Description
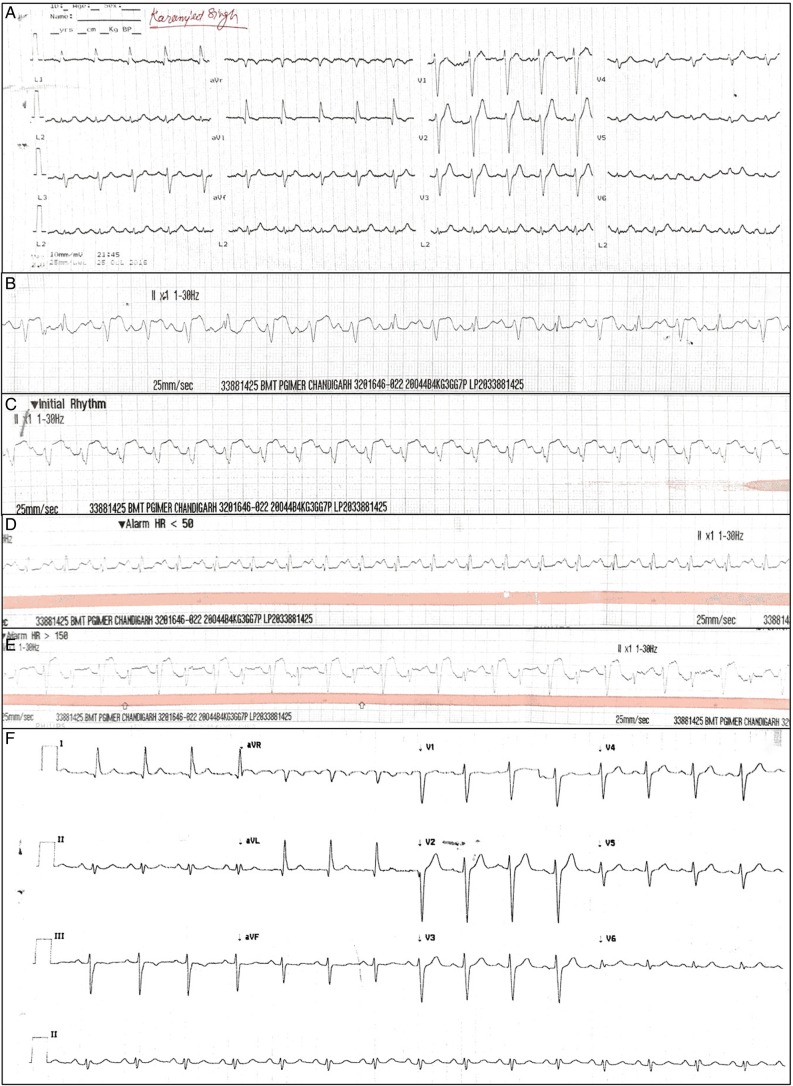
A 61-year-old man, with multiple myeloma (IgA lambda), was planned for autologous stem cell transplantation (ASCT). He also suffered a left thalamic haemorrhagic stroke in 1998 secondary to aneurysmal bleed. Following melphalan infusion of 200 mg at 45 mL/min, he developed acute onset rhythm abnormalities (intermittent ventricular ectopic and ventricular tachycardia). The patient's premelphalan evaluation was unremarkable with normal ECG (1A), ejection fraction (55%) and renal/hepatic function. He was evaluated by sequential 2D echocardiography and troponin I (immediately, 2, 4, 12 and 24 hours) which were normal excluding acute STEMI. These rhythm abnormalities were dynamic probably secondary to the plasma melphalan concentrations and normalised by 18 h postinfusion (figure 1B–F). There has been no recurrence of these rhythm abnormalities thereafter. He was transplanted with abbreviated melphalan conditioning.
Figure 1.
(A) Normal ECG before starting melphalan infusion. (B) ECG after 200 mg of melphalan infusion, showing acute onset ventricular rhythm with atrio-ventricular (AV) dissociation. Subsequent ECG showing dynamic ventricular rhythm at (C) 4 hours (D) 8 hours and (E) 12 hours postinitiation of melphalan infusion. (F) Normalisation of rhythm after 18 hours postinitiation of melphalan infusion.
Cardiac toxicity in the form of atrial fibrillation (AF, 6.6–11%) and supraventricular tachycardia are common after high dose melphalan.1 Melphalan is considered the most arrhythmogenic chemotherapeutic agent used in ASCT.1 Increased age (>60 years), higher baseline creatinine, larger left atrium size and previous cardiac comorbidities are noted risk factors for upraventricular tachycardia (SVT), of which our patient only had age as a risk factor.1 2 Other conditioning drugs in ASCT with similar cardiotoxicity include cyclophosphamide, anthracyclines and fludarabine. The presence of amyloidosis in multiple myeloma (MM) increases the risk of arrhythmias during ASCT conditioning. Investigators used amifostine to counter cardiac toxicity of increased dose melphalan (∼280 mg/m2).3 We present this case due to the rarity of ventricular arrhythmias secondary to melphalan and reversal of normal rhythm chronologically coinciding with excretion t½ of the drug.
Learning points.
Monitoring for cardiac toxicity in patients receiving high-dose melphalan is mandatory.
Melphalan should be infused with constant monitoring of rhythm, heart rate, blood pressure and oxygen saturation until four times of the excretion t½ of the drug.
Patients should be screened for the risk factors for SVT in all patients planned for high-dose melphalan therapy.
Acknowledgments
The authors thank Dr Ganesh Kasinadhuni, Cardiology senior resident, for evaluating the case from cardiology department.
Footnotes
Contributors: UY, SG, AK and PM involved in the management of the patient. UY involved in the manuscript preparation. All authors vetted the submitted manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Feliz V, Saiyad S, Ramarao SM et al. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol 2011;34:356–9. 10.1002/clc.20904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mileshkin LR, Seymour JF, Wolf MM et al. Cardiovascular toxicity is increased, but manageable, during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for patients aged 60 years and older. Leuk Lymphoma 2005;46:1575–9. 10.1080/10428190500235884 [DOI] [PubMed] [Google Scholar]
- 3.Phillips GL, Meisenberg B, Reece DE et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: a phase I study. Biol Blood Marrow Transplant 2004;10:473–83. 10.1016/j.bbmt.2004.03.001 [DOI] [PubMed] [Google Scholar]