Abstract
A 58-year-old man with medical history of thrombocytopenia was admitted to an outside hospital for a 6-day history of worsening dyspnoea requiring mechanical ventilator support. He was transferred to our institution for extracorporeal membrane oxygenation (ECMO) given his refractory hypoxaemia. On arrival, H1N1 influenza virus was confirmed and all measures to improve oxygenation were ineffective. Thus, the decision was made to start venovenous (VV)-ECMO. Although a low baseline platelet count was recognised (60–70×109/L), a sudden further decrease occurred (30×109/L) and platelet transfusion was initiated. A substantial increase in the pressure across the ECMO oxygenator was identified, and the diagnosis of type II heparin-induced thrombocytopenia was suspected and confirmed. Heparin was discontinued, the oxygenator was exchanged and argatroban was used for anticoagulation. After 28 days on VV-ECMO support, the decision was made to withdraw organ support in conjunction with the patient and family wishes.
Background
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is a salvage therapy used in critically ill patients with isolated respiratory failure but preserved cardiac function.1 It has demonstrated to improve the overall survival in patients with influenza A virus (H1N1) infection.2 Unfractioned heparin (UFH) is the most widely used systemic anticoagulant to prevent the risk of thrombosis during ECMO.3 Hence, it is the causal factor for heparin-induced thrombocytopenia (HIT); an immune-mediated complication resulting from the development of IgG antibodies against complexes of platelet factor-4 (PF4) and heparin.4 Two clinical entities of HIT have been described: type I and type II. Type I HIT is a benign condition characterised by decreased platelet count within 24–48 hours after heparin initiation and lacks of clinical value.5 6 Conversely, type II HIT is a life-threatening condition characterised by bleeding and thrombosis and is considered the most common cause of drug-induced thrombocytopenia.5 6 The diagnosis of HIT is based on clinical suspicion and further laboratory confirmation.7–9 Diagnostic tests available are not only laborious but are also time-dependent.7 8 The diagnosis of HIT in the critical care setting is cumbersome, as thrombocytopenia is present in about 50% of patients at some point during their intensive care unit stay.10 Likewise, mechanical circulatory support is also correlated with thrombocytopenia through consumption of platelets within the ECMO circuit.11 Thus, a suspicion of HIT in patients under ECMO is not only rare but also a challenging diagnosis for the intensivist.
To date, the incidence of HIT in patients undergoing ECMO support is estimated to be lower than 1%.12 Only a few cases have been reported in the literature of HIT in patients under ECMO support.13–15 We present a case of laboratory-confirmed HIT type II in a patient with VV-ECMO who developed an acute oxygenator failure from thrombosis. This case highlights the diagnostic challenge and need for rapid recognition of this clinical condition in patients that are almost totally dependent on this form of mechanical ventilatory support. Likewise, it adds evidence to limit platelet transfusion in patients with clinically suspected HIT who are under ECMO support.
Case presentation
A 58-year-old man with a medical history of hypertension, hyperlipidaemia, obstructive sleep apnoea and mild chronic thrombocytopenia (100–150×109/L) was admitted to an outside hospital with a 6-day history of worsening dyspnoea, cough, chills, diarrhoea and myalgia. Laboratory tests on admission were remarkable for an abnormally elevated D-dimer (832 ng/mL; normal range: <250 ng/mL). A spiral chest CT scan was found to be negative for pulmonary embolism. Chest X-ray and CT scan were suggestive of pneumonia and pulmonary oedema. The patient was started on empiric antiviral and antibiotic medications while the results of a rapid streptococcal antigen test and H1N1 influenza virus infection were pending. Although both tests were negative, the empiric medication coverage was sustained during the entire outside hospital stay.
On hospital day 5, the patient's respiratory status continued to deteriorate and he was intubated after a failed trial of Bi-level Positive Airway Pressure (BiPAP). Progressively worsening thrombocytopenia (87×109/L) was also identified at this time. Nevertheless, due to a positive family history of myelodysplastic syndrome as well as a medical history of baseline chronic thrombocytopenia, the patient was characterised as having an acute on chronic reduced platelet count secondary to a respiratory infection.
One day later, he was transferred to our institution due to persistently worsening hypoxoemia and potential requirement of ECMO support. H1N1 diagnostic test was repeated and reported positive. During the first 24 hours after admission, mechanical ventilation (tidal volume 439 mL; positive end-expiratory pressure of 18 mm Hg; FiO2 100%; respiratory rate 18 and peak inspiratory pressure of 35 mm Hg), inhaled nitric oxide at 20 parts per million, deep sedation and paralysis were provided. In addition, chest tubes for bilateral pneumothoraces were inserted in an attempt to improve the refractory hypoxoemia. However, these measures only resulted in a transient improvement in oxygenation. Thus, given his normal cardiac function and haemodynamic stability, the patient was initiated on VV-ECMO support.
Following ECMO initiation, hourly visual inspection of the oxygenator and the heparin-coated circuit (BIOLINE coating; Maquet Cardiopulmonary AG, Hirrlingen, Germany) was unremarkable during days 1–10. We routinely apply an institutional protocol that include a sequential approach to assess fibrin strands on the ECMO system; a handheld flashlight is initially applied to the visible (external) side of the integrated diffusion oxygenator membrane of the centrifugal pump (The Cardiohelp system; Maquet Cardiopulmonary AG) followed by a thorough inspection of the heparin-coated ECMO cannulas (BIOLINE coating; Maquet Cardiopulmonary AG). A checklist is subsequently filled by the nursing team to confirm proper functioning of the ECMO system and document the absence of blood clotting and/or fibrin formation.
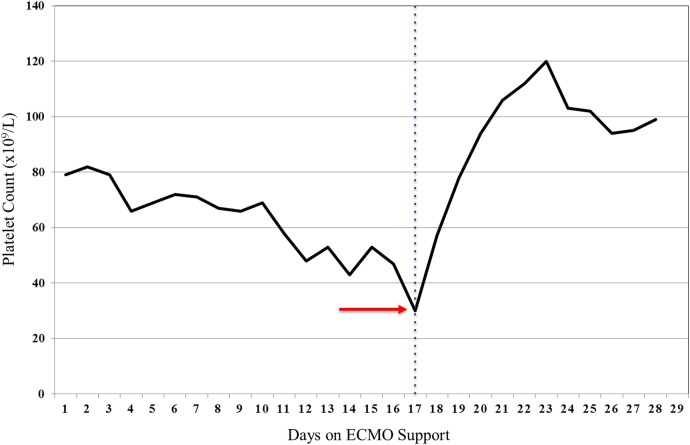
Although no initial complications were detected following VV-ECMO placement (heparin infusion at 10 units/kg/hour), some fibrin strands were identified in the venous side of the circuit on day 10. The heparin infusion dose was increased (14 units/kg/hour) and the activated partial thromboplastin time (aPTT) goal was modified (from 40 to 50 to 50–60 s). At this time, thrombocytopenia (ranging from 60 to 70×109/L) remained unchanged (figure 1).
Figure 1.
Platelet count variation during ECMO support. Representative variation of the platelet count during 28 days of VV-ECMO support. The red arrow indicates the nadir (30×109/L) of the platelet count (day 17). The blue dotted line differentiates the platelet count before and after the successful management of the HIT-induced thrombosis. ECMO, extracorporeal membrane oxygenation; HIT, heparin-induced thrombocytopenia.
One week later, the platelet count dropped (from 55×109/L to 30×109/L) while on heparin infusion (13 units/kg/hour). At the point of time, the calculated 4T's score (3 of 8) revealed a low pretest probability of HIT (≤1%).9 The decision was made to transfuse three units of platelets (figure 1). By the end of the transfusion, a severe impairment on ECMO flow was identified (from 3.94 L/min to <1.0 L/min) (table 1). Despite increasing the revolutions per minute (RPM) on the ECMO circuit, the cardiac flow remained low. Subsequent desaturation and worsening PaO2 on arterial blood gas were identified. In addition, multiple bright red clots and, most importantly, an acute increase in the pressure difference across the ECMO oxygenator (ΔP=Pint−Part) were noted (table 1). The diagnosis of HIT was considered at this time.
Table 1.
Characteristics of VV-ECMO haemodynamics following acute oxygenator failure
| Time after the event (in hours) |
||||
|---|---|---|---|---|
| ECMO parameters | 0 | 1 | 1.15 | 5 |
| Flow (L/min) | 3.94 | 0.98 | 2.16 | 4.05 |
| Pump speed setting (RPM) | 3150 | 3135 | 4000 | 3000 |
| SvO2 (%) | 61.4 | 78.4 | 76.8 | 65.1 |
| P art (mm Hg) | 159 | 50 | 89 | 177 |
| P ven (mm Hg) | −34 | 20 | 7 | –52 |
| P int (mm Hg) | 227 | 302 | 460 | 194 |
| ΔP (mm Hg) | 68 | 252 | 371 | 19 |
| T art (°C) | 37.1 | 36.9 | 37.1 | 37.1 |
| Comments | Initial thrombotic reaction | Peak failure of oxygenator | Increase in RPM's in an attempt to increase flow | After oxygenator replacement |
ECMO, extracorporeal membrane oxygenation; ΔP, P int−P art; P art, arterial pressure in the ECMO circuit; P ven, venous pressure in the ECMO circuit; P int, pressure inside the ECMO oxygenator; RPM, revolutions per minute; SVO2, mixed venous oxygen saturation; T art, temperature of the arterial blood.
Investigations
As a result of the abrupt drop in platelet count followed by a thrombotic event while on VV-ECMO support, a diagnosis of HIT was considered. An ELISA-based immunoassay (Asserachrom HPIA; Diagnostica Stago, Asnières, France) revealed the presence of antibodies directed against the complexes of UFH and PF4. The diagnosis of type II HIT was confirmed.
Differential diagnosis
The differential diagnosis included disseminated intravascular coagulation (DIC); immune thrombocytopenia; post-transfusion purpura; myelodysplastic syndrome.
Treatment
Given our set up with immediate availability of prime ECMO circuits in the operating room and its proximity to the intensive care unit (ICU), the patient was emergently transferred to the operating room by the cardiothoracic surgical and ICU teams. A massive clotting of the oxygenator was found. The decision was made to replace the oxygenator, the ECMO circuit and both cannulas. A heparin-free coating system was used to prevent HIT recurrence (SOFTLINE coating; Maquet Cardiopulmonary AG). Overall, the replacement was performed in 45 min, and no complications arose during the procedure. Heparin was discontinued, and patient's anticoagulation was switched to argatroban. The infusion was started at low dose (0.1–0.3 µg/kg/min) and carefully titrated to achieve a goal of activated partial thromboplastin time (aPTT) between 60 and 90 s.16 Periodic monitoring of the aPTT (six times per day) ensured proper monitoring of the anticoagulation therapy. The maintenance infusion of argatroban ranged between 1.5 and 1.7 µg/min/min, and no bleeding complications were reported during the remaining 11 days of VV-ECMO support.
Outcome and follow-up
With the discontinuation of heparin and exchange of the ECMO system, the platelet count significantly rose (range 100–120×109/L) and the patient did not require additional transfusion of platelets throughout the rest of the hospitalisation (figure 1). Likewise, the haemodynamic parameters in the ECMO system returned to baseline (table 1).
After 11 days under anticoagulation with argatroban, no evidence of further clotting events were noted. However, due to lack of overall clinical improvement and debilitation after 28 days on VV-ECMO support, the decision was made to withdraw organ support in conjunction with the patient and family wishes.
Discussion
HIT type II is a potentially life-threatening immune-mediated condition resulting from the exposure to low-molecular weight heparins or most commonly UFHs.4 It is caused by pre-existing IgG antibodies directed against a complex formed between heparin molecules and PF4.17 18 Circulating complexes (IgG-PF4-heparin) bind to platelet Fc receptors and induce procoagulant microparticle release as well as monocyte activation.19–22 Clinically, this immune phenomenon is manifested by thrombocytopenia (defined as platelet count <150×109/L or <50% of baseline platelet count) following 5–15 days of heparin exposure. Concurrent venous/arterial thrombotic events (50–75%) or DIC (10–20%) are well-known complications displayed by patients with laboratory-confirmed HIT.18
In the ICU setting, testing for HIT is typically performed in 13% of patients.10 Nevertheless, despite the elevated number of participants assessed, the incidence of laboratory-confirmed HIT remain <1%.23 The frequency of this condition has also been estimated in patients under mechanical circulatory support. In a retrospective cohort study of 115 patients with newly implanted ventricular-assisted devices managed with UFH, 51 patients were tested for HIT; 24% had a positive heparin-PF4 immunoassay and only 12% displayed a positive platelet aggregation assay.24 Likewise, a prospective study including 581 patients who developed postoperative thrombocytopenia after cardiopulmonary bypass further revealed the low incidence of laboratory-confirmed HIT (0.5%).25 Only one retrospective study including 119 patients has estimated the incidence (19%) of this condition in patients under ECMO support;12 laboratory confirmation was provided in only one patient.12 Additional studies in this condition include few case reports.13–15 26 Laboratory-confirmed type II HIT is certainly needed to be reported given the drastic and life-threatening nature of this condition in patients under ECMO support.
The diagnosis of HIT requires a high degree of clinical suspicion followed by pathological confirmation.18 Clinical suspicion is based on the 4Ts' score, a pretest probability scoring system.9 It includes four distinctive features of HIT: degree of thrombocytopenia, timing of thrombocytopenia according to heparin exposure, thrombosis and/or other sequelae and probability of other causes of thrombocytopenia.9 Laboratory confirmation requires immunoassays (identify IgG antibodies against heparin-PF4 complexes) and/or functional assays (estimate the activation capacity of heparin-PF4-IgG complexes).7 18 Both diagnostic options are not only laborious but also time-consuming.7 8 Hence, HIT-laboratory results are not typically available in critically ill patients requiring immediate therapeutic interventions.
In the critical care setting, thrombocytopenia (defined as a platelet count of 150×109/L or less) is present in about 50% of patients at some point during their ICU stay.10 It is not only associated with higher bleeding risk but is also considered a risk factor for increased length of stay, higher in-hospital mortality and failure to wean from VV-ECMO.27–29 Previous reports have also established that ECMO circuits predispose to thrombocytopenia due to platelet activation and aggregation.3 30 31 Indeed, two cohort studies among patients with acute respiratory failure under ECMO support have demonstrated the association between mechanical circulatory support use and development of thrombocytopenia.32 33
Overall, a timely diagnosis of HIT in our case was impeded by inherent limitations of diagnostic testing (timely availability of results in a critical care scenario), a low pretest probability of HIT (<1%) and multiple comorbidities that might explain concurrent thrombocytopenia (medical history of thrombocytopenia, VV-ECMO support, critical illness and confirmed H1N1 viral infection). Thus, platelet transfusion was not contraindicated before the acute episode of oxygenator thrombosis and failure. Certainly, HIT was strongly suspected following this complication. We provided standard measures once the oxygenator thrombosis was recognised, and no additional platelet transfusion was administered afterward.
Although multiple factors precluded a prompt diagnosis of type II HIT in this case, we propose that the intensivist should exercise a systematic approach that facilitates a timely recognition and treatment of this life-threatening condition. Daily evaluation of the 4T's pretest probability score, thorough inspection of the ECMO oxygenator, monitoring of pressures among the circuit and careful evaluation of the system efficiency (lower PaO2 and higher PaCO2) were considered valuable surveillance strategies in our case.
Few reports are available on the effect of platelet transfusion on thrombotic events or bleeding in HIT patients. Recent studies proposed that platelet transfusions in laboratory-confirmed HIT are safe and efficient. Hopkins and Goldfinger34 reported four cases of laboratory-confirmed HIT who required multiple platelet transfusions; no thromboembolic events were noted. Likewise, similar results were obtained in a retrospective case series of 36 patients with confirmed HIT.35 Nevertheless, platelet transfusions are not currently indicated because of the fear of thrombotic events. Indeed, a recent retrospective study in 6332 patients with confirmed HIT suggested that platelet transfusions were associated with a high age and gender adjusted odds of arterial but not venous thrombosis.36
Up to date, there is limited evidence of platelet transfusion in patients under ECMO support with suspected HIT. A recent case report suggested that platelet transfusions are safe in patients under ECMO support with HIT diagnosis.14 Conversely, our case highlights that platelet transfusion in patients with suspected HIT should be avoided due to the increased risk of thrombotic complications; in our case an acute oxygenator failure.
Although difficult to diagnose in the ICU setting, intensivists should increase their awareness of HIT in patients receiving ECMO support. The effect of platelet transfusion in patients with suspected HIT while receiving ECMO support deserves further investigation.
Learning points.
Type II heparin-induced thrombocytopenia must be suspected in patients who develop thrombocytopenia and are on ECMO support.
Thrombocytopenia in critically ill patients is a common condition and mask the diagnosis of HIT in a critical care scenario.
Platelet transfusion should be avoided in patients undergoing ECMO in order to prevent thrombotic complications.
Direct thrombin inhibitors, though costly, may be the norm in the future for anticoagulation during ECMO because of their predictable pharmacokinetics and greater reduction of thrombin generation.
Footnotes
Contributors: RAR, JGR, LLK and JLD-G interpreted data, researched and wrote the paper. RAR and JLD-G revised and contributed critically important intellectual content. All authors contributed substantially to the work. All authors discussed the results and implications and commented on the paper at all stages.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166–76. 10.3978/j.issn.2072-1439.2015.07.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351–63. 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 3.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 2009;13:154–75. 10.1177/1089253209347384 [DOI] [PubMed] [Google Scholar]
- 4.Linkins LA, Dans AL, Moores LK et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e495S–530S. 10.1378/chest.11-2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase M, Bellomo R, Rocktaeschel J et al. Use of fondaparinux (ARIXTRA) in a dialysis patient with symptomatic heparin-induced thrombocytopaenia type II. Nephrol Dial Transplant 2005;20:444–6. 10.1093/ndt/gfh544 [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Tiruvoipati R, Green C et al. Heparin induced thrombocytopenia in critically ill: diagnostic dilemmas and management conundrums. World J Crit Care Med 2015;4:202–12. 10.5492/wjccm.v4.i3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood 1986;67:27–30. [PubMed] [Google Scholar]
- 8.Warkentin TE, Sheppard JI, Moore JC et al. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost 2008;6:1304–12. 10.1111/j.1538-7836.2008.03025.x [DOI] [PubMed] [Google Scholar]
- 9.Lo GK, Juhl D, Warkentin TE et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 2006;4:759–65. 10.1111/j.1538-7836.2006.01787.x [DOI] [PubMed] [Google Scholar]
- 10.Crowther MA, Cook DJ, Meade MO et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care 2005;20:348–53. 10.1016/j.jcrc.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Dewald O, Fischlein T, Vetter HO et al. Platelet morphology in patients with mechanical circulatory support. Eur J Cardiothorac Surg 1997;12:634–41. 10.1016/S1010-7940(97)00151-6 [DOI] [PubMed] [Google Scholar]
- 12.Glick D, Dzierba AL, Abrams D et al. Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J Crit Care 2015;30:1190–4. 10.1016/j.jcrc.2015.07.030 [DOI] [PubMed] [Google Scholar]
- 13.Dolch ME, Frey L, Hatz R et al. Extracorporeal membrane oxygenation bridging to lung transplant complicated by heparin-induced thrombocytopenia. Exp Clin Transplant 2010;8:329–32. [PubMed] [Google Scholar]
- 14.Welp H, Ellger B, Scherer M et al. Heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2014;28:342–4. 10.1053/j.jvca.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 15.Koster A, Weng Y, Bottcher W et al. Successful use of bivalirudin as anticoagulant for ECMO in a patient with acute HIT. Ann Thorac Surg 2007;83:1865–7. 10.1016/j.athoracsur.2006.11.051 [DOI] [PubMed] [Google Scholar]
- 16.Phillips MR, Khoury AI, Ashton RF et al. The dosing and monitoring of argatroban for heparin-induced thrombocytopenia during extracorporeal membrane oxygenation: a word of caution. Anaesth Intensive Care 2014;42:97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak U, Yacobobich J, Tamary H et al. Heparin-induced thrombocytopenia and extracorporeal membrane oxygenation: a case report and review of the literature. J Extra Corpor Technol 2011;43:5–12. [PMC free article] [PubMed] [Google Scholar]
- 18.Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Semin Thromb Hemost 2015;41:49–60. 10.1055/s-0034-1398381 [DOI] [PubMed] [Google Scholar]
- 19.Madeeva D, Cines DB, Poncz M et al. Role of monocytes and endothelial cells in heparin-induced thrombocytopenia. Thromb Haemost 2016;116:806–12. 10.1160/TH16-02-0162 [DOI] [PubMed] [Google Scholar]
- 20.Aster RH. Heparin-induced thrombocytopenia and thrombosis. N Engl J Med 1995;332:1374–6. 10.1056/NEJM199505183322011 [DOI] [PubMed] [Google Scholar]
- 21.Rauova L, Hirsch JD, Greene TK et al. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood 2010;116:5021–31. 10.1182/blood-2010-03-276964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warkentin TE, Hayward CP, Boshkov LK et al. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 1994;84:3691–9. [PubMed] [Google Scholar]
- 23.Verma AK, Levine M, Shalansky SJ et al. Frequency of heparin-induced thrombocytopenia in critical care patients. Pharmacotherapy 2003;23:745–53. 10.1592/phco.23.6.745.32188 [DOI] [PubMed] [Google Scholar]
- 24.Schenk S, El-Banayosy A, Prohaska W et al. Heparin-induced thrombocytopenia in patients receiving mechanical circulatory support. J Thorac Cardiovasc Surg 2006;131:1373–81.e4. 10.1016/j.jtcvs.2006.01.048 [DOI] [PubMed] [Google Scholar]
- 25.Selleng S, Malowsky B, Strobel U et al. Early-onset and persisting thrombocytopenia in post-cardiac surgery patients is rarely due to heparin-induced thrombocytopenia, even when antibody tests are positive. J Thromb Haemost 2010;8:30–6. 10.1111/j.1538-7836.2009.03626.x [DOI] [PubMed] [Google Scholar]
- 26.Parlar AI, Sayar U, Cevirme D et al. Successful use of fondaparinux in a patient with heparin-induced thrombocytopenia while on extracorporeal membrane oxygenation after mitral valve redo surgery. Int J Artif Organs 2014;37:344–7. 10.5301/ijao.5000302 [DOI] [PubMed] [Google Scholar]
- 27.Vanderschueren S, De Weerdt A, Malbrain M et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28:1871–6. 10.1097/00003246-200006000-00031 [DOI] [PubMed] [Google Scholar]
- 28.Baughman RP, Lower EE, Flessa HC et al. Thrombocytopenia in the intensive care unit. Chest 1993;104:1243–7. 10.1378/chest.104.4.1243 [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Kim DJ, Kim JS et al. Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. ASAIO J 2015;61:184–9. 10.1097/MAT.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 30.Haneya A, Philipp A, Diez C et al. Comparison of two different minimized extracorporeal circulation systems: hematological effects after coronary surgery. ASAIO J 2009;55:592–7. 10.1097/MAT.0b013e3181be2f5c [DOI] [PubMed] [Google Scholar]
- 31.Peek GJ, Firmin RK. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J 1999;45:250–63. 10.1097/00002480-199907000-00003 [DOI] [PubMed] [Google Scholar]
- 32.Panigada M, Artoni A, Passamonti SM et al. Hemostasis changes during veno-venous extracorporeal membrane oxygenation for respiratory support in adults. Minerva Anestesiol 2016;82:170–9. [PubMed] [Google Scholar]
- 33.Weingart C, Lubnow M, Philipp A et al. Comparison of coagulation parameters, anticoagulation, and need for transfusion in patients on interventional lung assist or veno-venous extracorporeal membrane oxygenation. Artif Organs 2015;39:765–73. 10.1111/aor.12464 [DOI] [PubMed] [Google Scholar]
- 34.Hopkins CK, Goldfinger D. Platelet transfusions in heparin-induced thrombocytopenia: a report of four cases and review of the literature. Transfusion 2008;48:2128–32. 10.1111/j.1537-2995.2008.01822.x [DOI] [PubMed] [Google Scholar]
- 35.Refaai MA, Chuang C, Menegus M et al. Outcomes after platelet transfusion in patients with heparin-induced thrombocytopenia. J Thromb Haemost 2010;8:1419–21. 10.1111/j.1538-7836.2010.03861.x [DOI] [PubMed] [Google Scholar]
- 36.Goel R, Ness PM, Takemoto CM et al. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood 2015;125:1470–6. 10.1182/blood-2014-10-605493 [DOI] [PMC free article] [PubMed] [Google Scholar]