedish case-control studies on exposure to pesticides as a risk-factor for soft-tissue sarcoma including the relation to tumor-localization and histopathological type. Int J Oncol 1995;6:847–51. [DOI] [PubMed] [Google Scholar]
- 15.Hardell L. Pesticides, soft-tissue sarcoma and non-Hodgkin lymphoma—historical aspects on the precautionary principle in cancer prevention. Acta Oncol 2008;47:347–54. 10.1080/02841860701753697 [DOI] [PubMed] [Google Scholar]
- 16.Greenwald P, Kovasznay B, Collins DN et al. Sarcomas of soft tissues after Vietnam service. J Natl Cancer Inst 1984;73:1107–9. [PubMed] [Google Scholar]
- 17.The association of selected cancers with service in the US military in Vietnam. II. Soft-tissue and other sarcomas. The Selected Cancers Cooperative Study Group. Arch Intern Med 1990;150:2485–92. [PubMed] [Google Scholar]
- 18.Kang H, Enzinger FM, Breslin P et al. Soft tissue sarcoma and military service in Vietnam: a case-control study. J Natl Cancer Inst 1987;79:693–9. [PubMed] [Google Scholar]
- 19.Fingerhut MA, Halperin WE, Marlow DA et al. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med 1991;324:212–18. 10.1056/NEJM199101243240402 [DOI] [PubMed] [Google Scholar]
- 20.Wingren G, Fredrikson M, Brage HN et al. Soft tissue sarcoma and occupational exposures. Cancer 1990;66:806–11. [DOI] [PubMed] [Google Scholar]
- 21.Kogevinas M, Becher H, Benn T et al. Cancer Mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. Am J Epidemiol 1997;145:1061–75. [DOI] [PubMed] [Google Scholar]
- 22.Hooiveld M, Heederik DJ, Kogevinas M et al. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am J Epidemiol 1998;147:891–901. [DOI] [PubMed] [Google Scholar]
- 23.Miligi L, Costantini AS, Veraldi A et al. Cancer and pesticides: an overview and some results of the Italian multicenter case-control study on hematolymphopoietic malignancies. Ann N Y Acad Sci 2006;1076:366–77. 10.1196/annals.1371.036 [DOI] [PubMed] [Google Scholar]
- 24.Ghadimi MP, Liu P, Peng T et al. Pleomorphic liposarcoma: clinical observations and molecular variables. Cancer 2011;117:5359–69. 10.1002/cncr.26195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downes KA, Goldblum JR, Montgomery EA et al. Pleomorphic liposarcoma: a clinicopathologic analysis of 19 cases. Mod Pathol 2001;14:179–84. 10.1038/modpathol.3880280 [DOI] [PubMed] [Google Scholar]
- 26.Hornick JL, Bosenberg MW, Mentzel T et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol 2004;28:1257–67. [DOI] [PubMed] [Google Scholar]
- 27.Brower V. Gulf War syndrome revisited. EMBO Rep 2003;4:551–3. 10.1038/sj.embor.embor874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razavi SM, Abdollahi M, Salamati P. Cancer events after acute or chronic exposure to sulfur mustard: a review of the literature. Int J Prev Med 2016;7:76 10.4103/2008-7802.182733 [DOI] [PMC free article] [PubMed] [Google Scholar]


