Abstract
In the presented case, the authors describe an obese middle-aged man that presented to the emergency department for persistent oedema, scleral icterus and fatigue. He was admitted to the hospital and diagnosed with liver cirrhosis via transjugular liver biopsy. He continued to bleed from the biopsy site for 5 days from accelerated intravascular coagulation and fibrinolysis (AICF) requiring multiple transfusions of packed red blood cells, fresh-frozen plasma and cryoprecipitate. The authors then used thromboelastography (TEG) to further characterise the patient's coagulopathy, which revealed platelet inhibition. The results of the TEG significantly changed future transfusion management. Finally, the authors conducted a literature review to summarise the current literature available for the use of TEG in the management of liver cirrhosis with AICF.
Background
Hepatic cirrhosis results in complex changes of haemostasis due to an imbalance between anticoagulant and procoagulant factors. Conventional coagulation tests (ie, prothrombin time (PT) and activated partial thromboplastin time (aPTT)) do not accurately predict the risk of bleeding because they only assess procoagulant factors. Thromboelastography (TEG) characterises the interaction between procoagulant factors, anticoagulant factors, platelets and the fibrinolytic system and has potential for assessing bleeding risk and guiding therapy.1
TEG is a popular means of monitoring for haemostasis and transfusion management in major surgery, trauma and haemophilia.2 TEG is performed with whole blood and assesses the viscoelastic property of clot formation under low shear conditions. TEG can be performed with different activators and inhibitors, at various concentrations, and characterises the most important factors for clot formation.3 Although a useful tool, TEG is not yet widely used in the management of cirrhosis. Some suggest that TEG may have use in characterising and assisting in the management of patients with cirrhosis. In the presented case, we describe the application of TEG in an attempt to understand and manage a patient with cirrhosis and haemostatic complications. We attempt to answer the clinical question, ‘In an actively bleeding patient with liver disease, does TEG testing improve morbidity, mortality, blood product utilization, or cost, compared with conventional coagulation parameters?’.
Case presentation
An obese middle-aged man presented to the emergency department (ED) for new-onset painless jaundice and worsening lower extremity oedema over the previous 6 months. He intentionally lost 40 lbs one year prior, but in the 3 months leading to presentation had gained all of the weight back, despite no behavioural changes. One month before presentation, he saw his primary care manager who prescribed furosemide 20 mg daily, which was then increased to 40 mg daily 2 weeks later. He presented to the ED because of yellowing of his eyes and persistent oedema. He denied dyspnoea, orthopnoea, cough, fevers, chills, night sweats, nausea or vomiting. He reported recent onset of amber-coloured urine, but denied dysuria, urgency, frequency, nocturia or hesitancy. The remainder of the review of systems was unremarkable.
Past medical and surgical history were significant for sickle cell trait, obstructive sleep apnoea controlled with the use of nocturnal continuous positive airway pressure and gastro-oesophageal reflux managed with ranitidine 150 mg as needed. He took no other medications and had no known drug allergies.
His family and social history were significant for multiple direct relatives with lung and breast cancer. He reported consumption of six to eight alcoholic drinks per week and was a former smoker.
All of the vital signs were within normal limits. Physical examination was notable for scleral icterus, a distended abdomen and diffuse oedema with 4+ pitting oedema of the bilateral lower extremities, 2+ pitting oedema over the sacrum and 1+ pitting oedema extending to the midthorax.
An echocardiogram was normal and demonstrated an ejection fraction of 65–70%. Abdominal CT scan revealed a cirrhotic morphology of the liver, a normal-sized spleen, mesenteric oedema, diffuse anasarca and markedly dilated collateral vessels secondary to portal hypertension. Transjugular biopsy of the liver on hospital day 7 (HD7) confirmed cirrhosis. The aetiology of cirrhosis was suspected to be non-alcoholic steatohepatitis; however, alcoholic cirrhosis could not be ruled out. The patient continued to ooze blood from the transjugular biopsy site for 5 continuous days, despite hours of manual pressure, compression bandages and Quick-clot haemostatic dressings. The patient's daily coagulation abnormalities persisted with a PT of 25–30 s, aPTT of 45–52 s and international normalised ratio (INR) of 2.3–2.7, presumably from his liver disease. He was not on prophylactic or therapeutic anticoagulation and was not taking any antiplatelet agents. Labs were otherwise notable for a consistently low fibrinogen of 120–170 mg/dL, an elevated d-dimer of 10.49 μg/mL (laboratory reference range <0.5 μg/mL) and declining platelets from 108 000 the day of the biopsy (HD7) to 78 000/μL on HD11. Factor VIII level was 380% of the normal value. Additional studies can be seen in table 1.
Table 1.
Additional laboratory studies
| Test: CBC W/Diff (day: HD1) | Result | Units | Ref Rng | Fibrin degradation products (HD2) | Result | Units | Ref Rng |
| WCC | 9.4 | ×103/μL | 3.6–10.6 | Fibrinogen degradation products | 20 (H) | μg/mL | <5 |
| RBC | 2.95 (L) | ×106/μL | 4.21–5.92 | IgA+IgG+IgM panel (HD2) | Result | Units | Ref Rng |
| Haemoglobin | 8.0 (L) | g/dL | 12.8–17.7 | IgA | 905 (H) | mg/dL | 70–400 |
| Haematocrit | 24.3 (L) | % | 37.5–50.9 | IgG | 3804 (H) | mg/dL | 700–1600 |
| MCV | 82.3 | fL | 79.5–96.8 | IgM | 138 | mg/dL | 40–230 |
| Platelets | 165 | ×103/μL | 162–427 | Kappa+lambda light chains free W/kappa/lambda ratio (HD2) | Result | Units | Ref Rng |
| Comprehensive metabolic panel (HD1) | Result | Units | Ref Rng | Immunoglobulin light chains kappa free | 138.44 (H) | mg/L | 3.30–19.40 |
| Sodium | 139 | mmol/L | 136–145 | Immunoglobulin light chains lambda free | 55.55 (H) | mg/L | 5.71–26.30 |
| Potassium | 4.1 | mmol/L | 3.5–5.1 | Immunoglobulin light chains kappa/lambda | 2.49 (H) | 0.26–1.65 | |
| Chloride | 100 | mmol/L | 98–107 | α-1-antitrypsin (HD3) | Result | Units | Ref Rng |
| Carbon dioxide | 23 | mmol/L | 22–29 | α-1-antitrypsin | 116 | mg/dL | 90–200 |
| Urea nitrogen | 14.3 | mg/dL | 6–20 | HIV-1/O/2 Ab (HD3) | Result | ||
| Creatinine | 1.2 | mg/dL | 0.7–1.2 | HIV-1/O/2 Ab | Negative | ||
| Glucose | 110 (H) | mg/dL | 74–106 | Hepatitis virus panel acute (HD2) | Result | ||
| Alkaline phosphatase | 140 (H) | U/L | 40–129 | Hepatitis B virus surface Ag | Non-reactive | ||
| Alanine aminotransferase | 24 | U/L | 0–41 | Hepatitis B virus surface Ab | Reactive | ||
| Aspartate aminotransferase | 73 (H) | U/L | 0–40 | Hepatitis B virus core Ab IgM | Non-reactive | ||
| Total bilirubin | 7.4 (H) | mg/dL | 0.15–1.2 | Hepatitis B virus core Ab IgG | Reactive | ||
| Direct bilirubin | 2.9 (H) | mg/dL | <0.3 | Hepatitis A IgM | Non-reactive | ||
| Protein | 8.6 | g/dL | 6.6–8.7 | Hepatitis C virus Ab | Non-reactive | ||
| Albumin | 2.4 (L) | g/dL | 3.5–5.2 | Hepatitis B virus quant PCR (HD2) | Result | Units | Ref Rng |
| Coagulation studies (HD1) | Result | Units | Ref Rng | Hepatitis B copies/mL | None detected | IU/mL | |
| Fibrinogen | 138 (L) | mg/dL | 207–454.0 | Liver kidney microsomal Ab (HD14) | Result | Units | Ref Rng |
| Protime | 31.5 (H) | s | 11.8–14.6 | Liver kidney microsomal Ab | 3.5 | Units | 0.0–20.0 |
| APTT | 62.4 (H) | s | 23.8–35.5 | Mitochondrial Ab (HD3) | Result | Units | Ref Rng |
| Thrombin time | 23.8 (H) | s | 14.0–20.0 | Mitochondrial Ab | Negative | Negative | |
| INR | 3 | 2.0–4.5 | Nuclear Ab panel (HD2) | Result | Units | Ref Rng | |
| Fibrin D-Dimer | 10.49 (H) | μg/mL | <0.5 | Nuclear Ab | Positive (H) | Negative | |
| Probrain natriuretic peptide N-terminal (HD1) | Result | Units | Ref Rng | Nuclear Ab titre | Positive at 1:160 dilution (H) | Titre units | Negative <1:80 |
| Pro-BNP | 928.0 (H) | pg/mL | (5.0–125.0) | Nuclear Ab pattern | Diffuse | ||
| Epstein-Barr virus panel (HD16) | Result | Units | Ref Rng | Smooth muscle Ab panel (HD14) | Result | Units | Ref Rng |
| Epstein-Barr virus capsid Ab IgG | >600.0 (H) | U/mL | 0.0–17.9 | Smooth muscle Ab | <1:10 dilution | Titre units | Negative |
| Epstein-Barr virus nuclear Ab IgG | >600.0 (H) | U/mL | 0.0–17.9 | Soluble liver Ab (HD 3) | Result | Units | Ref Rng |
| Factor VIII activity (HD3) | Result | Units | Ref Rng | Soluble liver antigen Ab | 3.2 | Units | 0.0–20.0 |
| Factor VIII activity actual/normal | 380 (H) | % | (50–150) | ||||
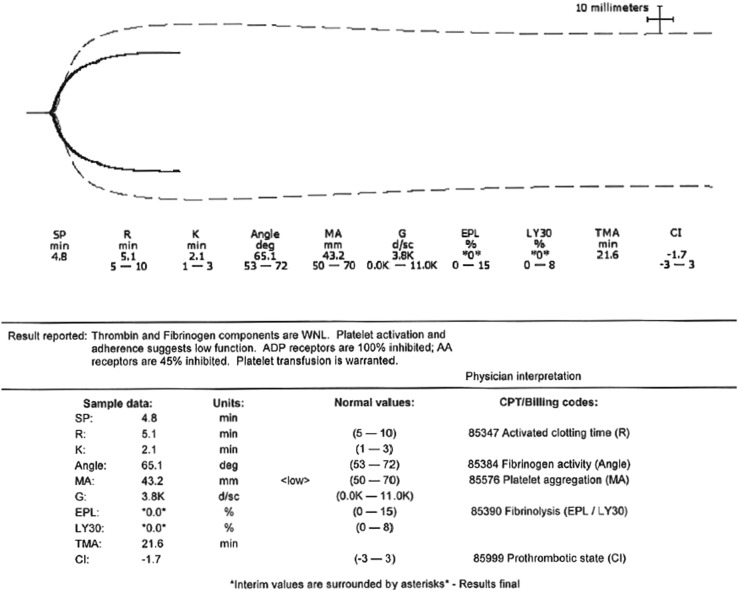
The patient required multiple transfusions from HD7 to 11 in accordance with current transfusion guidelines, including 7 units of packed red blood cells (pRBC), 6 units of fresh-frozen plasma (FFP) and 2 units of cryoprecipitate, with no improvement in the bleeding from the transjugular biopsy site. A thromboelastogram (TEG) was then performed to further characterise the patient's bleeding diathesis on the evening of HD11. The TEG revealed that thrombin and fibrinogen were within normal limits; however, platelet activation and adherence suggested low function. ADP receptors were 100% inhibited; arachidonic acid (AA) receptors were 45% inhibited (figure 1). The TEG indicated the need for platelet transfusion, given platelet inhibition and function, independent of total platelet number (figure 2). Platelet transfusion was then initiated and the biopsy site bleeding temporarily ceased. The amount of future blood product transfusions was significantly reduced. From HD12 to 17, the patient only required 2 units of pRBC (71% reduction), 0 units of FFP (complete reduction), 1 unit of cryoprecipitate (100% reduction) and 4 units of platelets.
Figure 1.
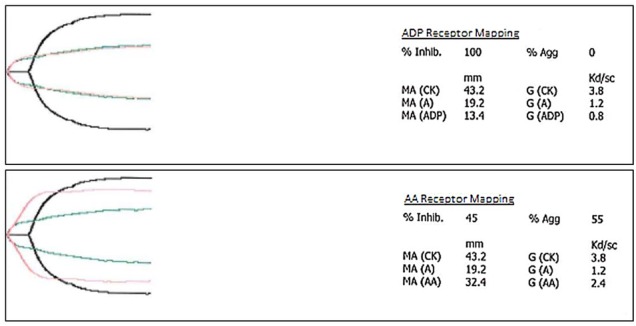
Thromboelastography (TEG) platelet receptor mapping function report. ADP or AA receptors can be isolated in TEG to determine their function by adding ADP or AA to the whole blood sample, respectively. These receptors are often tested to determine if a patient is on antiplatelet agents like clopidogrel, prasugrel, ticagrelor (ADP receptors) or aspirin (AA receptors). The patient's ADP receptors were 100% inhibited and AA receptors were 45% inhibited. The patient was not taking any antiplatelet agents; his platelet dysfunction was attributed to his high levels of fibrin degradation products that have been shown to inhibit ADP receptors. High levels of fibrin degradation products are a hallmark of accelerated intravascular coagulation and fibrinolysis. AA, arachidonic acid; ADP, adenosine diphosphate; TEG, thromboelastography.
Figure 2.
Final thromboelastography (TEG) report. The TEG reveals a low maximum amplitude (MA), which is a marker for platelet aggregation and maximum clot strength. Platelet activation and adherence are reported as inhibited, suggesting low function and warranting a platelet transfusion. Thrombin and fibrinogen are within normal limits. This report changed our management because current transfusion guidelines do not recommend a platelet transfusion in a bleeding patient with a platelet count >50 000/μL with normal levels of fibrinogen and thrombin. TEG, thromboelastography; MA, maximum amplitude.
Outcome and follow-up
On HD17, the patient began to have bright red blood per rectum with an estimated loss of 1000 mL in a 3-hour period. He was transferred to the medical intensive care unit (MICU) where he received further blood products. Computed topography revealed a ruptured bowel diverticulum that was not apparent on initial imaging when the patient was admitted to the hospital. In the MICU, he began to show signs of hepatorenal syndrome, and with increasing model for end-state liver disease (MELD) score on HD20, he was transferred to a regional liver transplant centre. The regional liver transplant centre continued to medically manage the patient with multiple transfusions of pRBC, platelets and FFP. Once stabilised, he was discharged home from the regional liver transplant centre to continue treatment on an outpatient basis. Unfortunately, the patient died before receiving a liver transplant.
Discussion
Multiple studies have described the complex haematological changes in liver disease.1 4–16 High-quality data are lacking in predicting bleeding risk in this disease, but many studies agree that conventional coagulation studies (PT, aPTT, INR) are not specific for predicting bleeding risk in liver disease.1 4–16 Additionally, evidence-based guidelines regarding acute therapy for haemorrhage in liver disease are lacking.1 This presents a challenge for practicing physicians in clinical care and cost. We performed a literature search with the clinical question, ‘In an actively bleeding patient with liver disease, does TEG testing improve morbidity, mortality, blood product utilization, or cost, compared with conventional coagulation parameters?’
Our literature search was conducted with the help of a clinical librarian. Using medical subject headings (MeSH), we searched the US National Library of Medicine MEDLINE database via PubMed.gov for any published literature with the following criteria: ((liver(tw) AND cirrho*(tw)) OR ‘Liver Cirrhosis’(mh) OR ‘End Stage Liver Disease’(mh) OR (end stage(tw) AND (liver disease(tw) OR liver failure(tw)))) AND (thromboelast*(tw) OR ROTEG(tw) OR ROTEM(tw) OR ‘Thromboelastography’(mh)). We found 16 publications in the MEDLINE search. We also broadened our search using the Embase database which found an additional 10 abstracts, for a total of 26 publications. Four of the 26 publications were interim updates before a final research study publication, resulting in 22 unique abstracts found. These consisted of 12 research studies, 6 conference abstracts and 4 review or editorial articles. Fifteen of these 22 publications addressed our specific clinical question.
In our search, 13 of the 22 publications suggested that TEG testing could predict bleeding risk and guide transfusion therapy in patients with liver disease.1 5–11 13–15 In a study of 273 patients with cirrhosis, Stravitz14 conclude that TEG studies can be within normal limits in patients with stable cirrhosis, despite abnormal conventional coagulation studies, suggesting that TEG may be more specific at detecting bleeding risk than conventional coagulation studies. Premkumar et al10 described TEG as a strong predictor of bleeding (OR 2.1, p=0.05) and mortality (OR 3.1, p=0.05) in a trial of 114 patients (mean age 44.3±11.7 years, males 90%) with acute-on-chronic liver failure.
De Pietri et al17 reported that even with factoring in additional costs with performing a TEG test on every patient with liver disease, overall transfusion costs were lower in the patient population that received a TEG test compared with those with conventional coagulation testing only.
Other studies have suggested that TEG may be useful in predicting the risk or presence of infection in hospitalised patients with cirrhosis. Montalto et al18 identified one possible mechanism as an exogenous heparin effect induced by infection. TEG parameters became more hypocoagulable in patients, even days before an infection was identified.8 14 TEG parameters for patients that did not develop an infection did not change and conventional tests did not change in either group.8 14 18 This suggests that TEG may be a more sensitive test for determining changes in coagulation status in patients with cirrhosis.8 14 18
Conventional coagulation studies are often inadequate for managing bleeding in a patient with liver disease because these studies do not account for the role of platelets in haemostasis. Platelet dysfunction is widely reported in liver disease and cirrhosis, but specific dysfunctions cannot reliably be predicted.1 The TEG reported that our patient's ADP receptors were 100% inhibited. ADP receptors, also known as the P2Y12 and P2Y1 receptors, must be activated in order for platelet aggregation and conformational changes to occur.19 Additionally, fibrin degradation products competitively inhibit the ADP receptors on platelets.20
Some studies have attempted to characterise a pattern of predicting whether a patient with cirrhosis will be hypercoagulable or hypocoagulable, and initial data indicate that there is a statistically significant association with cirrhosis secondary to primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC). Stravitz14 reported that hypercoagulable TEGs were detected in 43% of patients with PSC, 23% of patients with PBC, 5% of patients with non-cholestatic liver disease and 0% in healthy controls. In contrast, conventional coagulation studies did not differ between patients with cholestatic compared with non-cholestatic liver disease.14 Pihusch et al9 observed a similar relationship; that cholestatic disease predisposes patients to a hypercoagulable state in a cohort of 37 non-cirrhotic patients with PBC or PSC compared with 53 cirrhotic patients with hepatitis C. New et al21 also observed this hypercoagulable relationship in PBC and PSC patients in their study of 164 patients who had liver transplants.
Conclusion
We chose to perform a TEG because our patient continued to bleed from his biopsy site for 5 days, despite multiple transfusions of pRBC, FFP and cryoprecipitate. Current guidelines recommend initiating platelet transfusion in an actively bleeding patient when platelet counts are under 50 000/μL.22 The TEG results indicating severe platelet dysfunction had a direct impact on the management of the patient, suggesting that the 50 000/μL threshold for platelet transfusion in a bleeding patient may not be applicable to patients with advanced liver disease.
This case demonstrates the potential utility of TEG in a patient with cirrhosis and active bleeding, and that TEG could improve bleeding risk estimations and patient care while reducing costs and complications in the management of liver disease. The major limitations of TEG include lack of standardisation among test protocols, availability of TEG machines and training of technicians. Further research should be conducted in a large setting to further characterise the utility of TEG in managing acute bleeding in patients with liver disease.
Learning points.
Thromboelastography (TEG) shows potential to improve bleeding risk estimations, patient care and reduce cost in managing liver disease.
TEG is a sensitive, specific and fast test that can significantly affect patient management.
Current transfusion guidelines state that the 50 000/μL threshold for platelet transfusion in a bleeding patient may not be applicable to patients with advanced liver disease.
The major limitations of TEG include lack of standardisation among test protocols, availability of TEG machines and training of technicians.
Acknowledgments
A special thanks to Sarah Cantrell, Darnal Medical Library Clinical Librarian, for her help and collaboration in making this literature search possible.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Army or Navy, Department of Defense, or the United States Government.
Contributors: TJP wrote the article and reviewed all of the articles in the literature search. BV and AMBW reviewed the articles in the literature search and edited the original manuscript to prepare for submission.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematol Am Soc Hematol Educ Program 2015;2015:243–9. 10.1182/asheducation-2015.1.243 [DOI] [PubMed] [Google Scholar]
- 2.Bianchini M, De Pietri L, Di Maira T et al. Thrombelastography decreases hemoderivates requirement before invasive procedures in cirrhotic patients with coagulation disorders. A randomized controlled trial. Hepatology 2013;58:292A–3A. [Google Scholar]
- 3.Cheng R, Kanazaki R, Chen FW et al. Thromboelastography for predicting differences in cirrhosis-associated coagulopathy. J Gastroenterol Hepatol 2013;28:71.24251708 [Google Scholar]
- 4.Ben-Ari Z, Panagou M, Patch D et al. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol 1997;26:554–9. 10.1016/S0168-8278(97)80420-5 [DOI] [PubMed] [Google Scholar]
- 5.De Pietri L, Bianchini M, Montalti R et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology 2016;63:566–73. 10.1002/hep.28148 [DOI] [PubMed] [Google Scholar]
- 6.Jairath V, Hall D, Harrison P et al. Rotational thromboelastometry in cirrhosis: hypercoagulable and hyperfibrinolytic. Gut 2012;61:A30. [Google Scholar]
- 7.Lentschener C, Flaujac C, Ibrahim F et al. Assessment of haemostasis in patients with cirrhosis: relevance of the ROTEM tests?: a prospective, cross-sectional study. Eur J Anaesthesiol 2016;33:126–33. 10.1097/EJA.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 8.Papatheodoridis GV, Patch D, Webster GJ et al. Infection and hemostasis in decompensated cirrhosis: a prospective study using thrombelastography. Hepatology 1999;29:1085–90. 10.1002/hep.510290437 [DOI] [PubMed] [Google Scholar]
- 9.Pihusch R, Rank A, Gohring P et al. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol 2002;37:548–55. 10.1016/S0168-8278(02)00239-8 [DOI] [PubMed] [Google Scholar]
- 10.Premkumar M, Rangegowda D, Mirza R et al. Thromboelastography accurately interprets coagulation failure in patients with ACLF. Hepatol Int 2016;10:S173. [Google Scholar]
- 11.Shah NL, Xavier E, Northup PG et al. The use of thromboelastography platelets, and INR in a clinical model for bleeding risk in cirrhotic patients. Gastroenterology 2009;136:A795–A96. [Google Scholar]
- 12.Shin KH, Kim IS. Thromboelastographic evaluation of coagulation in patients with liver disease. Blood 2015;126:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somani VS, Amarapurkar DN. Comparing the TEG with conventional coagulation tests in cirrhotics undergoing invasive procedures. Hepatol Int 2016;10:S382. [Google Scholar]
- 14.Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (N Y) 2012;8:513–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Tripodi A, Primignani M, Chantarangkul V et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res 2009;124:132–6. 10.1016/j.thromres.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 16.Umgelter A, Mößmer G, Schmid RM et al. Thrombelastography and platelet function assay in cirrhosis and sepsis. Intensive Care Med 2009;35:S160. [Google Scholar]
- 17.De Pietri L, Bianchini M, Montalti R et al. Thrombelastography (TEG) decreases blood products requirement before invasive procedures in cirrhotic patients with coagulation tests derangement. A randomized controlled trial. Dig Liver Dis 2014;46:e5–6.24423608 [Google Scholar]
- 18.Montalto P, Vlachogiannakos J, Cox DJ et al. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol 2002;37:463–70. 10.1016/S0168-8278(02)00208-8 [DOI] [PubMed] [Google Scholar]
- 19.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther 2005;108:180–92. 10.1016/j.pharmthera.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Thorsen LI, Brosstad F, Gogstad G et al. Competitions between fibrinogen with its degradation products for interactions with the platelet-fibrinogen receptor. Thromb Res 1986;44:611–23. 10.1016/0049-3848(86)90163-5 [DOI] [PubMed] [Google Scholar]
- 21.New S, Stratton K, Benson AB et al. Associations between thromboelastography (TEG) and severity of cirrhosis. Gastroenterology 2014;146(5):S-987 10.1016/S0016-5085(14)63587-3 [DOI] [Google Scholar]
- 22.Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program 2013;2013:638–44. 10.1182/asheducation-2013.1.638 [DOI] [PubMed] [Google Scholar]