Abstract
♦ Background:
Early peritonitis was confirmed to be associated with a higher risk of early technique failure. However, literature concerning peritonitis within the first 3 months of peritoneal dialysis (PD) initiation is scarce. The present study was to investigate risk factors associated with early-onset peritonitis in PD patients.
♦ Methods:
In this retrospective observational cohort study, all incident PD patients from January 1, 2006, to December 31, 2013, were recruited and followed up until December 31, 2014. According to time-to-first episode of peritonitis, patients were divided into early-onset (≤ 3 months) peritonitis and late-onset (> 3 months) peritonitis. Baseline demographic, clinical, and laboratory data, as well as episodes of peritonitis, were collected. Risk factors associated with early-onset peritonitis were evaluated using logistic regression model.
♦ Results:
Of 1,690 patients on PD, 503 (29.8%) developed at least 1 episode of peritonitis and 118 (7.0%) patients presented the first episodes of peritonitis within the first 3 months. A multivariate logistic analysis showed that higher body mass index (BMI) (odds ratio [OR] 1.08, 95% confidence interval [CI] 1.01 – 1.15, p = 0.034), hypoalbuminemia (OR 1.75, 95% CI 1.11 – 2.78, p = 0.017), and catheter exit-site infection (OR 4.14, 95% CI 2.45 – 7.00, p < 0.001) were risk factors independently associated with early-onset peritonitis. Compared to those with late-onset, patients with early-onset peritonitis had a higher overall peritonitis rate (0.76 vs 0.38 per patient-year, p < 0.001) and worse technique survival (p < 0.001), while patient survival did not differ significantly between the 2 groups during the long-term follow-up (p > 0.05).
♦ Conclusions:
Higher BMI, hypoalbuminemia, and catheter exit-site infection were the risk factors associated with early-onset peritonitis in PD patients.
Keywords: Early-onset peritonitis, outcome, peritoneal dialysis, risk factor
Peritoneal dialysis (PD)-related peritonitis is one of the most common complications during PD therapy. It has been reported that PD-related peritonitis contributes directly to ~ 20% of PD technique failures (1) and 2 – 6% of deaths (2). Prior reports of US cohorts showed low mortality rates in PD during the first year of renal replacement therapy, while the risk of peritonitis and switch to hemodialysis (HD) were high during the first 3 – 6 months (3). Also, in a recent study, early peritonitis was confirmed to be associated with a higher risk of early technique failure, which could limit the utilization of PD in end-stage renal disease (ESRD) patients (4). However, information about specific risk factors associated with heightened risk of early peritonitis in PD patients is not fully understood, and literature concerning peritonitis within the first 3 months of PD initiation is scarce. We therefore aimed to identify the risk factors associated with “early-onset” peritonitis and to evaluate its outcomes.
Subjects and Methods
Study Population
This was a retrospective observational cohort study. All incident PD patients at The First Affiliated Hospital of Sun Yat-sen University were recruited from January 1, 2006, to December 31, 2013. Inclusion criteria were age ≥ 18 years old and Tenckhoff catheters placed using sterile surgical technique in our hospital. Patients who were on PD less than 3 months, catheterized in other hospitals, transferred from permanent HD, or failed renal transplantation were excluded. Patients and their caregivers had received standard training after catheterization, and they did the exchange themselves only after successful training by primary nurses. Conventional PD solutions (Dianeal 1.5%, 2.5%, or 4.25% dextrose; Baxter Healthcare, Guangzhou, China), Y-sets and twin-bag systems were utilized in almost all of the PD patients. The study was conducted in compliance with the ethical principles of the Helsinki Declaration and approved by the Human Ethics Committees of Sun Yat-sen University.
All patients were followed up until death, transfer to HD, kidney transplantation, transfer to another center, or censoring on December 31, 2014. Baseline data at the first 1 – 3 months of the PD initiation were collected, including demographic (age [years], gender, cause of ESRD, presence of diabetes, body mass index [BMI] [kg/m2], educational level, income) and biochemical data (white blood cell [WBC], hemoglobin, neutrophil to lymphocyte [N/L] ratio in peripheral blood; serum albumin, serum creatinine, serum potassium, serum calcium, serum phosphorus, total cholesterol), PD adequacy indices (renal and peritoneal Kt/V urea [urea clearance index]), and nutritional parameters (normalized protein catabolic rate [nPCR] [g/kg/d]), which were measured in the center laboratory of our hospital. The comorbidity score was determined according to the Charlson Comorbidity Index (CCI) (5). Glomerular filtration rate (GFR) was measured as the mean of urea and creatinine clearance, calculated from 24-hour urine collections and indexed for body surface area.
Study Definitions
In accordance with the recommendations of the International Society for Peritoneal Dialysis (ISPD) guidelines, peritonitis was diagnosed if 2 of the following 3 criteria were satisfied: (1) abdominal pain or tenderness and/or cloudy fluid; (2) peritoneal effluent containing WBC count > 100 cells/mL, with more than 50% polymorph nuclear leukocytes; and (3) positive result of the effluent culture. Catheter exit-site infection (ESI) is defined by the presence of purulent drainage, with or without erythema in the catheter interface (6). Early-onset peritonitis was defined as peritonitis occurring within 3 months (90 days) after PD catheter insertion while peritonitis occurring beyond this period was classified as late-onset. Relapse was defined as an episode occurring more than 4 weeks after completion of therapy of prior episode with the same organism. Recurrence referred to an episode occurring within 4 weeks of completion of therapy of a prior episode but with a different organism (6). Complete cure was defined as the resolution of peritonitis without relapse or recurrence by antibiotics alone (7). Death-censored technique failure was defined as permanent transfer to HD because of inadequate dialysis, exit-site or tunnel infection, peritonitis, the presence of any mechanical problem or the patient's choice. Hypoalbuminemia was defined as serum albumin < 3.5 g/dL and hypokalemia defined as serum potassium < 3.5 mmol/L.
Statistical Analysis
Results were expressed as frequencies and percentages for categorical variables, mean ± standard deviation (SD) for continuous variables, and median and interquartile range (IQR) for skewed distributions. Differences between the patients with early-onset and late-onset peritonitis were tested using Chi-square test or Student's t test. Survival was calculated using Kaplan-Meier method, and differences between distributions of survival were assessed by log rank test. Univariate logistic regression model was used to determine the risk factors associated with early-onset peritonitis. Factors with p < 0.10 on univariate analysis and those thought to be related to peritonitis were chosen for multivariate logistic regression. A p value less than 0.05 was considered statistically significant. Statistical evaluation was performed using SPSS version 13.0 for Windows (SPSS, Chicago, IL, USA).
Results
Baseline Characteristics in the Study Population
During the study period, 1,777 subjects were referred to the dialysis center, and 87 subjects dropped from PD within 3 months (39 patients died, 19 patients transferred to HD, 28 patients underwent renal transplantation, and 1 transferred out of the unit), but none of these patients suffered early peritonitis. Thus, 1,690 incident PD patients included in the study were followed for a median of 32.6 months (IQR 17.2 to 50.6 months), and 503 (29.8%) patients suffered at least 1 episode of peritonitis during a cumulative follow-up period of 5,113 patient-years, of whom 118 (7.0%) developed the first peritonitis within the initial 3 months. The mean age of the population was 47.6 ± 15.4 years, 59.5% were male and 24.5% had diabetes mellitus. The most common primary renal disease was chronic glomerulonephritis (59.2%), followed by diabetic nephropathy (22.5%) and hypertension (6.4%). Additional demographic and laboratory characteristics of the study population are presented in Table 1.
TABLE 1.
Baseline Characteristics of the Study Population and Outcomes
Causative Organisms
Table 2 lists the organisms causing the first episode of peritonitis in 503 patients. Microbiological information was not reported in 59 (11.7%) episodes, and negative culture was observed in 132 (26.2%) episodes. Of the 312 cases with etiologic definition, 171 (34.0%) were caused by gram-positive organisms, 112 (22.3%) by gram-negative agents, and 26 (5.2%) by fungi. Among the gram-positive organisms in the early-onset group, the most frequent was Streptococcus (41.7%), followed by Staphylococcus epidermidis (16.7%), coagulase-negative Staphylococci (12.5%), Staphylococcus aureus (12.5%), and Enterococcus (6.2%) and other gram-positive organisms (10.4%). Among the gram-negative organisms, Escherichia was the main germ causing 59.1% of the cases, followed by Klebsiella pneumoniae (22.7%), Pseudomonas aeruginosa (4.5%), and other gram-negative organisms (13.7%). The distribution of pathogens was somewhat different in peritonitis occurring in different vintages of PD: the rate of gram-positive germ infection and sterile culture was higher in early-onset peritonitis than in late-onset (40.7% vs 31.9%; 34.7% vs 23.4%, p < 0.001). In a subgroup analysis of sterile culture rate in the early peritonitis group, the rate during the initial 2 weeks, and 3 weeks to 3 months were 47.8% and 31.6%, respectively. Fungi infection was not found in early-onset peritonitis, but accounted for 26 (6.5%) episodes of late-onset peritonitis.
TABLE 2.
Organism and Outcome of Different Vintages of Peritonitis (n, %)
Risk Factors for Early-Onset Peritonitis
Variables in Table 1 were tried in a univariate logistic regression model, and only variables with p value < 0.10 or traditional risk factors for peritonitis were depicted in Table 3. It was found that higher BMI, hypoalbuminemia, and ESI were associated with risk for the first episode of early-onset peritonitis in the univariate logistic analysis. After adjustment for confounders depicted in Table 3, higher BMI (odds ratio [OR] 1.08, 95% confidence interval [CI] 1.01 – 1.15, p = 0.034), hypoalbuminemia (OR 1.75, 95% CI 1.11 – 2.78, p = 0.017), and ESI (OR 4.14, 95% CI 2.45 – 7.00, p < 0.001) were independently associated with risk for early-onset peritonitis.
TABLE 3.
Risk Factor Associated with Early-Onset Peritonitis
Outcomes
As shown in Table 2, early-onset first episode of peritonitis had a higher cure rate (89.8% vs 80.8%), lower rate of transferring to HD (0.8% vs 9.9%), and less mortality (0.8% vs 4.2%) compared to late-onset first episode of peritonitis (p < 0.001).
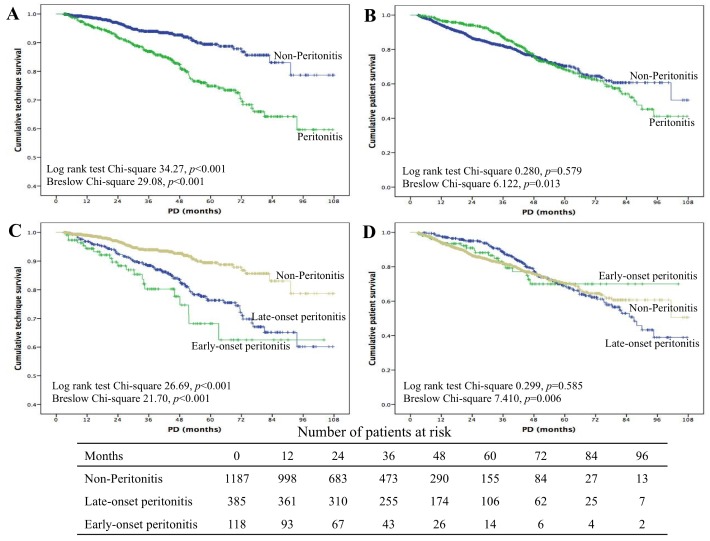
By the end of the study, 807 episodes of peritonitis occurred in 503 patients, and the peritonitis rate was 0.158 episodes per patient-year. Peritonitis occurred more frequently in the early-onset peritonitis group than in the late-onset group (0.76 vs 0.38 per patient-year, p < 0.001). In a subgroup analysis of peritonitis rate by different dialysis vintage, it was found that the rates of peritonitis during the initial 2 weeks, 3 weeks to 3 months, and after the initial 3 months were 1.4%, 5.6%, and 22.8%, respectively. Compared with non-peritonitis, technique survival was lower in the peritonitis group (log rank 34.27, p < 0.001, Figure 1A), but patient survival did not differ between the 2 groups (log rank 0.280, p = 0.579, Figure 1B). Again, subgroup analysis showed that technique survival in the early peritonitis group was lower than in the non-peritonitis as well as late peritonitis groups (log rank 26.69, p < 0.001, Figure 1C). Also, patient survival did not differ among the 3 groups (log rank 0.299, p = 0.585, Figure 1D).
Figure 1 —
Kaplan-Meier survival analysis of clinical outcomes: technique survival (A, C) and patient survival (B, D). PD = peritoneal dialysis.
Discussion
Our retrospective cohort study of 1,690 PD patients showed that 118 (7%) patients in our center presented the first episodes of peritonitis within the first 3 months of PD, which was seldom reported in studies on peritonitis. Higher BMI, hypoalbuminemia, and ESI were risk factors independently associated with early-onset peritonitis. Moreover, our results showed that patients with early-onset peritonitis had higher overall peritonitis rate and worse technique survival than those with late-onset peritonitis. However, patient survival did not differ significantly between the 2 groups during long-term follow-up.
A previous study from our center indicated that elderly patients, male gender, poor education level, and lower serum albumin were risk factors associated with the first episode of peritonitis (8). In the current study, apart from hypoalbuminemia, higher BMI and ESI were the specific risk factors associated with early-onset peritonitis. A study from the USA showed a correlation between a 5-kg/m2 increment in BMI and an 8% increased risk for peritonitis (hazard ratio [HR] 1.08; 1.04 – 1.12 per 5 kg/m2) (9); while the risk for peritonitis was not significantly different from that in patients with BMI between 20.0 – 24.9 (10). In accordance with this report, we found that increasing BMI, especially in patients with a BMI > 24.9 kg/m2 was associated with a higher risk for peritonitis in the current study. It might be hypothesized that there is an association between high BMI and peritonitis through colonization of PD catheters shortly after their insertion, resulting from increased wound area, reduced resistance of fat to infection, accentuated abdominal wall trauma stemming from a need for more vigorous retraction, and an inability to obliterate dead space in abdominal wall fat (11). The association between malnutrition and the risk for peritonitis has been widely recognized and is of special interest because a great proportion of patients were malnourished at the initiation of PD treatment (12). We found that hypoalbuminemia, as a mark of malnutrition status or inflammatory response, increased the risk of early-onset peritonitis by 75% in the current study. It was reported that low albumin levels were associated with a higher risk for peritonitis (HR 1.67; per 10 g/L decrease (11) and OR 1.2 per 1 mg/L (13), respectively), which is consistent with our results. It is generally accepted that ESI may lead directly to peritonitis. The suggested mechanism includes a movement of bacteria from the exit site into the peritoneum via peri-luminal migration along the PD catheter tunnel. The risk of peritonitis in patients following an ESI was significantly higher compared with those with no previous ESI (14), and the increased risk was particularly pronounced with gram-positive organisms such as S. aureus and coagulase-negative Staphylococcus (15). Recently, a post hoc analysis of a randomized controlled trial (RCT) demonstrated a very high risk of peritonitis 15 days post-ESI (HR 11.1; 95% CI 4.9 – 25.1) (16), similar to our findings in early-onset peritonitis.
The current study identified that early-onset peritonitis was usually caused by gram-positive organisms, and the overall peritonitis rate was twice as high as that of the late-onset group, which is consistent with findings by Fourtounas et al. (17). They observed that patients who developed peritonitis within the first year of PD start had both higher peritonitis rates and more gram-positive peritonitis than did their PD counterparts who developed their first episode of peritonitis later (17), presumably due to contamination of exchange (18). Another study from Taiwan also reported that Streptococcus species were the most common organisms in the early-onset group (19).The explanation of high overall peritonitis rates in the early peritonitis group might be a high risk of pathogenic colonization in the catheter, which may lead to subsequent peritonitis (19). Interestingly, early-onset peritonitis has a higher cure rate but lower technique survival. Several reasons could explain this phenomenon. Firstly, as shown in Table 1, early-onset peritonitis is mainly caused by gram-positive organisms and less fungi infection compared with late-onset peritonitis, which may contribute to its higher cure rate. Secondly, during the follow-up period, patients with early peritonitis exhibited an increased peritonitis rate. It is well documented that frequent peritonitis is a risk factor for peritoneal membrane deterioration and therefore affects technique survival (20). Thirdly, patients with early peritonitis may be in poor health. As shown in this study, patients in the early peritonitis group exhibited a lower serum albumin level and higher proportion of hypokalemia, either of which can negatively impact patient outcomes.
The main strength of this study is the fact that it is a large cohort analysis of incident PD patients, incorporating several potentially important demographic and socioeconomic factors beyond relevant clinical pre-existing conditions. Moreover, this is the first study designed to address whether these factors are predictors of early-onset peritonitis risk. However, our study has several limitations. Firstly, our data set did not include data on individual patients' training (according to different educational level), dedicated space to dialysis procedure, catheter exit-site care and appropriate dialysis procedure, all of which could contribute to the higher risk of peritonitis. Also, in this study there were a high proportion of culture-negative episodes, which may result from the use of antibiotic in the local hospital before transfer to our hospital. In this study, 53 (10.5%) patients had a history of prior antibiotic use. Another reason for a higher sterile culture rate in early-onset peritonitis might be associated with prophylactic antibiotics administered before catheterization. In this study, it was found that the sterile culture rate during the initial 2 weeks was higher than that of 3 weeks to 3 months (47.8%, 31.6%). However, it should stimulate the review of laboratory methods currently utilized and generate new protocols to improve positive culture rate. It is also noticeable that this is only a single center in Southern China, which may lack generalization to other ethnicities and race.
In conclusion, the present study demonstrated that higher BMI, hypoalbuminemia, and ESI were the independent risk factors associated with early-onset peritonitis. Also, we identified that patients with early-onset peritonitis had higher peritonitis rates compared to those with late-onset peritonitis during a median of 32.6 months follow-up. An improved understanding of the characteristics and risk factors associated with the development of early-onset peritonitis allows for establishing prevention measures and delaying its appearance as long as possible.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
We thank all doctors and nurses in our PD center for their patient care and data collection.
This work was supported by grants from the Key Clinical Program of the Ministry of Health, China (2010-439), Natural Science Foundation of China (81570614), the National Key Technology R&D Program (2011BAI10B08), and the Guangdong Natural Science Foundation of China (2014A030313139, 2015A030310252).
REFERENCES
- 1. Brown F, Gulyani A, McDonald S, Hurst K. Chapter 6: peritoneal dialysis. In: ANZDATA 2012 Annual Report. 35th ed. http://www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c06_peritoneal_v3.pdf Accessed November 21, 2013.
- 2. Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012; 23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pulliam J, Li NC, Maddux F, Hakim R, Finkelstein FO, Lacson E., Jr First-year outcomes of incident peritoneal dialysis patients in the United States. Am J Kidney Dis 2014; 64:761–9. [DOI] [PubMed] [Google Scholar]
- 4. Béchade C, Guittet L, Evans D, Verger C, Ryckelynck JP, Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant 2014; 29:2127–35. [DOI] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 6. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 7. Feng X, Yang X, Yi C, Guo Q, Mao H, Jiang Z, et al. Escherichia coli peritonitis in peritoneal dialysis: the prevalence, antibiotic resistance and clinical outcomes in a south China dialysis center. Perit Dial Int 2013; 34:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan X, Huang R, Wang J, Ye H, Guo Q, Yi C, et al. Risk factors for the first episode of peritonitis in southern Chinese continuous ambulatory peritoneal dialysis patients. PLOS ONE 2014; 9:e107485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 2004; 24:340–6. [PubMed] [Google Scholar]
- 10. Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology 2005; 10:192–7. [DOI] [PubMed] [Google Scholar]
- 11. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005; 25:374–9. [PubMed] [Google Scholar]
- 12. Prasad N, Gupta A, Sharma RK, Sinha A, Kumar R. Impact of nutritional status on peritonitis in CAPD patients. Perit Dial Int 2007; 27:42–7. [PubMed] [Google Scholar]
- 13. Oygar DD, Yalin AS, Altiparmak MR, Ataman R, Serdengecti K. Obligatory referral among other factors associated with peritonitis in peritoneal dialysis patients. Nefrologia 2011; 31:435–40. [DOI] [PubMed] [Google Scholar]
- 14. Abraham G, Savin E, Ayiomamitis A, Izatt S, Vas SI, Mathews RE, et al. Natural history of exit-site infection (ESI) in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1988; 8:211–6. [Google Scholar]
- 15. Lloyd A, Tangri N, Shafer LA, Rigatto C, Perl J, Komenda P, et al. The risk of peritonitis after an exit-site infection: a time-matched, case-control study. Nephrol Dial Transplant 2013; 28:1915–21. [DOI] [PubMed] [Google Scholar]
- 16. Van Diepen ATN, Tomlinson GA, Jassal SV. The association between exit-site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol 2012; 7:1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fourtounas C, Savidaki E, Dousdabanis P, Hardalias A, Kalliakmani P, Papachchristou E, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial 2006; 22:50–4. [PubMed] [Google Scholar]
- 18. Piraino B. Peritonitis as a complication of peritoneal dialysis. J Am Soc Nephrol 1998; 9:1956–65. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh YP, Chang CC, Wen YK, Chiou PF, Yang Y. Predictors of peritonitis and the impact of peritonitis on clinical outcomes of continuous ambulatory peritoneal dialysis patients in Taiwan—10 years' experience in a single center. Perit Dial Int 2014; 34:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown MC, Simpson K, Kerssens JJ, Mactier RA, Scottish Renal Registry Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post millennium (2000–2007). Perit Dial Int 2011; 31:639–50. [DOI] [PubMed] [Google Scholar]