Abstract
We studied the expression of a subset of chemokines, including RANTES/CCL5, MIP-1α/CCL3, IP-10/CXCL10, and MCP-1/CCL2, in Toll-like receptor (TLR)-competent and -deficient mice after infection with Leishmania major. Chemokine expression at the site of infection (the footpad), in the draining lymph nodes and in the spleens of infected animals was determined by using two different methods of analysis. The results indicate that L. major infection causes overall upregulation of RANTES/CCL5, MIP-1α/CCL3, IP-10/CXCL10, and MCP-1/CCL2 in the footpads and lymph nodes, while expression of these chemokines is constitutive in the spleens of TLR4-competent mice (C57BL/10ScSn) and TLR4-deficient mice (C57BL10/ScN). Different patterns of expression were detected depending on the time postinfection, but there was little variation in the expression of these four chemokines in the presence or absence of TLR4.
The leishmaniases are caused by more than 20 Leishmania species that are transmitted to humans by the bite of an infected female sand fly (17). These parasites can cause a heterogeneous group of clinical manifestations, ranging from cutaneous to visceral forms of disease. To date, there is no vaccine against leishmaniases in routine use anywhere in the world, although several vaccine preparations are in advanced stages of testing (17; http://www.who.int/tdr/diseases/leish/).
In humans, the cutaneous leishmaniases can develop into self-healing localized cutaneous leishmaniasis (LCL) or progressive diffuse cutaneous leishmaniasis (DCL). These forms of disease differ in their cytokine profile and in the composition of the cellular infiltrate at the site of infection. Lesions from the LCL form are associated with a T helper 1 (Th1) response, and lesions from the DCL form are associated with a Th2 response (28, 38, 40). Differences in chemokine profiles are also observed (38, 40). In skin biopsies of patients with LCL, high levels of MCP-1/CCL2 and IP-10/CXCL10 are found together with moderate levels of MIP-1α/CCL3, associated with infiltration of macrophages into the lesions and stimulation of leishmanicidal activity. In contrast, MIP-1α/CCL3 expression predominates in lesions of patients suffering from chronic DCL, in which the concentration of MCP-1/CCL2 is much lower (38, 39). Therefore, the correlation of opposing expression patterns of chemokines in these forms of disease is a strong indication that these effector molecules play an important role during the immune response to Leishmania. Furthermore, many studies have described chemokine expression patterns in mouse models of experimental leishmaniasis (21, 29, 30, 32, 41, 44, 45). For instance, lymph nodes draining the site of Leishmania major infection in both resistant C57BL/6 and susceptible BALB/c mice display significant levels of IP-10/CXCL10, MCP-1/CCL2, RANTES/CCL5, MIP-1α/CCL3, and lymphotactin/XCL expression shortly after infection (46, 47). Summarizing these and other studies, the expression of MCP-1/CCL2, MIP-1α/CCL3, RANTES/CCL5, and IP-10/CXCL10 is clearly relevant in leishmaniasis, although their role in regulation of host immunity following infection is still not clear. In addition, many of these chemokine studies have focused on the early phase of the immune response, omitting the later stages of infection.
Studies on Toll-like receptor (TLR) signaling pathways have emphasized the role of chemokines in linking innate and adaptive immunity (1). Activation of TLRs by various microbial products triggers the expression and subsequent production of specific chemokines (12, 13, 26), which are also induced by agonists of both TLR2 and TLR4 in macrophages and dendritic cells (37). For example, IP-10/CXCL10 (which is also associated with a Th1 response) is preferentially induced by TLR4 agonists (lipopolysaccharide), while interleukin-8 (IL-8)/CXCL8 is preferentially induced by TLR2 agonists (zymosan and peptidoglycan) (37). A growing number of studies have shown TLR involvement in immunity to protozoan parasites (5, 6, 34). TLR2 expression on human NK cells is upregulated and stimulated by purified L. major lipophosphoglycan (2), while the involvement of MyD88 signaling in L. major infection has been shown in vitro and in vivo (8, 9, 10, 18, 33).
We recently described the specific contribution of TLR4 to the control of parasite growth in both innate and adaptive immune responses following L. major infection (23, 24). In our studies, a comparison of infections in C57BL10/ScN mice (which carry a null mutation in TLR4) (35, 36) with those in TLR4-competent (C57BL/10ScSn) mice demonstrated that parasite loads are significantly higher in the TLR4-deficient mice during infection. These data correlate with early induction of nitric oxide synthase in TLR4-competent mice, whereas increased parasite survival in host cells from TLR4-deficient mice correlates with higher activity of arginase (23). To extend these studies and determine whether TLR4 activation influences the expression of chemokines after infection with L. major, we examined here the expression of chemokines during the early and late phases of the immune response. We analyzed the chemokine profile at the site of parasite entry (footpad), at the site of initiation of antigen-specific immune responses (the lymph node draining the lesion), and at a distant site, the spleen. Our findings indicate that both TLR4-competent and -deficient mice infected with L. major stationary-phase promastigotes show organ-specific induction of RANTES/CCL5, MIP-1α/CCL3, IP-10/CXCL10, and MCP-1/CCL2 but that there is little variation in the expression of these four chemokines in the presence or absence of TLR4.
MATERIALS AND METHODS
Animals.
C57BL/10ScSn (wild-type) and C57BL/10ScN (TLR40/0) mice were bred under specific-pathogen-free conditions in the animal facilities at the Max-Planck-Institut für Immunbiologie, Freiburg, Germany. C57BL/10ScN mice have a homozygous deletion of 74,723 bp at the tlr4 locus, removing all three exons (35, 36). Six- to 10-week-old animals of both sexes were used in this study.
Parasites and infection.
L. major LV39 (MRHO/SU/59/P strain) was maintained in a virulent state by monthly passage in mice (22). For infections, 2 × 106 stationary-phase parasites were injected subcutaneously into the hind footpad.
Quantitative PCR assay.
Total RNA was extracted from footpads, lymph nodes, or spleens with Tri-Reagent (Sigma-Aldrich, St. Louis, Mo.) at the indicated time points after infection, according to the manufacturer's protocol. After DNase I (Ambion) treatment, 2 μl of total RNA from each sample was used as template for cDNA synthesis. Reverse transcription reactions were performed with the Omniscript reverse transcriptase kit (Qiagen).
Quantification of chemokine transcripts (MCP-1, RANTES, IP-10, and MIP-1α) in tissue samples was carried out by real-time PCR, using the ABI PRISM 7700 system (Applied Biosystems) with the DNA binding SYBR Green I dye for the detection of PCR products. The hypoxanthine phosphoribosyl transferase gene was used as a reference. The forward and reverse specific primer sequences used for RANTES/CCL5, MIP-1α/CCL3, IP-10/CXCL10, and MCP-1/CCL2 are described elsewhere (19, 27). DNA standards were prepared from products amplified from infected organs. PCR products were isolated by using the QIAquick kit (Qiagen). The amount of extracted DNA was quantified by spectrophotometry and expressed as molecule number (copy number). A serial dilution was used to generate each standard curve. For real-time quantitative PCR, each reaction contained 1× SYBR Green master mix (Quantitect SYBR Green PCR kit; Qiagen), a 0.3 μM concentration of each primer, and 2 μl of cDNA in a final volume of 25 μl. The reaction conditions were as follows: 95°C for 15 min and then 40 cycles of three 94°C for 15 s, 60°C for 20 s, and 72°C for 20 s. Emitted fluorescence for each reaction was measured during the annealing-extension phase, and amplification plots were analyzed by using the ABI PRISM 7700 sequence detection system (software version 1.7). Product specificity was determined by melting curve analysis with the ABI PRISM dissociation curve software. Quantity values (given as copy number) for gene expression were generated (in triplicate) by comparison of the fluorescence generated by each sample with standard curves of known quantities. The results are expressed as ratios of chemokine transcripts to hypoxanthine phosphoribosyl transferase transcripts.
RPA.
RNase protection assays (RPAs) were performed as described previously (14, 31, 43). Briefly, total RNA was extracted as described above. Chemokine RNA levels were analyzed by using the RiboQuant multiprobe RNase protection assay system (PharMingen, San Diego, Calif.) according to the manufacturer's protocol, with the custom-made template set specific for Lymphotactin/XCL1, RANTES/CCL5, MIP-1β/CCL4, MIP-1α/CCL3, IP-10/CXCL10, MCP-1/CCL2, TCA-3/CCL1, and eotaxin/CCL11. Internal standards for RNA quantity (GAPDH [glyceraldehyde-3-phosphate dehydrogenase] and L32) and quality (yeast tRNA) were included for each assay, as were samples from uninfected control mice. Transcript levels were quantified by autoradiography and densitometric scanning of autoradiograms with a BAS 1500 phosphorimager and TINA 2.10g software (Fuji Photo Film Co., Tokyo, Japan). RNA loading was estimated by measuring the intensities of protected fragments of the housekeeping GAPDH gene. Undetectable levels were defined as equivalent to the background of the assay. The level of expression for chemokine mRNA is given as a percentage of expression of the housekeeping GAPDH gene.
RESULTS
To examine the patterns of chemokine expression in TLR4-competent and -deficient mice during L. major infection, we analyzed the chemokine expression profiles at the site of parasite entry (footpad), at the site of initiation of antigen-specific immune responses (the lymph node draining the lesion), and at a distant site, the spleen. Wild-type and TLR4-deficient mice were infected subcutaneously in one hind footpad with 2 × 106 stationary-phase L. major promastigotes, and expression of the chemokines RANTES/CCL5, MIP-1α/CCL3, IP-10/CXCL10, and MCP-1/CCL2, was determined by two detection methods, RPA and real-time PCR.
Chemokine expression at the site of parasite entry.
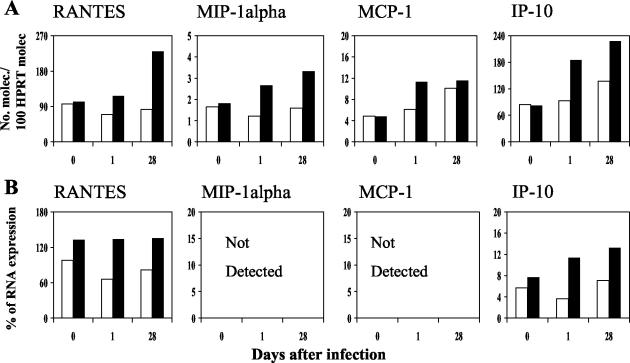
The profiles of chemokine expression at the local site of parasite inoculation (the footpad) of TLR4-competent and -deficient mice are shown in Fig. 1. During the early phase of infection (after 1 day), expression of the chemokine genes for MIP-1α/CCL3 and MCP-1/CCL2 was clearly upregulated in both groups of mice. IP-10/CXCL10 and RANTES/CCL5 were also detected, but at lower levels of expression (Fig. 1). In the later phase of L. major infection (28 days), RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2, and IP-10/CXCL10 were strongly upregulated in the footpads of both control and TLR4-deficient mice, compared to the early phase (Fig. 1). MCP-1/CCL2 and IP-10/CXCL10 gene expression was slightly higher in the absence of TLR4 (Fig. 1). These data were reproducible in two independent experiments, and the profiles detected by real-time PCR and RPA were similar. Cumulatively, these results show an upregulation of chemokines at early and late stages after infection in both TLR4-competent and -deficient mice, with no significant differences between the two strains of mice.
FIG. 1.
Chemokine gene expression in the footpads of mice in the early and late phases of infection with L. major. Real-time PCR (A) and RPA analysis (B) of total RNA isolated from footpads of TLR-competent (white bars) and -deficient (black bars) mice at 1 and 28 days following infection with L. major were performed as described in Materials and Methods. The results shown are from a single experiment and are representative of those from two independent experiments.
Chemokine expression in the draining lymph nodes.
Since TLR activation has been implicated in the signaling pathways involved in the induction of chemokine synthesis, we investigated whether differences in the induction of chemokines could be detected in the lymph nodes draining the lesions of TLR4-competent and -deficient mice.
Following infection, chemokine gene expression for RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2, and IP-10/CXCL10 in the lymph nodes of both groups of mice was measured (Fig. 2). The results show that the patterns of chemokine gene expression in the lymph nodes draining the lesions of TLR4-competent and -deficient mice were strikingly different from the expression profiles seen in the footpad and spleen tissue (described below). A pronounced increase in gene expression of IP-10/CXCL10 (∼7-fold increase) and MCP-1/CCL2 (∼5-fold increase) was detected 1 day after infection (Fig. 2), with the levels of expression of these chemokines being similar in the presence or absence of TLR4. However, these chemokine expression levels had changed considerably by the late phase of infection (day 28): IP-10/CXCL10 expression had returned to constitutive levels, whereas MCP-1/CCL2 expression remained similar to that detected at 1 day postinfection in both groups (Fig. 2). While RANTES/CCL5 expression was similar in the lymph nodes of both strains of mice during the course of infection, there was a downregulation of expression at 24 h in the draining lymph node, with a return to baseline levels by 28 days postinfection (Fig. 2). This was the only chemokine showing a downregulation of transcription early after infection. MIP-1α/CCL3 expression in the lymph node was not altered compared to that in noninfected groups at the time points evaluated (Fig. 2). Real-time PCR analysis showed low constitutive expression levels early and late after infection for both groups, whereas MIP-1α/CCL3 was barely detected in the wild-type group by RPA (Fig. 2). These differences can be attributed to the limits of sensitivity of RPA. No differences between groups were detected. Our results show different chemokine expression profiles in the lymph nodes and the footpad. The analysis suggests that, for the chemokines tested here, production of IP-10/CXCL10 is most significant early after infection, while MCP-1/CCL2 is most significant at a later time point. Expression of RANTES/CCL5 and MIP-1α/CCL3 is maintained at the same levels as in the noninfected controls, an observation consistent with the use of CCR5 as a common receptor for these two chemokines.
FIG. 2.
Chemokine gene expression in the lymph nodes of mice in the early and late phases of infection with L. major. Real-time PCR (A) and RPA analysis (B) of total RNA isolated from draining lymph nodes of TLR-competent (white bars) and -deficient (black bars) mice at 1 and 28 days following infection with L. major were performed as described in Materials and Methods. The results shown are from a single experiment and are representative of those from two independent experiments.
Chemokine expression in the spleen.
Expression of RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2, and IP-10/CXCL10 in the spleen was measured by both analytical methods (Fig. 3). MIP-1α/CCL3 and MCP-1/CCL2 were not detected by RPA and were detected only at low (constitutive) levels by real-time PCR (Fig. 3). In these experiments (Fig. 3A), infection with L. major resulted in slight upregulation of IP-10/CXCL10, MCP-1/CCL2, and MIP-1α/CCL3 after 1 and 28 days in the TLR4-deficient mice but not in the TLR4-competent control mice. The expression of RANTES was not altered 1 day after infection but was upregulated in TLR4-deficient mice after 28 days. In TLR4-competent and -deficient mice infected with L. major, the production of RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2, and IP-10/CXCL10 in the spleen, a distant organ from the site of infection, appeared to be unaffected by infection.
FIG. 3.
Chemokine gene expression in the spleens of mice in the early and late phases of infection with L. major. Real-time PCR (A) and RPA analysis (B) of total RNA isolated from spleens of TLR-competent (white bars) and -deficient (black bars) mice at 1 and 28 days after infection with L. major were performed as described in Materials and Methods. The results shown are from a single experiment and are representative of those from two independent experiments.
The chemokines constitutively expressed in the spleens of naive mice that were detected by RPA were RANTES/CCL5 and IP-10/CXCL10, while the levels of MIP-1α/CCL3 and MCP-1/CCL2 mRNA were below the level of RPA detection. (Fig. 3B).
DISCUSSION
In the present study, we investigated the chemokine expression profile at the site of parasite entry (footpad), at the site of initiation of antigen-specific immune responses (the lymph node draining the lesion), and at a distant site, the spleen, during the early and late phases following infection with L. major. Our data show that L. major selectively activates expression of four chemokine genes (RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2 and IP-10/CXCL10) in TLR4-competent and -deficient mice, with distinct organ-specific differences. Different patterns of chemokine expression are observed at the site of infection and the draining lymph node, at early and late stages of infection. However, differences between wild-type and TLR4-deficient mice are not detected. In the footpads, RANTES/CCL5, MIP-1α/CCL3, MCP-1/CCL2, and IP-10/CXCL10 are induced at the early time point, and expression is more pronounced in the late phase of infection. However, the patterns of chemokine expression in the lymph node have different characteristics. IP-10/CXCL10 is mostly upregulated during the innate phase, while RANTES/CCL5 is downregulated at this early time point, returning to constitutive levels late in the infection. MIP-1α/CCL3 expression does not change at the times of infection evaluated. MCP-1/CCL2 transcription levels appear to be upregulated throughout infection, correlating with MCP-1 protein detection by enzyme-linked immunosorbent assay (23). The expression profiles of these chemokines in the spleen do not indicate alterations to the constitutive pattern of expression at the time points postinfection studied.
We used two different methods to determine chemokine expression patterns in L. major-infected mouse tissues, RPA and real-time PCR. The major advantage of RPA is the simultaneous detection of at least six different mRNA species, using commercially available multiprobe template sets in a single experimental setting. However, detection of very low mRNA levels is not always reproducible (see, e.g., reference 21). Our data show that the low level of expression of MIP-1α/CCL3 could be quantified reliably only by real-time PCR (Fig. 3). A second limitation of RPA is the effect of polymorphism in the target genes (16, 25). Given these potential problems, we used real-time PCR (20) to compare and confirm the results obtained by RPA in this study. Most of our results obtained with both techniques were similar, although the sensitivity of detection is clearly higher with real-time PCR (Fig. 3A). In the type of analysis carried out here, we believe that real-time PCR is the method of choice, given its sensitivity with small amounts of template and its reproducibility.
It is well established that the outcome of experimental infection with L. major is strongly influenced by the genetic background of mice. Infection induces a pronounced Th1 response in resistant strains of mice such as C57BL/6 and a Th2 response in susceptible strains of mice (BALB/c), leading to cure or uncontrolled progressive disease, respectively (42). The chemokine expression profile during infection is also dependent on the genetic background (4). Expression of MCP-1/CCL2, IP-10/CXCL10, and lymphotactin/XCL1 is upregulated 1 day after infection in the draining lymph nodes of resistant C57BL/6 mice but not in those of susceptible BALB/c mice (46). Our experiments with TLR4-competent and -deficient mice on a genetically resistant background show that the lack of TLR4 alone does not have a direct effect on chemokine expression at the time points postinfection evaluated. To date, studies on the involvement of TLRs in Leishmania infection have focused on MyD88 knockout mice with a genetically resistant background (C57BL/6) (2, 8, 9, 10, 18, 33). While MyD88 is a key mediator of signal transduction from all TLRs, it is also involved in the signaling pathways of IL-1R, T1/ST2, and IL-18. We have recently shown that TLR4-deficient mice on a genetically resistant background show an increased permissiveness for parasite growth during both the innate and adaptive phases of the immune response and a significant delay in healing of lesions, compared to TLR4-competent mice (23, 24). Although induction of nitric oxide synthase in TLR4-competent mice correlates with increased parasite survival and higher arginase activity in cells from TLR4-deficient mice, it is important to note that a shift from a Th1 to a Th2 response is not observed in the TLR4-deficient mice (23, 24). This could explain why the chemokine expression levels in L. major-infected TLR4-competent and TLR4-deficient mice are similar.
Induction of chemokine expression is one of the earliest known responses to Leishmania infection and potentially provides a signal for the initiation of in vivo downstream immunological responses such as cytokine induction and chemotaxis or activation of different subsets of cells. Following infection with L. major, the chemokines MIP-2 and KC (the functional murine homologues of IL-8) are very rapidly produced in the skin, underscoring the predominant role of CC chemokines in early neutrophil accumulation (30). Besides chemokine gene expression at the site of infection, the production of chemotactic factors by the parasites themselves also influences the site-directed migration and activation of cells of the innate immune response. Leishmania promastigotes can release a granulocyte chemotactic factor and inhibit IP-10/CXCL10 production by neutrophil granulocytes (45). Importantly, the transcription and expression of the NK cell-activating chemokine IP-10/CXCL10 correlates with migration of NK cells 24 h after infection in resistant mice (30, 46). It has been suggested that the roles of NK cells, gamma interferon secretion, and cytotoxic activity during the innate phase of Leishmania infection are associated with the concomitant chemokine expression and function of IP-10/CXCL10, RANTES/CCL5, and lymphotactin/XCL1 (46). Recently, it has been shown that lipophosphoglycan is recognized by TLR2 expressed on human NK cells and that this results in NK cell activation (2). In this study, we observed no changes in IP-10/CXCL10 and RANTES/CCL5 expression during the innate phase of Leishmania infection in the footpad, suggesting that the cytotoxic activity of NK cells probably is not altered in TLR4-competent or -deficient mice. However, a higher cell recruitment (Mac1+ cells) was observed in the TLR4-deficient mice (24).
The contribution of chemokines in the adaptive phase following Leishmania infection has also been extensively documented. In this context, RANTES/CCL5, MIP-1β/CCL4, MIP-1α/CCL3, and lymphotactin/XCL1 are reported to have a role not only as chemotactic factors but also as coactivators of macrophages; they can function together with gamma interferon as Th1 cytokines (11). This “functional unit” could be used by NK cells in the innate phase and by CD8+ T cells and CD4+ Th1 cells in the adaptive phase of the immune defense, thus bridging the two components of a Th1 immune reaction. In another study, the induction of RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4, MIP-2, and MCP-1/CCL2 has been observed in the footpads of resistant mice infected with L. major or Leishmania amazonensis, at early and late phases of infection (21). Upregulation of these chemokines was maximal at 2 weeks following infection with L. major, but production of the same chemokines was delayed in L. amazonensis infection (21). In experimental visceral leishmaniasis, Leishmania donovani infection induced a rapid hepatic accumulation of mRNAs encoding MCP-1/CCL2, IP-10/CXCL10, and MIP-1α/CCL3, in both BALB/c mice and immunodeficient scid mice (7). The role of MCP-1/CCL2 in Leishmania infection is a more controversial and complex issue. MCP-1/CCL2 is induced after infection with L. major (46) and has been shown to stimulate parasite killing by human monocytes (39). Genetically resistant CCR2-deficient mice (MCP-1/CCL2 receptor) become susceptible to infection with L. major, possibly due to a defect in Langerhans cell migration to the draining lymph node (44). On the other hand, MCP-1/CCL2 has been shown to inhibit IL-12 production and may be involved in the development of polarized Th2 responses (3, 15). Here, we demonstrate high expression of MCP-1/CCL2, IP-10/CXCL10, RANTES/CCL5, and MIP-1α/CCL3 in the footpads and high expression of MCP-1/CCL2, RANTES/CCL5, and MIP-1α/CCL3 in the lymph nodes of both groups of mice during the adaptive phase of infection. The expression patterns are not affected directly by the presence or absence of TLR4. To our knowledge, this is the first study showing chemokine expression in the innate and adaptive phases of the immune response to L. major infection in TLR-competent and -deficient strains of mice.
Our current knowledge of the function and significance of TLRs in Leishmania infections is very limited. This study on tissue-specific chemokine expression in TLR4-competent and -deficient mice contributes to our understanding of the role played by chemokines in the development of the inflammatory and immune response to Leishmania infection. It will be interesting to verify which subset of tissue-specific cells is responsible for production of these chemokines during infection.
Acknowledgments
We thank B. Askonas and R. Tewari for critical reading of the manuscript and for helpful discussions and Laurence Bugeon for assistance with real-time PCR.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), The Wellcome Trust (program grant 061343 support to D.F.S.; I.M. is supported by grant 063289), The St Mary's Development Trust, the Deutsche Forschungsgemeinschaft, CNPq-Brasil (proc. 202733/02-5; S.A.), and CAPES (proc. BEX: 0247/03-0; S.A.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Becker, I., N. Salaiza, M. Aguirre, J. Delgado, N. Carrillo-Carrasco, L. G. Kobeh, A. Ruiz, R. Cervantes, A. P. Torres, N. Cabrera, A. Gonzalez, C. Maldonado, and A. Isibasi. 2003. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 130:65-74. [DOI] [PubMed] [Google Scholar]
- 3.Braun, M. C., E. Lahey, and B. L. Kelsall. 2000. Selective suppression of IL-12 production by chemoattractants. J. Immunol. 164:3009-3017. [DOI] [PubMed] [Google Scholar]
- 4.Brenier-Pinchart, M. P., H. Pelloux, D. Derouich-Guergour, and P. Ambroise-Thomas. 2001. Chemokines in host-protozoan-parasite interactions. Trends Parasitol. 17:292-296. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. C., and R. S. Corral. 2002. Stimulation of B lymphocytes, macrophages, and dendritic cells by protozoan DNA. Microbes Infect. 4:969-974. [DOI] [PubMed] [Google Scholar]
- 6.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like-receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 7.Cotterell, S. E., C. R. Engwerda, and P. M. Kaye. 1999. Leishmania donovani infection initiates T cell-independent chemokine responses, which are subsequently amplified in a T cell-dependent manner. Eur. J. Immunol. 29:203-214. [DOI] [PubMed] [Google Scholar]
- 8.Debus, A., J. Glasner, M. Röllinghoff, and A. Gessner. 2003. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 71:7215-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Trez, C., M. Brait, O. Leo, T. Aebischer, F. A. Torrentera, Y. Carlier, and E. Muraille. 2004. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect. Immun. 72:824-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822-2831. [DOI] [PubMed] [Google Scholar]
- 11.Dorner, B. G., A. Scheffold, M. S. Rolph, M. B. Huser, S. H. Kaufmann, A. Radbruch, I. E. Flesch, and R. A. Kroczek. 2002. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc. Natl. Acad. Sci. USA 99:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 13.Fan, J., and A. B. Malik. 2003. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat. Med. 9:315-321. [DOI] [PubMed] [Google Scholar]
- 14.Gilman, M. 1993. Ribonuclease protection assay, p. 4.7.1-4.7.8. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Stuhl (ed.), Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 15.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 16.Hallensleben, W., L. Biro, C. Sauder, J. Hausmann, V. C. Asensio, I. L. Campbell, and P. Staeheli. 2000. A polymorphism in the mouse crg-2/IP-10 gene complicates chemokine gene expression analysis using a commercial ribonuclease protection assay. J. Immunol. Methods 234:149-151. [DOI] [PubMed] [Google Scholar]
- 17.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawn, T. R., A. Ozinsky, D. M. Underhill, F. S. Buckner, S. Akira, and A. Aderem. 2002. Leishmania major activates IL1-α expression in macrophages through a MyD88-dependent pathway. Microbes Infect. 4:763-771. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, F., T. K. Means, and A. D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660-2669. [DOI] [PubMed] [Google Scholar]
- 20.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 21.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kropf, P., K. Brunson, R. Etges, and I. Müller. 1998. Immunology of infection, p. 419-458. Academic Press, San Diego, Calif.
- 23.Kropf, P., M. A. Freudenberg, M. Modolell, H. P. Price, S. Herath, S. Antoniazi, C. Galanos, D. F. Smith, and I. Müller. 2004. Toll-like receptor 4 contributes to the efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72:1920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kropf, P., N. Freudenberg, C. Kalis, M. Modolell, S. Herath, C. Galanos, M. Freudenberg, and I. Müller. 2004. Infection of C57BL/10ScCr and C57BL/10ScNCr mice with Leishmania major reveals a role for toll-like receptor 4 in the control of parasite replication. J. Leukoc. Biol. 76:48-57. [DOI] [PubMed]
- 25.Luckow, B., H. Maier, S. Chilla, and G. Perez de Lema. 2000. The mCK-5 multiprobe RNase protection assay kit can yield erroneous results for the murine chemokines IP-10 and MCP-1. Anal. Biochem. 286:193-197. [DOI] [PubMed] [Google Scholar]
- 26.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 27.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165-5175. [DOI] [PubMed] [Google Scholar]
- 28.Melby, P. C., Y. Z. Yang, J. Cheng, and W. Zhao. 1998. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect. Immun. 66:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moll, H. 1997. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst. Mitt. 99:73-78. [PubMed] [Google Scholar]
- 30.Müller, K., G. van Zandbergen, B. Hansen, H. Laufs, N. Jahnke, W. Solbach, and T. Laskay. 2001. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. (Berlin) 190:73-76. [DOI] [PubMed] [Google Scholar]
- 31.Müller, K., S. Ehlers, W. Solbach, and T. Laskay. 2001. Novel multi-probe RNase protection assay (RPA) sets for the detection of murine chemokine gene expression. J. Immunol. Methods 249:155-165. [DOI] [PubMed] [Google Scholar]
- 32.Müller, K., S. Bischof, F. Sommer, M. Lohoff, W. Solbach, and T. Laskay. 2003. Differential production of macrophage inflammatory protein 1γ (MIP-1γ), lymphotactin, and MIP-2 by CD4+ Th subsets polarized in vitro and in vivo. Infect. Immun. 71:6178-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237-4241. [DOI] [PubMed] [Google Scholar]
- 34.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via TLR2, and confers protection against lethal infection. J. Immunol. 168:6366-6374. [DOI] [PubMed] [Google Scholar]
- 35.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 36.Poltorak, A., T. Merlin, P. J. Nielsen, O. Sandra, I. Smirnova, I. Schupp, T. Boehm, C. Galanos, and M. A. Freudenberg. 2001. A point mutation in the IL-12R beta 2 gene underlies the IL-12 unresponsiveness of Lps-defective C57BL/10ScCr mice. J. Immunol. 167:2106-2111. [DOI] [PubMed] [Google Scholar]
- 37.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 38.Ritter, U., H. Moll, T. Laskay, E. Brocker, O. Velazco, I. Becker, and R. Gillitzer. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699-709. [DOI] [PubMed] [Google Scholar]
- 39.Ritter, U., and H. Moll. 2000. Monocyte chemotactic protein-1 stimulates the killing of L. major by human monocytes, acts synergystically with IFN-γ and is antagonized by IL-4. Eur. J. Immunol. 30:3111-3120. [DOI] [PubMed] [Google Scholar]
- 40.Ritter, U., and H. Korner. 2002. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 24:295-301. [DOI] [PubMed] [Google Scholar]
- 41.Rousseau, D., S. Demartino, F. Anjuere, B. Ferrua, K. Fragaki, Y. Le Fichoux, and J. Kubar. 2001. Sustained parasite burden in the spleen of Leishmania infantum-infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. Eur. Cytokine Netw. 12:340-347. [PubMed] [Google Scholar]
- 42.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch., and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 10.27-10.37. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor CCR2 is required for Langerhans cell migration and localization of Th1-inducing dendritic cells. Absence of CCR2 shifts the L. major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B-cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Zandbergen, G., N. Hermann, H. Laufs, W. Solbach, and T. Laskay. 2002. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70:4177-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vester, B., K. Muller, W. Solbach, and T. Laskay. 1999. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect. Immun. 67:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaph, C., and P. Scott. 2003. Interleukin-12 regulates chemokine gene expression during the early immune response to Leishmania major. Infect. Immun. 71:1587-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]