Abstract
A woman aged 77 years with a history of rheumatoid arthritis (RA) presented with inflammatory colitis confined to her rectum, which was incidentally found by a screening colonoscopy. Histopathological examination of colonic biopsies showed non-specific inflammatory infiltrates of lymphocytes, the cause of which was unknown. She had been diagnosed with RA 5 years before, and she was receiving methotrexate 6 mg weekly, to which tocilizumab had been added 4 years earlier, which achieved stable control of her disease. She had no gastrointestinal symptoms or other health problems. Tocilizumab-induced colitis was considered likely, and the drug was discontinued. Metronidazole was also prescribed because of possible Clostridium difficile-associated colitis. 3 months later, a repeat colonoscopy showed no improvement of the colitis. The methotrexate was also discontinued, and folinic acid was prescribed daily for 2 weeks, leading to complete resolution of the colitis observed at repeat colonoscopy.
Background
In 1948, methotrexate was introduced as a chemotherapeutic agent for the treatment of leukaemia.1 In 1988, methotrexate was approved for treating rheumatoid arthritis (RA) in the USA. In recent years, methotrexate has played a key role in the treatment of RA, so the European League Against Rheumatism and the American College of Rheumatology recommended that methotrexate should be part of the first-line treatment strategy in patients with RA.2 3 Methotrexate has many side effects; therefore, it should be administered with caution. In RA, methotrexate is administered as long-term, low-dose therapy. The major side effects of low-dose methotrexate are gastrointestinal (GI) problems, such as nausea, stomach upset and loose stools, stomatitis and abnormal liver chemistry. Others include a macular punctate cutaneous eruption, central nervous system symptoms, alopecia, fever, haematological abnormalities and pulmonary toxicity. Although GI problems are common side effects, there are few case reports describing colitis due to low-dose methotrexate.4 Here, we present a case of colitis induced by methotrexate and improved by discontinuing the drug.
Case presentation
The patient was a woman aged 77 years diagnosed with RA about 5 years earlier. She was on methotrexate 6 mg weekly, folic acid 5 mg weekly, esomeprazole 20 mg daily and tocilizumab 480 mg monthly. At the time of presentation, she had been receiving methotrexate for 5 years and had started tocilizumab 4 years earlier.
Although there were no remarkable symptoms such as nausea, stomach upset and diarrhoea, and the findings on physical examination were unremarkable, screening colonoscopy revealed colonic inflammation confined to the rectum (figure 1). At that time, she was not treated with non-steroidal anti-inflammatory drugs. On peripheral blood examination, red blood count was 3.77×106/mm3, haemoglobin was 11.7 g/dL and haematocrit was 35.2%. White cell count was 2900/mm3 with 48% neutrophils, 35% lymphocytes, 9% monocytes, 7% eosinophils and 1% basophils. Blood chemistry revealed total serum albumin 4.3 g/dL. Blood urea nitrogen was 19.7 mg/dL, creatinine 0.61 mg/dL and uric acid 4.9 mg/dL. Sodium was 140 mEq/L, potassium 4.3 mEq/L and chloride 105 mEq/L. Liver function tests were normal. Urinalysis was unremarkable. Stool culture yielded Escherichia coli and Enterococcus faecalis. Histopathological examination of colonic biopsies showed non-specific inflammatory infiltrates of lymphocytes (figure 2).
Figure 1.
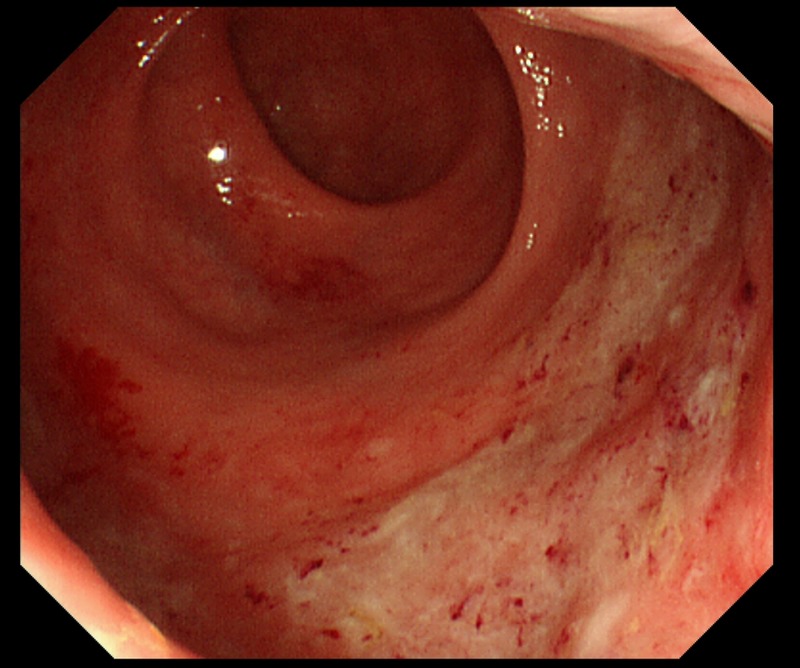
First colonoscopy shows the rectal inflammation.
Figure 2.
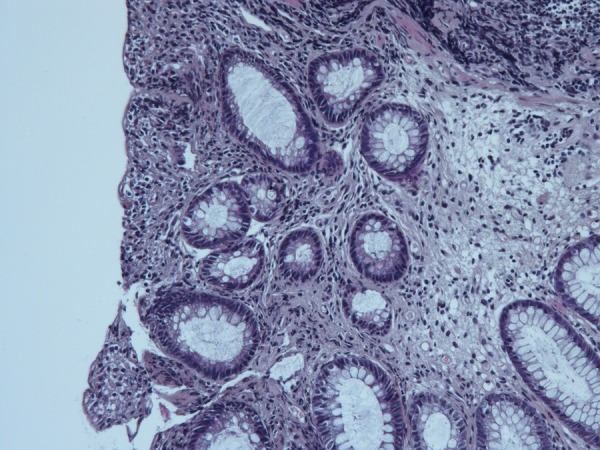
Histopathological examination of colonic biopsies showed non-specific inflammatory infiltrates of lymphocytes (HE stain).
Outcome and follow-up
Tocilizumab-induced colitis was considered likely, and the drug was discontinued. Metronidazole was also prescribed because of possible Clostridium difficile-associated colitis.
After 3 months, a repeat colonoscopy showed no improvement of the colitis (figures 3 and 4). Methotrexate was also discontinued, and folinic acid was prescribed for 3 weeks.
Figure 3.
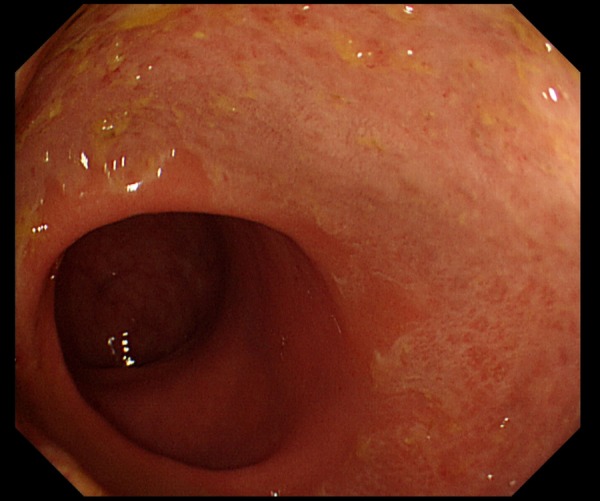
Second colonoscopy shows no improvement.
Figure 4.
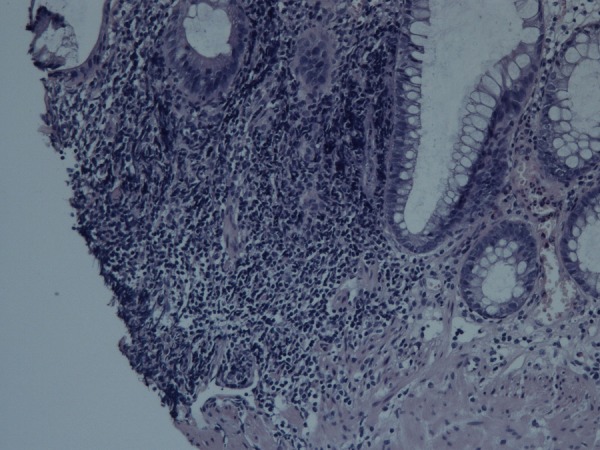
Histopathological examination in second colonoscopy showing non-specific inflammatory infiltrates of lymphocytes (HE stain).
One week after stopping methotrexate, a third colonoscopy revealed complete resolution of the colitis (figure 5). We made a diagnosis of low-dose methotrexate-induced colitis and restarted tocilizumab for the treatment of RA.
Figure 5.
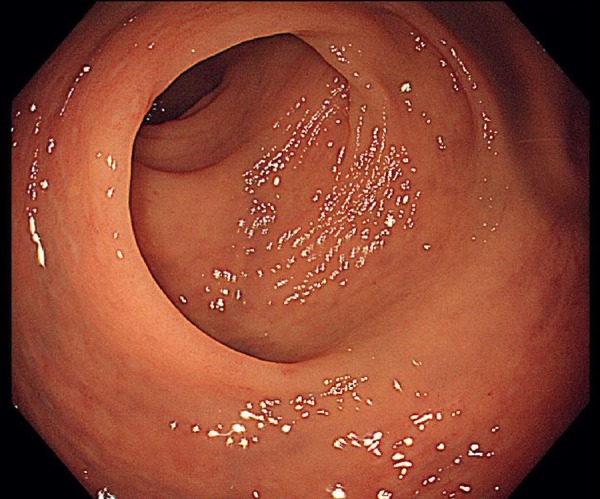
Third colonoscopy shows remarkable improvement.
Discussion
Methotrexate is known to cause many side effects, but there are few reports describing colitis due to low-dose methotrexate. In this report, we discontinued tocilizumab initially, but there were no changes in colonoscopy results. After discontinuing methotrexate and administering folinic acid, we found notable improvement on colonoscopy. Infectious enteritis and ulcerative colitis were other possible causes of colitis, but were unlikely considering the findings on histopathological examination, stool culture and clinical course.
GI symptoms are common adverse events during the first 1 or 2 years of methotrexate therapy. GI side effects are generally dose-dependent and could be alleviated by dose reduction.5 Toxicity is more a function of duration of drug exposure than the serum concentration of drug.6 For this reason, daily administration of the drug contributes to the development of drug toxicity. Other factors such as renal impairment, ageing, diabetes mellitus, obesity and alcohol abuse also increase the risk of drug toxicity.7 Although our patient was a woman aged 77 years, she had no other risk factors.
It should be noted that although our patient received weekly low-dose methotrexate, she developed methotrexate-induced colitis. Although Novak and Kessinger,6 Iveson and Chan8 and Taylor et al9 reported the occurrence of colitis in patients who were administered methotrexate as chemotherapy, there have been few reports about colitis due to weekly low-dose methotrexate for RA.4 It also should be noted that in our case, the patient developed colitis without any symptoms and we could only confirm the improvement by colonoscopy.
Learning points.
We have reported a rare case of asymptomatic colitis induced by low-dose methotrexate. The presence of colitis in a patient receiving methotrexate should raise a high index of suspicion for not only infectious colitis due to immunosuppression, but also methotrexate-induced colitis, even though the patient may only be on weekly low-dose methotrexate.
Footnotes
Contributors: TO drafted the article and revised it critically in cooperation with YF.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Farber S, Diamond LK, Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787–93. 10.1056/NEJM194806032382301 [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL Jr et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé R, Breedveld FC et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toquet S, Nguyen Y, Sabbagh A et al. Severe apoptotic enteropathy caused by methotrexate treatment for rheumatoid arthritis. Joint Bone Spine 2016;83:217–19. 10.1016/j.jbspin.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Borchers AT, Keen CL, Cheema GS et al. The use of methotrexate in rheumatoid arthritis. Semin Arthritis Rheum 2004;34:465–83. 10.1016/j.semarthrit.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Novak RA, Kessinger A. Methotrexate induced colitis. Nebr Med J 1976;61: 84–7. [PubMed] [Google Scholar]
- 7.Willkens RF, Watson MA. Methotrexate: a perspective of its use in the treatment of rheumatic diseases. J Lab Clin Med 1982;100:314–21. [PubMed] [Google Scholar]
- 8.Iveson TJ, Chan A. Pseudomembranous colitis complicating chemotherapy. Lancet 1992;339:192–3. 10.1016/0140-6736(92)90271-4 [DOI] [PubMed] [Google Scholar]
- 9.Taylor SG, Hass GM, Crumrine JL et al. Toxic reactions of 4-amino-pteroylglutamic acid (aminopterin) in patients with far-advanced neoplastic disease. Cancer 1950;3:493–503. [DOI] [PubMed] [Google Scholar]


