Abstract
The intracellular organism Anaplasma phagocytophilum causes human granulocytic ehrlichiosis and specifically infects and multiplies in neutrophilic granulocytes. Previous reports have suggested that, for its survival, this bacterium suppresses the neutrophil respiratory burst. To investigate the mechanism of survival, we first assessed the kinetics of A. phagocytophilum entry into neutrophils by using double-labeling confocal microscopy. At 30, 60, 120, and 240 min of incubation, 25, 50, 55, and 70% of neutrophils contained bacteria, respectively. The neutrophil respiratory burst in the presence of A. phagocytophilum was assessed by a kinetic cytochrome c assay and by measurement of oxygen consumption. Neutrophils in the presence of A. phagocytophilum did not produce a significant respiratory burst, but A. phagocytophilum did not inhibit the neutrophil respiratory burst when phorbol myristate acetate was added. Immunoelectron microscopy of neutrophils infected with A. phagocytophilum or Escherichia coli revealed that NADPH oxidase subunits gp91phox and p22phox were significantly reduced at the A. phagocytophilum phagosome after 1 and 4 h of incubation. In neutrophils incubated simultaneously with A. phagocytophilum and E. coli for 30, 60, and 90 min, gp91phox was present on 20, 14, and 10% of the A. phagocytophilum phagosomes, whereas p22phox was present in 11, 5, and 4% of the phagosomes, respectively. Similarly, on E. coli phagosomes, gp91phox was present in 62, 64, and 65%, whereas p22phox was detected in 54, 48, and 48%. We conclude that A. phagocytophilum does not suppress a global respiratory burst and that, under identical conditions in the same cells, A. phagocytophilum, but not E. coli, significantly reduces gp91phox and p22phox from its phagosome membrane.
Human granulocytic ehrlichiosis (HGE) is a tick-transmitted febrile illness caused by an intracellular organism recently renamed Anaplasma phagocytophilum, which exclusively infects neutrophilic granulocytes (4, 10, 16, 23). Clinical manifestations include fever, headache, myalgias, neutropenia, and thrombocytopenia and are occasionally complicated by secondary opportunistic infections (1, 15, 20). In areas of endemicity, the HGE incidence among tick-associated diseases is second only to Lyme disease (21). A major property of A. phagocytophilum is that it survives in a cytoplasmic vacuole and it inhibits fusion with lysosomes (40). Thus, if this pathogen enters and survives in neutrophils, it must circumvent or endure the effects of the respiratory burst, which is an important mechanism of killing by these polymorphonuclear cells (PMN). Indeed, several investigators have reported complete inhibition of the respiratory burst in PMN and in HL-60 cells infected with A. phagocytophilum (5, 26, 27).
Activation of the respiratory burst coincides with the assembly of the NADPH oxidase, leading to the rapid generation of superoxide (O2−) and other toxic reactive oxygen species (ROS) such as hydrogen peroxide and hypochlorous acid. The importance of the NADPH oxidase system to the innate host defense is demonstrated by frequent and severe infections resulting from the ineffective killing of microorganisms in patients with chronic granulomatous disease (12). The chronic granulomatous disease phenotype resulting in an ineffective NADPH oxidase is caused by mutations in one of several subunits of the NADPH oxidase (3). To target the toxic effects of ROS at microorganisms while limiting the collateral damage to host cells, activation of the NADPH oxidase complex is both spatially and temporally regulated. NADPH oxidase activation and termination coincides with the assembly and disassembly of its constituents. In unstimulated neutrophils, the NADPH oxidase is unassembled and inactive, with its components stored in different locations (for reviews, see references 3, 13, 14, and 28). Upon activation, the complete NADPH oxidase is assembled rapidly from its presynthesized subunits and consists of the cytoplasmic subunits p40phox, p47phox, p67phox (phox stands for “phagocyte oxidase”) that translocate en bloc to bind to membrane-bound flavocytochrome b558, which itself is a heterodimer of the membrane proteins gp91phox and p22phox. In unstimulated neutrophils, the majority of flavocytochrome b558 is present on the membrane of secretory vesicles and to a lesser extent on the cell membrane (24). Upon activation, the secretory vesicles deliver flavocytochrome b558 by fusing with either the cell membrane or the phagosomal membrane, resulting in the generation of ROS at two different compartments, extracellularly and intracellularly in phagosomes.
A. phagocytophilum can be propagated in culture with HL-60 cells, and most investigations in the laboratory have used HL-60 cells (19). However, HL-60 cells are not normal phagocytes, and the use of HL-60 cells has several disadvantages: undifferentiated HL-60 cells have no respiratory burst and differentiated HL-60 cells display some respiratory burst but constitutively lack secretory vesicles. Thus, HL-60 cells are of limited use when studying changes in the respiratory burst upon interaction with pathogens. Therefore, we have focused our studies of the respiratory burst by using PMN. In the present study we have examined the kinetics of entry of A. phagocytophilum and its effects on the global respiratory burst. Furthermore, we provide evidence that the number of A. phagocytophilum phagosomes containing cytochrome b558 is significantly reduced compared to phagosomes containing Escherichia coli, suggesting that A. phagocytophilum manipulates its local intracellular environment.
MATERIALS AND METHODS
Cultivation of A. phagocytophilum and E. coli.
The human promyelocytic cell line HL-60 (ATCC 240-CCL; American Type Culture Collection, Rockville, Md.) was infected with A. phagocytophilum and maintained in culture in Iscove's modified Dulbecco's medium supplemented with 20% fetal calf serum at 37°C with 5% carbon dioxide (19). The A. phagocytophilum strain used for all experiments was the HGE bacterial isolate NCH-1 (36). Cell density was maintained between 0.5 × 106 and 2.0 × 106 cells/ml by changing the culture medium twice per week, and the percentage of infected HL-60 cells was ≥85%, as determined by counting 200 HL-60 cells by indirect immunofluorescence. For the preparation of fresh A. phagocytophilum, infected HL-60 cells were lysed at 4°C by passage through a 21-gauge needle five times, followed by a low-spin centrifugation (1,800 × g). The supernatant containing the A. phagocytophilum was then subjected to centrifugation (13,500 × g) at 4°C to pellet the bacteria. The pellet was dissolved in sterile endotoxin-free Hanks' buffered salt solution (HBSS) and washed three times in HBSS (Biowhittaker, Wakersville, Md.). The concentration of A. phagocytophilum was measured spectrophotometrically at the A600 (comparing the A600 with the A600 = 0.5, equaling 2.0 × 108 bacteria). Final A. phagocytophilum concentrations for all experiments were adjusted to yield a ratio of 50 bacteria per neutrophil, unless stated otherwise. E. coli containing the pMAL-c2X vector encoding the maltose binding protein (MBP) (NEB, Beverly, Mass.) was grown overnight to a concentration of 2 × 108 cells/ml. The presence of this plasmid allowed for rapid antibody detection with anti-MBP in immunofluorescence experiments. For opsonization experiments, A. phagocytophilum cells were incubated with human serum at a dilution of 1:3 for 30 min at 37°C. Bacteria were heat killed at 65°C for 15 min.
Isolation of human peripheral neutrophils.
Heparinized venous blood was obtained from healthy individuals in accordance with a protocol approved by the institutional review board for human subjects at the University of Iowa. Neutrophils were freshly isolated for each experiment by 3% dextran sedimentation and Ficoll-Hypaque (Amersham, Piscataway, N.J.) density gradient separation, followed by hypotonic lysis of erythrocytes, as described previously (6). Neutrophils were gently resuspended in HBSS (with Ca and Mg) supplemented with 10 mM d-glucose and kept on ice until use. The concentration of purified cells was determined by counting with a hemocytometer. Purified cells contained >95% neutrophils, as assessed by diff-Quick stain (Baxter Healthcare Corp., Miami, Fla.).
Incubation of neutrophils with A. phagocytophilum or E. coli.
Fresh neutrophils were incubated with freshly prepared A. phagocytophilum at a final bacteria/neutrophil ratio of 50:1 at 37°C under gentle agitation, and aliquots were collected at different time points. The final bacteria/neutrophil ratio was achieved by adding a spectrophotometrically measured concentration of bacteria to a known concentration of PMN. The ratio was confirmed by counting the total number of bacteria per 20 cells by immunofluorescence staining methods. Similarly, neutrophils were incubated with E. coli at the same concentrations as A. phagocytophilum cells. For simultaneous dual infections, A. phagocytophilum and E. coli were added to neutrophils at the same time and incubated as described above. After incubation, cells were used in the different assays described below. For experiments with synchronized entry, bacteria were allowed to bind for 30 min, followed by centrifugation of the neutrophils (400 × g), and resuspended in HBSS to remove unbound bacteria.
Indirect immunofluorescence and confocal immunofluorescence microscopy.
Immunostaining and fluorescence microscopy were performed as described previously (13, 14). Briefly, after incubation, cells were allowed to adhere to microscope slides for 10 min and then fixed with 10% formalin for 15 min. Cells were permeabilized with acetone at 20°C, washed, and then blocked for 1 h in HBSS containing 5 mg of bovine serum albumin/ml, 10% normal horse serum, and 0.02% sodium azide. Primary antibodies included polyclonal rabbit anti-A. phagocytophilum, rabbit anti-MBP antibody to detect E. coli, antivinculin antibodies to counterstain neutrophils, and rhodamine-phalloidin to stain HL-60 cells. Monoclonal antibodies to gp91phox (54.1) and p22phox (44.1) were a gift from A. J. Jesaitis and J. B. Burritt (7, 8, 22, 40). Fixed and permeabilized cells were incubated with primary antibodies for 1 h at 25°C in a humidified chamber and then washed six times in HBSS. After incubation with secondary antibodies conjugated with fluorescein isothiocyanate or tetramethyl rhodamine isothiocyanate (TRITC) or incubation with rhodamine-phalloidin for an additional hour, slides were washed six times in HBSS, covered with glass coverslips by using mounting media containing the antifading agent 1,4-diazabicyclo-octane (Sigma, St Louis, Mo.). In double-labeling experiments, primary antibodies were incubated sequentially, followed by incubation with secondary antibodies. The specificity of the staining was assessed by the omission of primary antibodies and by the use of mouse and rabbit isotype control antibodies. For each incubation time point, 25 Z-stacks were examined and cells were scored for having A. phagocytophilum or E. coli bacteria intracellularly. A. phagocytophilum and E. coli bacteria were labeled with TRITC-conjugated secondary antibody bound to rabbit anti-A. phagocytophilum or rabbit anti-MBP, respectively, and neutrophils were labeled with antivinculin antibodies. Fluorescence was visualized by using an Axioplan2 epifluorescence microscope (Carl Zeiss, Thornwood, N.Y.), an LSM 510 laser-scanning confocal microscope (Carl Zeiss) at the Veterans Administration Medical Center, or a model 1024 laser-scanning confocal microscope (Bio-Rad, Hercules, Calif.) at the University of Iowa Central Microscopy Research Facility.
Electron microscopy and immunolabeling.
Uninfected neutrophils (controls), neutrophils incubated with A. phagocytophilum or E. coli at different time intervals, and neutrophils infected with both A. phagocytophilum and E. coli were fixed in phosphate-buffered saline with 4% paraformaldehyde, subsequently embedded in LR White resin, and incubated at 55°C for 48 h. After sectioning and placement on grids, samples were incubated sequentially with a first primary antibody, washed, and incubated with a secondary antibody conjugated with colloidal gold, followed by postfixation in 2.5% glutaraldehyde and silver enhancement. The samples were then incubated with the second primary antibody, washed, and incubated with a secondary antibody, followed by postfixation, further silver enhancement, and poststaining for contrast enhancement. The specificity of staining was assessed by the omission of primary antibodies or the omission of secondary antibodies in single-labeling studies. Samples were examined and photographed with a transmission electron microscope (Hitachi H-7000) operating at 75 kV at the University of Iowa Central Microscopy Research Facility.
Kinetic cytochrome c assay.
Neutrophils with or without preincubation with A. phagocytophilum or E. coli were aliquoted to wells of a 96-well microtiter plate (adjusted final concentration, 2.0 × 106 cells/ml in HBSS supplemented with 10 mM d-glucose). Ferricytochrome c (final concentration, 240 mM), phorbol myristate acetate (PMA) (final concentration, 50 ng/ml), superoxide dismutase (SOD) (final concentration, 0.125 mg/ml), or HBSS alone was added to the wells, and the final volume for each well was adjusted with HBSS to 200 μl. All experiments were done in duplicate wells. Plates were warmed to 37°C by using a Spectramax microplate spectrophotometer (Molecular Devices, Sunnyvale, Calif.), and the optical density at 550 nm was measured over time. The O2− generation was defined as the SOD-inhibitible reduction of ferricytochrome c at 550 nm (net difference of the optical density values of the wells containing SOD subtracted from those of the wells without SOD). The concentration of O2− was calculated by using the nanometer extinction coefficient of 21.1 and a light path length of 3 mm (volume of 200 μl per well).
Oxygen consumption.
Oxygen consumption was used as an alternate method to overcome several limitations inherent to the cytochrome c assay. Polarigraphic measurement of oxygen pressure in solution was performed by using a biologic oxygen monitor with a Clark-type electrode (YSI, Yellow Springs, Ohio). Neutrophils (preincubated with and without A. phagocytophilum), 5.0 × 106 cells in 0.99 ml, were placed in a sample chamber with a stir magnet at 37°C for 5 min to equilibrate. Oxygen consumption was measured for 15 min; subsequently, 10 μl of PMA (final concentration, 100 ng/ml) was added and oxygen consumption was measured for 30 min or until all oxygen was consumed. The maximal rate of oxygen consumption (Vmax) and the total amount of oxygen consumed were determined for resting neutrophils and PMA-stimulated neutrophils. The system was calibrated with oxygen-saturated water, which contains 217 nmol of O2/ml at sea level, at 37°C for 1 h. All oxygen consumption assays were repeated five times.
Xanthine and xanthine oxidase assay.
In a cell-free system, superoxide was generated by dissolving xanthine (final concentration, 50 μM) and ferricytochrome c (final concentration, 80 mM) with and without SOD (final concentration in the assay, 0.125 mg/ml) in HBSS from 10× stock solutions. Final volumes were adjusted with HBSS to 200 μl. The plate was warmed to 37°C by using a Spectramax microplate spectrophotometer, and xanthine oxidase (final concentration, 25 mU/ml) was added just before the plate was read. All experiments were done in triplicate wells. Freshly prepared A. phagocytophilum or HL-60 cells were added in different concentrations. The O2− generation was calculated from the measured SOD-inhibitible reduction of ferricytochrome c at 550 nm. Simultaneously, urate production was measured at 290 nm.
Statistical analysis.
Comparisons of the percentages of A. phagocytophilum and E. coli phagosomes, each labeled with gp91phox and p22phox, were performed by using the χ2 test. The statistical significance was set at a P value of <0.05.
RESULTS
A. phagocytophilum entry into neutrophils.
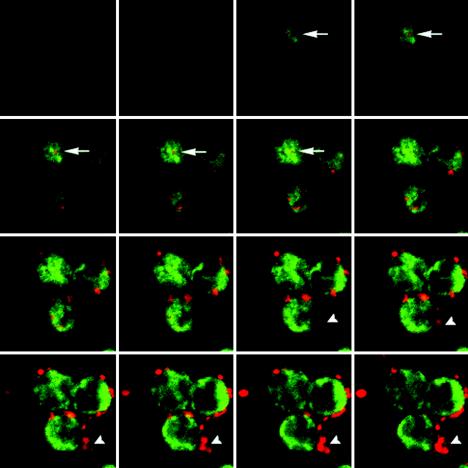
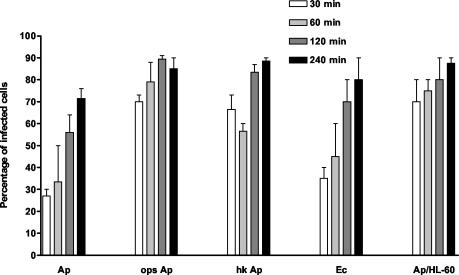
Prior to examining the respiratory burst generated by neutrophils incubated with A. phagocytophilum, we first established the kinetics of bacterial entry. Human neutrophils were incubated with A. phagocytophilum, and aliquots were taken at 0, 30, 60, 120, and 240 min and prepared for double immunolabeling with anti-A. phagocytophilum and antivinculin antibodies. Using confocal microscopy, 25 Z-stacks were generated for each time point (Fig. 1) and the percentage of neutrophils having one or more intracellular A. phagocytophilum cells was recorded. The percentage of infected cells increased over time (Fig. 2). The entry of opsonized and heat-killed A. phagocytophilum cells was significantly higher than that of nonopsonized A. phagocytophilum or nonopsonized E. coli. Nonopsonized A. phagocytophilum entry into HL-60 cells was also increased compared to entry into neutrophils. Extracellular organisms, but adherent to neutrophils, were seen in 95% of the cells examined at all time points. The scoring of intracellular organisms was done conservatively, and only organisms with a clearly surrounding vinculin stain at multiple sections were considered intracellular.
FIG. 1.
Confocal immunofluorescence microscopy (serial sections) of neutrophils incubated with unopsonized A. phagocytophilum for 4 h. Dual labeling shows A. phagocytophilum (TRITC) and vinculin (fluorescein isothiocyanate). An intracellular A. phagocytophilum organism is marked by an arrow. Many bacteria are still extracellular (arrowheads).
FIG. 2.
Percentages of neutrophils containing intracellular A. phagocytophilum after incubation for 30, 60, 120, and 240 min, determined by using double-labeling immunofluorescence microscopy. Unopsonized A. phagocytophilum (Ap), opsonized A. phagocytophilum (ops Ap), heat-killed A. phagocytophilum (hk Ap), and unopsonized E. coli (Ec) (as a control) are compared. HL-60 cells were also incubated with unopsonized A. phagocytophilum (Ap/HL-60).
Kinetic cytochrome c assay.
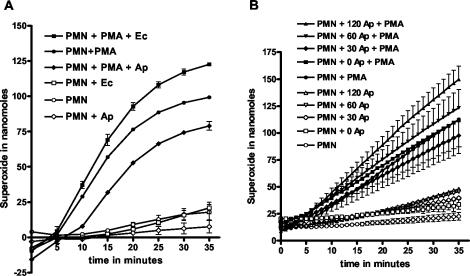
Superoxide generation by the neutrophil respiratory burst was assessed by measuring the SOD-inhibitible reduction of ferricytochrome c. Neutrophils alone or neutrophils in the presence of A. phagocytophilum or E. coli were examined with and without the addition of PMA at time zero (Fig. 3A). PMN without bacteria and without PMA did not produce significant O2−, whereas PMN (without bacteria) with PMA clearly produced a respiratory burst. Similarly, PMN in the presence of E. coli did not produce significant O2−, whereas PMN with E. coli plus PMA produced large amounts of O2−. Neutrophils in the presence of A. phagocytophilum did not produce O2−, whereas PMN with A. phagocytophilum plus PMA resulted in O2− production, albeit in lower amounts than the control PMN without bacteria. Differences in O2− production between corresponding samples with and without PMA were significant for time points at 10 min and greater, except for PMN plus PMA plus A. phagocytophilum at 10 min. Also, O2− production for all three samples with PMA were significantly different. Because the majority of bacteria were considered extracellular at time zero, we next preincubated PMN with A. phagocytophilum at various time points to examine the effect of intracellular A. phagocytophilum on the generation of O2−. The results are shown in Fig. 3B. PMN without A. phagocytophilum and without PMA did not show O2− production, whereas PMN without A. phagocytophilum and with PMA resulted in significant O2− production. PMN preincubated with A. phagocytophilum for increasing periods of time (0, 30, 60, and 120 min) but without PMA showed minute but increasing amounts O2−. PMN preincubated with A. phagocytophilum for the same increasing time periods showed a significant respiratory burst when PMA was added after the preincubation. Neutrophils preincubated with A. phagocytophilum for 30 min showed a slight overall reduction in the amounts of O2− measured, whereas PMN preincubated with A. phagocytophilum for 120 min showed an increased O2− production (Fig. 3B). In all cases, the addition of PMA showed a robust response regardless of the amount of time of preincubation with A. phagocytophilum compared to samples without PMA, indicating that the respiratory burst was not suppressed. Experiments were performed five times. The differences between samples with and without PMA were statistically significant. The maximal superoxide production after PMA stimulation (neutrophils alone) was between 3.6 and 4.9 nmol/min/106 PMN.
FIG. 3.
Kinetic cytochrome c assay measuring superoxide production. (A) PMN alone or in the presence of A. phagocytophilum (Ap) or E. coli (Ec) with or without PMA added at time zero; (B) PMN preincubated with A. phagocytophilum for 0, 30, 60, and 120 min. Unbound A. phagocytophilum was removed prior to the start of the cytochrome c assay.
Oxygen consumption.
To confirm the results obtained from the cytochrome c assay, we examined oxygen consumption as a measure of the respiratory burst of neutrophils incubated with A. phagocytophilum. Since the ROS produced by the NADPH oxidase are derived from molecular oxygen, the measurement of oxygen consumption in the medium containing the neutrophils should be equivalent to the amount of ROS produced, regardless of ROS (O2−, H2O2, or HOCl) or where (i.e., intracellularly or extracellularly) these compounds are produced. Unstimulated neutrophils consumed small amounts of oxygen at a constant rate (Fig. 4A). The addition of PMA at 15 min increased their oxygen consumption. PMN did not increase oxygen consumption when A. phagocytophilum (bacteria/PMN ratio, 50:1) was added at 15 min. PMN with A. phagocytophilum added at time zero showed an increased oxygen consumption only upon the addition of PMA at 15 min, similar to the PMN without A. phagocytophilum. To assess the effect of preincubation of neutrophils with A. phagocytophilum on ROS generation, oxygen consumption was measured for 15 min after PMN preincubation with A. phagocytophilum for 15, 45, and 105 min. Subsequently, PMA was added (at time 15 min) to these samples (at total incubation times of 30, 60 and 120 min with A. phagocytophilum) and resulted in increased oxygen consumption (Fig. 4B). The rate of increase of oxygen consumption among the different samples was similar, but the samples with longer preincubation times (45 and 105 min) displayed an increased lag phase to oxygen consumption after PMA stimulation. All oxygen consumption essays were repeated five times, and the results shown in Fig. 4 are representative. The maximum rate of oxygen consumption (Vmax) was between 6 and 9% per minute, and the calculated oxygen consumption was 2.6 to 3.9 nmol/min/106 PMN. These rates of oxygen consumption correlate with the measurements of superoxide production from the cytochrome c assays. Taken together, these data indicate that A. phagocytophilum does not stimulate a respiratory burst, nor does it inhibit neutrophil oxygen consumption when stimulated with PMA.
FIG. 4.
(A) Oxygen consumption of unstimulated PMN (open circles) before and after the addition of A. phagocytophilum (Ap) at 15 min (arrow). PMN with A. phagocytophilum added at 15 min without PMA (open squares) maintains constant oxygen consumption. PMN with A. phagocytophilum added at time zero (open diamonds) increases oxygen consumption after the addition of PMA at 15 min. (B) Oxygen consumption of PMN preincubated with A. phagocytophilum for 15, 45, and 105 min, followed by the addition of PMA (arrow).
Xanthine and xanthine oxidase assay.
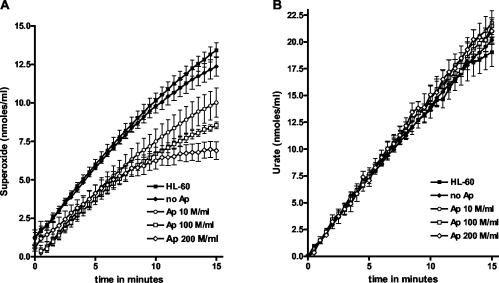
We considered the possibility that A. phagocytophilum might scavenge O2−, leading to reduced amounts of measured O2− in the cytochrome c assay (Fig. 3A). In a cell-free system, xanthine is converted by xanthine oxidase to O2− and urate. We first established the conditions such that the xanthine oxidase was the rate-limiting factor in the assay, resulting in a nearly constant rate of O2− production. The production of O2− was measured by the SOD-inhibitible reduction of ferricytochrome c, and simultaneously, the amount of newly formed urate was measured. An uninfected HL-60 control (uninfected cells were taken through the same bacterial purification steps as infected cells to have an exact comparison) and a negative control (no A. phagocytophilum) were included. The HL-60 control does not reduce the O2− production (Fig. 5A), excluding the possibility that HL-60 cell remnants could be scavenging O2−. The addition of A. phagocytophilum reduced the amount of measured O2− in a dose-dependent fashion, but the rate and total amount of urate produced did not change (Fig. 5B). The experiment was performed five times. Because urate formation was unaffected, inhibition of the xanthine oxidase by A. phagocytophilum did not account for the reduction in measured O2− production. Note that the concentration of 100 × 106 bacteria per ml is equivalent to the bacterial concentration used for experiments whose results are shown in Fig. 3. Thus, at least in vitro, A. phagocytophilum has the capacity to scavenge O2−. The Vmax of O2− production ranged between 1.2 and 1.3 nmol/ml, which is about two- to threefold less than the Vmax in the cytochrome c assays (Fig. 3) but is still in the physiologic range of O2−, as produced by stimulated neutrophils.
FIG. 5.
Xanthine conversion by xanthine oxidase in a cell-free system, producing O2− and urate. (A) O2− production was measured in the absence of A. phagocytophilum (Ap) (solid diamonds), in the presence of increasing concentrations of A. phagocytophilum, and in the presence of uninfected HL-60 control. (B) Urate production of the same samples at the same times as in panel A.
Dual-labeling electron microscopy.
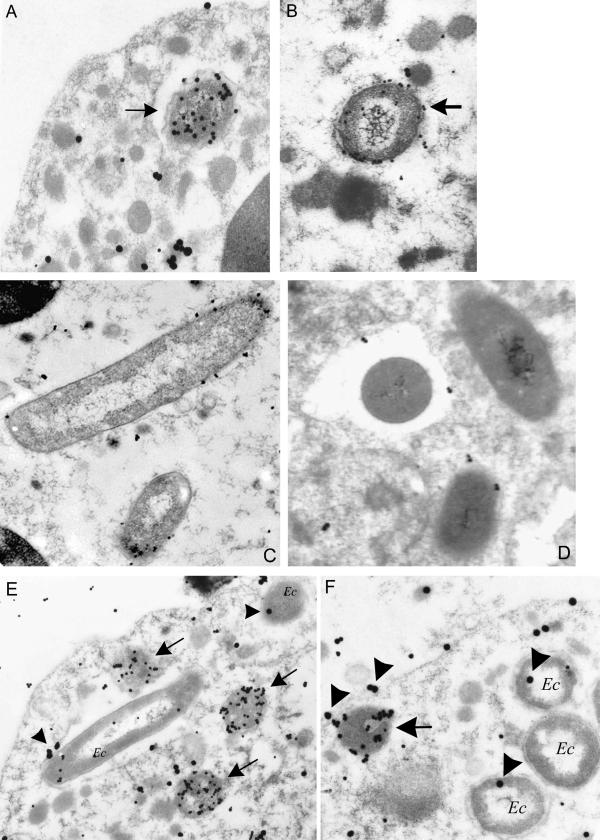
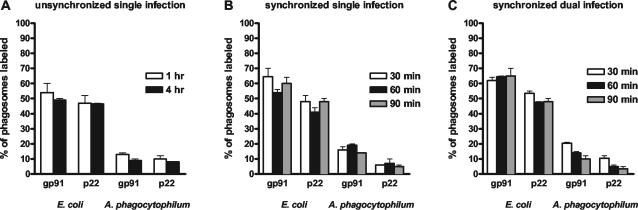
We hypothesized that if the overall respiratory burst is not inhibited, A. phagocytophilum could inhibit NADPH oxidase locally at the phagosome membrane to ensure survival. Neutrophils were incubated with A. phagocytophilum or E. coli for 1 and 4 h and processed for immunolabeling. Although E. coli phagosomes were identified easily, A. phagocytophilum cells were more difficult to distinguish from other cell organelles. Therefore, we used a double-labeling technique to visualize A. phagocytophilum and gp91phox or p22phox (Fig. 6). Of E. coli phagosomes, 54 and 50% showed the label for gp91phox and 48 and 48% showed the label for p22phox at 1 and 4 h of incubation, respectively (Fig. 7A). Of A. phagocytophilum phagosomes, 13 and 10% contained the label for gp91phox and 10 and 8% contained the label for p22phox at 1 and 4 h of incubation, respectively. Next, we repeated the experiment by using synchronized entry of bacteria into the neutrophils at 30, 60, and 90 min, and the results are shown in Fig. 7B. Of E. coli phagosomes, 64, 54, and 59% contained the gp91phox label and 47, 41, and 47% contained the p22phox label. Of A. phagocytophilum phagosomes, 16, 18, and 13% showed the label for gp91phox and 6, 7, and 5% showed label for p22phox. Subsequently, we incubated neutrophils simultaneously with A. phagocytophilum and E. coli for 30, 60, and 90 min, and only cells with both pathogens were scored (Fig. 7C). As was seen with incubation of neutrophils with single infections, dual infection of neutrophils showed a similar pattern: at 30, 60, and 90 min of incubation, 62, 64, and 65% of E. coli phagosomes contained gp91phox and 54, 48, and 48% of E. coli phagosomes contained p22phox, whereas 20, 14, and 10% of A. phagocytophilum phagosomes showed the label for gp91phox and 10, 5, and 4% of A. phagocytophilum phagosomes showed the label for p22phox, respectively. Thus, within the same cells, the percentage of A. phagocytophilum phagosomes labeled with gp91phox or p22phox was significantly lower than that of E. coli phagosomes (P < 0.001).
FIG. 6.
Electron microscopy of neutrophils incubated with A. phagocytophilum (A and B), E. coli (C and D), or both organisms simultaneously (E and F). Arrows indicate A. phagocytophilum-containing phagosomes. (A) Dual labeling with anti-A. phagocytophilum antibodies (small particles) and anti-gp91phox antibodies (large particles); (B) dual labeling with anti-A. phagocytophilum antibodies (small particles) and anti-p22phox antibodies (large particles); (C and D) single labeling (small particles only) of E. coli phagosomes labeled with anti-gp91phox (C) or anti-p22phox antibodies (D), respectively; (E) Neutrophils infected with A. phagocytophilum and E. coli (Ec), labeled with anti-A. phagocytophilum antibodies (small particles) and anti-gp91phox antibodies (large particles, indicated by arrowhead); (F) neutrophils infected with A. phagocytophilum and E. coli, labeled with anti-A. phagocytophilum antibodies (small particles) and anti-p22phox antibodies (large particles, indicated by arrowheads).
FIG. 7.
Percentage of phagosomes labeled with gp91phox and p22phox antibodies. Neutrophils were incubated with A. phagocytophilum and/or E. coli. (A) Single unsynchronized infection; (B) single synchronized infection; (C) synchronized dual infection.
DISCUSSION
The intracellular human pathogen A. phagocytophilum resides and multiplies in polymorphonuclear neutrophils (granulocytes), the primary early effector of innate defense in humans. To survive the major killing mechanism of this phagocyte, A. phagocytophilum must be able to circumvent the respiratory burst produced by neutrophils, an extraordinary feat shared by relatively few pathogens. We sought to investigate how A. phagocytophilum evades the toxic effects of oxygen radicals produced by the respiratory burst. Our data demonstrate that A. phagocytophilum does not induce a respiratory burst when incubated with neutrophils. Furthermore, it does not interfere with the overall neutrophil capacity to generate ROS, in contrast to the results of other reports (5, 26, 27). Moreover, in electron microscopy studies, we observed reduced labeling of cytochrome b558 at the A. phagocytophilum phagosome compared to E. coli phagosomes. These findings have important implications for understanding how A. phagocytophilum creates a safe haven within the hostile environment of the phagocyte.
A. phagocytophilum does not suppress the overall respiratory burst, in contrast to the results of previous reports (26, 27). However, it does interfere with the accumulation of NADPH oxidase locally at its own phagosome. We show evidence that the presence of gp91phox and p22phox in the phagosome containing A. phagocytophilum is reduced but not that in the E. coli phagosome. Dual infection of neutrophils with both organisms shows similar exclusion of cytochrome b558 from the A. phagocytophilum phagosome within the same cell. Thus, translocation of cytochrome b558 (and other subunits as well) to the A. phagocytophilum phagosome is selectively inhibited, a scenario that has been demonstrated for Salmonella enterica serovar Typhimurium (18, 37, 38). The fact that not all E. coli phagosomes are labeled with gp91phox and p22phox antibodies may reflect the limitations of the technique used or, alternatively, could be explained by degradation or disassembly of the NADPH oxidase complex over time. Certainly, our methods do not allow us to distinguish between exclusion or rapid degradation of the NADPH oxidase at the A. phagocytophilum phagosome, and this issue needs further investigation.
Several investigators have examined the effects of A. phagocytophilum infection on the respiratory burst (5, 9, 11, 26, 27, 39). Most studies have concluded that A. phagocytophilum infection results in a reduced or absent respiratory burst in neutrophils and/or HL-60 cells, whereas Choi and Dumler reported an early increase followed by a later suppression of the respiratory burst. Our results indicate that A. phagocytophilum does not significantly suppress the overall respiratory burst in infected neutrophils. How can these seemingly opposing results be explained?
First, it is important to distinguish studies of HL-60 cells from those examining mature neutrophils. While uninduced HL-60 cells do not have a respiratory burst and induced cells show some respiratory burst activity, they lack secondary granules, which is an important reservoir of flavocytochrome b558 (gp91phox and p22phox). The strength of the reports with HL-60 cells is primarily that they show that A. phagocytophilum is able to suppress the transcription of several components of the NADPH oxidase (such as gp91phox and Rac2) as one mechanism of ensuring prolonged intracellular survival (5, 9). This strategy may be very relevant but only if A. phagocytophilum is able to employ additional mechanisms to first successfully fend off an initial respiratory burst upon entry. The latter is addressed by studies, including ours, with mature neutrophils (11, 26, 27).
Second, because activation of the respiratory burst is rapid, within seconds to a few minutes, assays to measure O2− and/or its oxygen metabolites need to include early time points. We have presented data from two independent techniques (kinetic cytochrome c assay and oxygen consumption) that show that A. phagocytophilum does not significantly suppress a global respiratory burst when stimulated by PMA. We have not been able to confirm the complete elimination of the respiratory burst after stimulation with PMA, as reported by Mott and Rikihisa (26). Perhaps a somewhat different method, such as the time of measuring O2− after stimulation with PMA, the kinetic versus the end-point cytochrome c assay, and possible reoxidation over time of reduced cytochrome c, could account for these differences. In addition, chemiluminescent detection methods of O2− with lucigenin or luminol can be fraught with problems leading to inaccurate assessments of O2− production (17, 35).
Third, we suggest that scavenging of O2− may be yet another strategy used by A. phagocytophilum to ensure protection from ROS. Inherently, measured quantities of O2− produced by neutrophils infected with A. phagocytophilum may be affected by scavenging because all assays, except oxygen consumption, indirectly measure the amount of O2− and/or other ROS. In other words, the influence of O2− scavenging by A. phagocytophilum could lead to an underestimation of the degree of respiratory burst activity. In fact, lucigenin chemiluminescence has been used as a measure of SOD activity for E. coli (25). With the anticipated completion of the A. phagocytophilum genome sequencing project in the near future, several candidate genes involved in oxygen scavenging may well be identified.
Precisely to avoid the effects of O2− scavenging in assays measuring ROS, we examined oxygen consumption as a measure of the respiratory burst. Because molecular oxygen is the substrate for the generation of O2−, the change in oxygen pressure in a closed chamber containing neutrophils is a direct measure of the respiratory burst. Indeed, mature resting neutrophils consume small amounts of oxygen, whereas activation of the respiratory burst in neutrophils dramatically increases oxygen consumption. Based on our results obtained by measuring oxygen consumption, we conclude that A. phagocytophilum does not inhibit the overall respiratory burst.
Exclusion of NADPH oxidase at the phagosome by wild-type Salmonella, but not by mutants of pathogenicity island 2 (SPI-2), implies that the type III secretory system encoded by SPI-2 introduces factors into the host cytoplasm that play a role in the biogenesis of the Salmonella-containing vacuole (18). We hypothesize that A. phagocytophilum may employ a similar strategy. This notion is supported by the observation that A. phagocytophilum inhibits phagosome-lysosome fusion and by the recent identification of a type IV secretory system in A. phagocytophilum that could facilitate interference with intracellular trafficking (32, 40). Members of type IV systems have been identified in several intracellular organisms, including Helicobacter pylori, Rickettsia prowazekii, Rickettsia conorii, Brucella spp., and Bartonella henselae (2, 29-31, 33, 34). With the identification of these transporter systems, investigation of the action of effector proteins may provide further understanding of the basic mechanisms that lead to manipulation of the nascent phagosome containing these pathogens.
Acknowledgments
This work was supported in part by a grant (0160459Z to J.I.) from the American Heart Association Heartland Affiliate.
We thank Algirdas J. Jesaitis and James B. Burritt (Montana State University) for the gift of anti-gp91phox and p22phox monoclonal antibodies. We thank Jean Ross for technical assistance with the electron microscopy experiments performed at the Central Microscopy Facility at the University of Iowa. We are grateful to the members of the Interdisciplinary Inflammation Program at the University of Iowa for their stimulating discussions and support.
Editor: F. C. Fang
REFERENCES
- 1.Aguero-Rosenfeld, M. E., H. W. Horowitz, G. P. Wormser, D. F. McKenna, J. Nowakowski, J. Munoz, and J. S. Dumler. 1996. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann. Intern. Med. 125:905-908. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 3.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 5.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 6.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 97:77-89. [PubMed] [Google Scholar]
- 7.Burritt, J. B., S. C. Busse, D. Gizachew, D. W. Siemsen, M. T. Quinn, C. W. Bond, E. A. Dratz, and A. J. Jesaitis. 1998. Antibody imprint of a membrane protein surface. Phagocyte flavocytochrome b. J. Biol. Chem. 273:24847-24852. [DOI] [PubMed] [Google Scholar]
- 8.Burritt, J. B., M. T. Quinn, M. A. Jutila, C. W. Bond, and A. J. Jesaitis. 1995. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J. Biol. Chem. 270:16974-16980. [DOI] [PubMed] [Google Scholar]
- 9.Carlyon, J. A., W. T. Chan, J. Galan, D. Roos, and E. Fikrig. 2002. Repression of rac2 mRNA expression by anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 169:7009-7018. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, K. S., and J. S. Dumler. 2003. Early induction and late abrogation of respiratory burst in A. phagocytophilum-infected neutrophils. Ann. N. Y. Acad. Sci. 990:488-493. [DOI] [PubMed] [Google Scholar]
- 12.Clark, R. A. 1999. Activation of the neutrophil respiratory burst oxidase. J. Infect. Dis. 179(Suppl. 2):S309-S317. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo, F. R., L. A. Allen, M. Apicella, and W. M. Nauseef. 1999. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163:6732-6740. [PubMed] [Google Scholar]
- 14.DeLeo, F. R., and M. T. Quinn. 1996. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J. Leukoc. Biol. 60:677-691. [DOI] [PubMed] [Google Scholar]
- 15.Dumler, J. S., and J. S. Bakken. 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis. 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 16.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 17.Faulkner, K., and I. Fridovich. 1993. Luminol and lucigenin as detectors for O2. Free Radic. Biol. Med. 15:447-451. [DOI] [PubMed] [Google Scholar]
- 18.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 19.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 20.Hardalo, C. J., V. Quagliarello, and J. S. Dumler. 1995. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin. Infect. Dis. 21:910-914. [DOI] [PubMed] [Google Scholar]
- 21.IJdo, J. W., J. I. Meek, M. L. Cartter, L. A. Magnarelli, C. Wu, S. W. Tenuta, E. Fikrig, and R. W. Ryder. 2000. The emergence of another tick-borne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J. Infect. Dis. 181:1388-1393. [DOI] [PubMed] [Google Scholar]
- 22.IJdo, J. W., C. Wu, L. A. Magnarelli, and E. Fikrig. 1999. Serodiagnosis of human granulocytic ehrlichiosis by a recombinant HGE-44-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3540-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IJdo, J. W., Y. Zhang, E. Hodzic, L. A. Magnarelli, M. L. Wilson, S. R. Telford, S. W. Barthold, and E. Fikrig. 1997. The early humoral response in human granulocytic ehrlichiosis. J. Infect. Dis. 176:687-692. [DOI] [PubMed] [Google Scholar]
- 24.Kjeldsen, L., H. Sengelov, K. Lollike, M. H. Nielsen, and N. Borregaard. 1994. Isolation and characterization of gelatinase granules from human neutrophils. Blood 83:1640-1649. [PubMed] [Google Scholar]
- 25.Liochev, S. I., and I. Fridovich. 1997. Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc. Natl. Acad. Sci. USA 94:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mott, J., Y. Rikihisa, and S. Tsunawaki. 2002. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70:1359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauseef, W. M. 1999. The NADPH-dependent oxidase of phagocytes. Proc. Assoc. Am. Physicians 111:373-382. [DOI] [PubMed] [Google Scholar]
- 29.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 30.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 31.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi, N., N. Zhi, Q. Lin, and Y. Rikihisa. 2002. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect. Immun. 70:2128-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padmalayam, I., K. Karem, B. Baumstark, and R. Massung. 2000. The gene encoding the 17-kDa antigen of Bartonella henselae is located within a cluster of genes homologous to the virB virulence operon. DNA Cell Biol. 19:377-382. [DOI] [PubMed] [Google Scholar]
- 34.Schmiederer, M., and B. Anderson. 2000. Cloning, sequencing, and expression of three Bartonella henselae genes homologous to the Agrobacterium tumefaciens VirB region. DNA Cell Biol. 19:141-147. [DOI] [PubMed] [Google Scholar]
- 35.Tarpey, M. M., and I. Fridovich. 2001. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 89:224-236. [DOI] [PubMed] [Google Scholar]
- 36.Telford, S. R., J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Torres, A., and F. C. Fang. 2001. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect. 3:1313-1320. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 39.Wang, T., S. E. Malawista, U. Pal, M. Grey, J. Meek, M. Akkoyunlu, V. Thomas, and E. Fikrig. 2002. Superoxide anion production during Anaplasma phagocytophila infection. J. Infect. Dis. 186:274-280. [DOI] [PubMed] [Google Scholar]
- 40.Webster, P., J. W. IJdo, L. M. Chicoine, and E. Fikrig. 1998. The agent of human granulocytic ehrlichiosis resides in an endosomal compartment. J. Clin. Investig. 101:1932-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]