Various techniques have been described for catheter insertion—open surgical, i.e. mini-laparotomy, laparoscopic, peritoneoscopy, fluoroscopy-assisted percutaneous, and blind percutaneous techniques. In this issue of Peritoneal Dialysis International, George and colleagues have described their experience of inserting blind, bedside percutaneous Tenckhoff peritoneal dialysis (PD) catheters in a large cohort of patients. Their institute, Christian Medical College, has been at the forefront of nephrology in India for over 5 decades, and were the earliest to start hemodialysis and renal transplantation in the country.
In a country where more than half of the patients with chronic kidney disease stage 5 (CKD G5) present to the hospital with eGFR < 15mL/min/1.73m2 at their initial visit (1), planning for renal replacement therapy (RRT) becomes an immediate requirement without the luxury of abundant time. As the population of India largely lives in villages, PD ought to be the initial RRT modality of choice (2). There are many reasons for inadequate PD penetration in India, including absence of a “PD first” policy, it being perceived as a more expensive modality (though costs have been shown to be similar) (3), lesser remuneration for treating patients with CKD on PD compared to that for hemodialysis, etc. (4). However, perhaps one of the most important and least talked about is expertise in catheter placement. When the onus to insert catheters is on the treating nephrologist and each nephrologist is competent, PD penetration is almost guaranteed to increase.
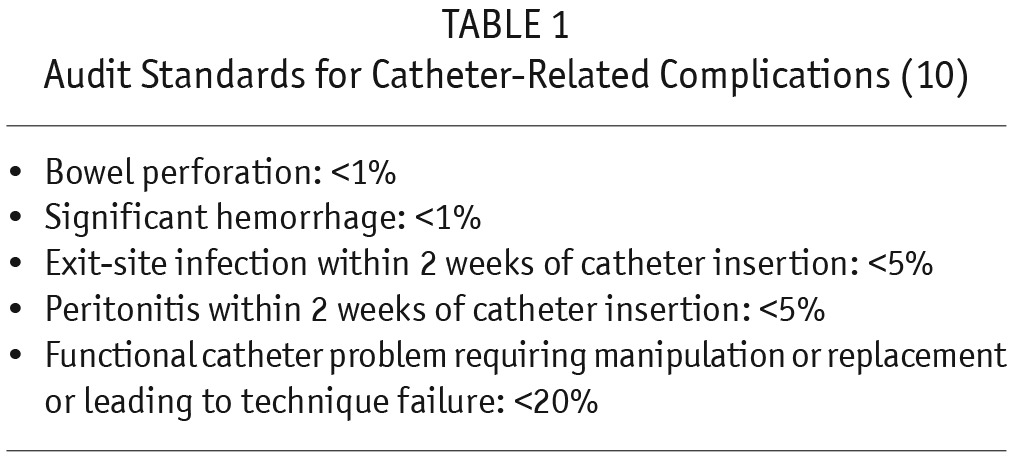
Percutaneous PD catheter insertion was described in 1984 (5), but its practice has not become routine in most parts of the world though, encouragingly, its use has recently increased. This article describes how, with only a 0.5-cm blind, midline incision, PD catheter insertion can be done using the Seldinger technique, a procedure that can be mastered with reasonable ease. Non-requirement of expensive operating time, surgeon, anesthetist, and a shorter hospital stay are all factors (6) that make this procedure economically more appealing. Additionally, the fact that a separate operation suite and fluoroscopy are not required may encourage other centers in developing countries to emulate the practice. Other reports have also shown that percutaneous PD catheter insertion has similar outcomes compared to open surgical placement (7,8). The rate of mechanical complications is lower than that reported elsewhere from India (9) and other countries (7,8). In their large cohort of 245 patients, the low incidences of visceral injury (2 patients), bleeding from the exit site (1 patient), and rectus sheath hematoma (1 patient) are testimony to the safety of this procedure. This article aims at setting new standards for PD practice. The International Society for Peritoneal Dialysis (ISPD) has set audit standards (Table 1) to be maintained with respect to PD catheter insertions (10). The authors report complication rates far less than those considered acceptable for a unit and could perhaps replace those in the ISPD guideline if successfully replicated by other centers that perform PD catheter insertions with the same technique. The percutaneous technique has previously been described as the preferred option in patients infected with blood-borne viral pathogens like hepatitis B and C and human immunodeficiency virus (11).
TABLE 1.
Audit Standards for Catheter-Related Complications (10)
The ISPD has hitherto recommended a break-in period of 2 weeks from catheter insertion to initiation of PD (10). The authors have successfully described an ‘ultra-short’ 2- to 3-day break-in period. This perhaps will influence the suggested break-in period in future PD access guidelines. Most patients who have not benefited from predialysis CKD education and planning are likely to start on hemodialysis. These constitute ‘dialysis-crash start’ patients and require an uncuffed temporary central venous dialysis catheter in their internal jugular veins. These patients are at a higher risk for mortality and hospitalization compared to their ‘planned-start’ counterparts (12) as they are likely to have more comorbidities and a worse biochemical profile (13). In developed countries, this cohort is elderly as well (14). Many of these ‘dialysis crash-start’ patients are unlikely to then switch to PD. The safety of starting PD without a break-in period was first described in a cohort of 51 patients (15). In this series, the results were excellent, with very low incidence of peri-catheter leak, catheter malfunction, and infections. No patient had injury to viscera or severe bleeding. The incision size was larger and was paramedian in location. Also, the deep cuff was secured in the anterior rectus sheath using purse-string sutures.
The ‘ultra-short’ break-in period described by the authors is ideally suited for these patients and will perhaps obviate some of the attendant risks of an unplanned start. All ‘dialysis crash-start’ patients barring those presenting with frank pulmonary edema, pericarditis, uremic colitis, severe hyperkalemia, or similar medical emergency should be offered PD as the initial RRT modality (2).
The authors have offered the blind percutaneous technique to patients who had undergone past abdominal surgeries that were less likely to have left behind any peritoneal adhesions. They have described this experience previously (16,17). Blind percutaneous PD catheter insertion in these patients, of course, is controversial and can by no means be applied universally. It will ultimately depend on the confidence of the individual who is attempting the procedure. The usual dictum as per the ISPD recommendation is to plan either a surgical or laparoscopic procedure for these patients (11,18), and any deviation from this routine must be done only after careful consideration of the clinical condition.
In summary, this article by George et al. describes how, in a developing nation, high standards of patient care can be met and surpassed. They have shown their technique to be safe, economic, and universal. Their PD catheter insertion technique and standards are worthy of emulation.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Varughese S, John GT, Alexander S, Deborah MN, Nithya N, Ahamed I, et al. Pre-tertiary hospital care of patients with chronic kidney disease in India. Indian J Med Res 2007; 126: 28–33. [PubMed] [Google Scholar]
- 2. Abraham G, Varughese S, Mathew M, Vijayan M. A review of acute and chronic peritoneal dialysis in developing countries. Clin Kidney J 2015; 8(3):310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeloka TK, Upase S, Chitikeshi S. Monthly cost of three exchanges a day peritoneal dialysis is same as of thrice a week hemodialysis in self-paying Indian patients. Indian J Nephrol 2012; 22(1): 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karopadi AN, Mason G, Rettore E, Ronco C. The role of economies of scale in the cost of dialysis across the world: a macroeconomic perspective. Nephrol Dial Transplant 2014; 29:885–92. [DOI] [PubMed] [Google Scholar]
- 5. Nakanishi T, Yanase M, Fujii M, Tanaka Y, Orita Y, Labe H. New acute peritoneal dialysis technique: guide wire insertion and long term indwelling of peritoneal catheter. Nephron 1984; 37:128–32. [DOI] [PubMed] [Google Scholar]
- 6. Varughese S, Sundaram M, Basu G, Tamilarasi V, John GT. Percutaneous continuous ambulatory peritoneal dialysis (CAPD) catheter insertion—a preferred option for developing countries. Trop Doct 2010; 40(2):104–5. [DOI] [PubMed] [Google Scholar]
- 7. Medani S, Shantier M, Hussein W, Wall C, Mellotte G. A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit Dial Int 2012; 32(6):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozener C, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 2001; 16(9):1893–9. [DOI] [PubMed] [Google Scholar]
- 9. Sampathkumar K, Mahaldar AR, Sooraj YS, Ramkrishnan M, Ajeshkumar, Ravichandran R. Percutaneous CAPD catheter insertion by a nephrologist versus surgical placement: a comparative study. Indian J Nephrol 2008; 18(1):5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9. [DOI] [PubMed] [Google Scholar]
- 11. Varughese S, Sundaram M, Basu G, Tamilarasi V. Percutaneous peritoneal dialysis catheter insertion in blood borne virus infected patients—a preferred option. Indian J Perit Dial 2009; 17(2):33–4. [Google Scholar]
- 12. Khan IH, Catto GR, Edward N, MacLeod AM. Death during the first 90 days of dialysis: a case control study. Am J Kidney Dis 1995; 25:276–80. [DOI] [PubMed] [Google Scholar]
- 13. Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of “unplanned” dialysis initiation: reframing the terminology to “suboptimal” initiation. BMC Nephrol 2009; 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivarsen P, Povlsen JV. Can peritoneal dialysis be applied for unplanned initiation of chronic dialysis? Nephrol Dial Transplant 2014; 29:2201–6. [DOI] [PubMed] [Google Scholar]
- 15. Jo YI, Shin SK, Lee JH, Song JO, Park JH. Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit Dial Int 2007; 27(2):179–83. [PubMed] [Google Scholar]
- 16. Varughese S, David VG, Basu G, Mohapatra A, Sundaram M, Tamilarasi V. Successful percutaneous CAPD catheter insertion in a patient with past abdominal surgeries. Saudi J Kidney Dis Transpl 2012; 23(2):353–4. [PubMed] [Google Scholar]
- 17. Varughese S, Sundaram M, Basu G, David VG, Mohapatra A, Alexander S, et al. Percutaneous PD catheter insertion after past abdominal surgeries. Indian J Nephrol 2012; 22(3):230–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crabtree JH. Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low hanging fruits. Perit Dial Int 2008; 28:134–7. [PubMed] [Google Scholar]


