Abstract
Objective: Endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) are safe and effective treatments for varicose veins caused by saphenous reflux. Deep venous thrombosis (DVT) and endovenous heat-induced thrombosis (EHIT) are known complications of these procedures. The purpose of this article is to investigate the incidence of postoperative DVT and EHIT in patients undergoing EVLA and RFA.
Methods: The patients were assessed by clinical examination and venous duplex ultrasonography before operation and at 24–72 hours, 1 month, and 1 year follow-up after operation. Endovenous ablation (EVA) had been treated for 1026 limbs (835 patients) using an RFA; 1174 limbs (954 patients) using a 1470-nm wavelength diode laser with radial two-ring fiber (1470R); and 6118 limbs (5513 patients) using a 980-nm wavelength diode laser with bare-tip fiber (980B).
Results: DVT was detected in 3 legs (0.3%) of RFA, 5 legs (0.4%) of 1470R, and 27 legs (0.4%) of 980B. One patient in three symptomatic DVT treated with 980B developed asymptomatic pulmonary embolus. In all, 31 of the 35 DVTs were confined to the calf veins. The incidence of EHIT classes 2 and 3 was 2.7% following RFA procedure, 6.7% after 1470R, and 7.5% after 980B.
Conclusion: The incidence of EHIT following EVA was low, especially the RFA procedure. EHIT resolves within 2–4 weeks in most patients. DVT rates after EVA were compared with those published for saphenous vein stripping. (This is a translation of J Jpn Coll Angiol 2015; 55: 153–161.)
Keywords: varicose vein, endovenous laser ablation, radiofrequency ablation, endovenous heat-induced thrombosis, deep vein thrombosis
Introduction
In Japan, endovenous laser ablation (EVLA) using 980-nm wavelength diode laser with bare-tip fiber (980B) has become covered under health insurance as a treatment for saphenous-type varicose veins in 2011. In 2014, EVLA using 1470-nm wavelength diode laser with radial two-ring fiber (1470R) and radiofrequency ablation (RFA) using a Closure FAST catheter (VNUS Medical Technologies, Inc., San Jose, CA, US) also have become covered under health insurance. In this study, we examined the course and correlation of thromboembolic complications, such as endovenous heat-induced thrombosis (EHIT) and deep vein thrombosis (DVT), after endovenous ablation (EVA) with respect to therapeutic devices.
Subjects and Methods
We investigated 8318 limbs (980B group: 6118 limbs, 1470R group: 1174, and RFA group: 1026) with primary varicose veins treated by EVA in our single clinic between January 2011 and March 2015. There were no differences in the patient background before surgery among the three groups (Table 1). The great saphenous vein (GSV) was treated in 6737 limbs, the small saphenous vein (SSV) in 1713 limbs, and the accessory saphenous vein (ASV) in 418 limbs. Concerning EVA, there was no patient with crossectomy, high ligation, or intraoperative sclerotherapy. The EVA indication rates were 91.5% in 2011, 95.3% in 2012, 96.9% in 2013, and 97.4% (as of April) in 2014. In the 980B group, the proportion of patients for whom EVLA was indicated increased. Overall, it was 95.1%. From May 2014, EVLA with 980B was not performed, and EVA with 1470R or RFA was indicated for 99.4%, showing an increase. All patients for whom EVA was not indicated underwent saphenous vein stripping under local anesthesia.
Table 1.
Demographic data for patients
| Group | RFA | 1470R | 980B | P* |
|---|---|---|---|---|
| Number of limbs | 1026 | 1174 | 6118 | |
| Gender (men:women) | 241:594 | 243:711 | 1555:3958 | NS |
| Mean age (range) | 60 ± 11 years (20–82) | 62 ± 11 years (22–87) | 60 ± 10 years (21–84) | NS |
| Treated veins | ||||
| GSV | 911 | 938 | 4888 | NS |
| SSV | 136 | 254 | 1323 | 0.001 |
| ASV | 20 | 64 | 334 | 0.001 |
| CEAP classification | ||||
| C2 and C3 | 830 | 965 | 4989 | NS |
| C4, C5, and C6 | 196 | 209 | 1129 | NS |
GSV: great saphenous vein; SSV: small saphenous vein; ASV: accessory saphenous vein; CEAP: Clinical Ethiological Anatomical Pathological classification; RFA: radiofrequency ablation; 1470R: endovenous laser ablation using 1470 nm diode lazer with radial 2rings fiber; 980B: endovenous laser ablation using 980 nm diode lazer with bare-tip fiber Value are number of limbs.
* Unpaired t test or χ2 test, NS: not significant
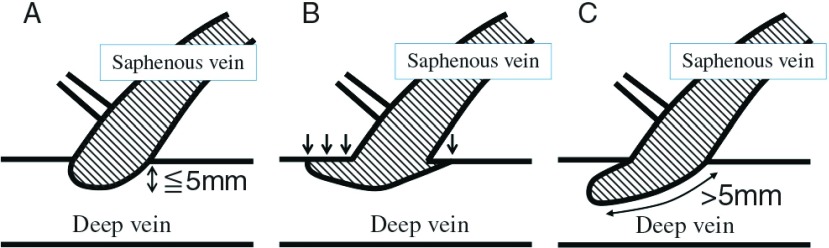
EHIT was classified into four types: Class 1, 2, 3, and 4 (C1, C2, C3, and C4, respectively) according to the classification proposed by Kabnick et al.1) In particular, C2 was morphologically classified into three subtypes: EHIT with a ≤5 mm protrusion into the deep vein lumen (protrusion type), EHIT adhering to the deep vein wall (creeping type), and EHIT protruding (length: >5 mm)/floating into the deep vein lumen (floating type) for the following reasons: the morphology and grade of C2 vary based on its definition, and therapeutic strategies differ among institutions. We compared the postoperative course among these types (Fig. 1). In patients with EHIT deterioration during follow-up, the most advanced state was regarded as EHIT.
Fig. 1.
Subclassification of endovenous heat-induced thrombus class 2. (A) protrusion type, (B) creeping type, (C) floating type.
DVT on the central side, involving the popliteal vein, was regarded as proximal type, and DVT peripheral to the popliteal vein as distal type. Its association with EHIT was examined.
EVA was indicated for primary varicose veins of the lower limbs with symptoms or congestive dermatitis according to the “Guidelines for Endovascular Treatment for Varicose Veins of the Lower Limbs”2) prepared by the Japanese Society of Phlebology. Venous incompetence or the postoperative state was evaluated using duplex ultrasonography (DUS) by vascular laboratory technicians. In each group, some unapproved medical instruments were used for a specific period, but health-insurance-covered or private practice was selected based on the patients’ wishes at that time. Others were covered under health insurance. With respect to indication criteria in our hospital, EVA with 980B was indicated for patients with a mean saphenous vein diameter of ≤9 mm, saccular venous aneurysm size of ≤15 mm in an area adjacent to the deep vein junction, or spindle venous aneurysm size of ≤20 mm. EVA with 1470R or RFA was indicated for those with a mean saphenous vein diameter of ≥9 mm, saccular venous aneurysm size of ≤20 mm in an area adjacent to the deep vein junction, or spindle venous size of ≤25 mm.
Ultrasonographs
For preoperative and postoperative DUS, a Xario (Toshiba Medical Systems, Tokyo, Japan) was used. For preoperative marking and intraoperative operations, iLook25 (SonoSite Japan, Tokyo, Japan) and Venue 40 Anesthesia (GE Health Care Japan, Tokyo, Japan) systems were used.
Laser devices and optical fiber
In the 980B group, 980-nm wavelength diode laser (Ceralas E980/15W, CeramOptec GmbH, Germany) and bare-tip fiber (fiber) were used. In the 1470R group, 1470-nm wavelength diode laser (Ceralas E1470/15W, CeramOptec GmbH) and radial two-ring fiber (ELVeS Radial-2ring fiber, CeramOptec GmbH) were used.
RFA systems and catheters
In the RFA group, a VNUS Closure FAST system (VNUS Medical Technologies, Inc., San Jose, CA, USA) consisting of an ablation catheter (catheter) with a shaft diameter of 7 Fr and generator was used.
Surgical procedures
Before surgery, marking the saphenous vein to be treated and varicose veins that could be confirmed under visual examination was performed under DUS. The patients were placed in a fowler position, and blood pressure, electrocardiogram, and oxygen saturation monitoring were performed using an automatic blood pressure meter, electrocardiographic monitor, and pulse oximeter, respectively. Tumescent local anesthesia (TLA) was basically selected. To avoid pain on puncture under TLA, local anesthesia was conducted at a site to be punctured using a 30G needle. Immediately before the start of surgery, propofol at 20 to 40 mg was intravenously injected to relieve patient tension.
For venous access, ultrasound-guided percutaneous approach was basically adopted. When puncture was difficult, minor incision methods were selected. In the 980B and 1470R groups, an introducer sheath (sheath) was inserted after guidewire insertion. In the RFA group, an 11-cm sheath was inserted after guidewire insertion, and a catheter was directly inserted into the saphenous vein through the sheath. If the insertion was difficult, then a guidewire measuring 0.025 inches in outer diameter was concurrently used. Subsequently, the position was changed to a head-down tilt, and TLA was started. TLA solution was prepared by mixing physiological saline at 500 mL with epinephrine-containing lidocaine (1% Xylocaine E) at 40 mL and sodium bicarbonate (7% Meylon) at 10 mL, with a total volume of 550 mL. Under ultrasound guidance, it was accurately infused into the saphenous compartment using a pump for TLA (Klein Pump; HK Surgical, Inc., USA). The fiber or catheter tip was placed just distal to the orifice of the superficial epigastric vein in the GSV or at a site not contacting the tibial nerve on the distal to the deep vein junction in the SSV. In the 980B and 1470R groups, while observing ablation of the vein under ultrasound, the laser energy was delivered manually pulling the optical fiber so as to adjust the linear endovenous energy density (LEED) to 70–100 J/cm. The laser power using a bare-tip and radial fiber was set at 8–10 and 10W, respectively. In the RFA group, ablation at 120°C for 20 seconds was conducted as a single session of basic ablation, and, on the proximal segment 2, the same site was ablated twice. When the saphenous vein diameter was large, additional ablation was performed based on surgeons’ evaluation. Following EVA, branch varicosities were resected with the stab avulsion method if necessary.
Postoperative compression therapy and follow-up
An elastic stocking over an elastic bandage was applied for 24–48 hours postoperatively, followed by wearing elastic stocking alone for 3 weeks. An oral analgesic was administered for 5 days, and an antibiotic for 3 days. Postoperative follow-up was basically performed within 48 hours after surgery, after 1 month, and after 1 year. If necessary, additional follow-up was conducted, and DUS was simultaneously performed. When DUS revealed EHIT, follow-up with elastic stockings alone was conducted for patients with C1, and additional ultrasonography was performed 1 week after the start of follow-up with elastic stockings alone for those with C2. The heparin and following oral administration of an anticoagulant, as well as follow-up by DUS were additionally conducted for those with C3. For those with C4 or DVT, hospital treatment was selected.
Results
In the RFA group, the mean frequency of ablation was 7.3 ± 1.9 times. The mean LEED in the 980B and 1470R groups was 86 ± 19 and 89 ± 22 J/cm, respectively. There were no differences in the mean cauterized vein length, mean cauterized saphenous vein diameter, or TLA solution volume among the three groups. The mean ablation time in the 980B, 1470R, and RFA groups was 348 ± 121, 266 ± 112, and 146 ± 39 seconds, respectively; it was significantly shorter in the RFA group (P <0.001).
After surgery, proximal-type DVT was observed in three (0.05%) patients in the 980B group and in one patient (0.09%) in the 1470R group, but not in any patient in the RFA group (Table 2). In the patient in the 1470R group, DUS did not show EHIT or DVT within 48 hours after GSV ablation. However, regular follow-up ultrasonography after 1 month revealed a small, asymptomatic, popliteal-vein-wall-adhered, and localized lesion of the popliteal vein without complete occlusion. Anticoagulant therapy and follow-up with elastic stockings at the outpatient clinic for 1 month led to a reduction in the lesion size/disappearance. In the 980B group, symptomatic DVT with swelling/pain of the lower extremity occurred 1 month after surgery in the three patients, and all of them required hospital treatment. All patients developed DVT after GSV ablation, and the extent of peripheral thrombus-related occlusion involved the popliteal vein. In one of these patients, C2 (floating type, length: 10.9 mm) was observed after surgery, but there were no changes in the EHIT state on follow-up after 1 week. In addition, thoracic computed tomography (CT) after admission revealed asymptomatic pulmonary embolism (PE). In the other two patients, there was no EHIT within 72 hours after surgery, but detailed examination by abdominal CT after admission revealed iliac compression. Thoracic CT did not show PE.
Table 2.
Deep vein thrombosis after endovenous ablation of the saphenous vein
| Group | Total | RFA | 1470R | 980B | P** |
|---|---|---|---|---|---|
| Treated limbs | 8318 | 1026 | 1174 | 6118 | |
| Total DVT | 35 (0.42) | 3 (0.29) | 5 (0.43) | 27 (0.44) | NS |
| Proximal type | 4 (0.05) | 0 (0) | 1 (0.09) | 3 (0.05)* | NS |
| Distal type | 31 (0.37) | 3 (0.29) | 4 (0.34) | 24 (0.39) | NS |
RFA: radiofrequency ablation; 1470R: endovenous laser ablation using 1470 nm diode lazer with radial 2rings fiber; 980B: endovenous laser ablation using 980 nm diode lazer with bare-tip fiber; DVT: deep vein thrombosis Value are number (%) of limbs proximai type is defined as occurring in the popliteal vein and above distal type is defined as occurring in the calf veins
* Anticoagulant therapy was administer at hospitalization. One patient have developed with asymptomatic pulmonary embolism.
** χ2 test, NS: not significant
Distal-type DVT (Table 2), thrombosis of the soleal vein in most cases, was noted in 24 (0.39%), 4 (0.34%), and 3 (0.29%) limbs in the 980B, 1470R, and RFA groups, respectively; there were no differences among the three groups. In patients for whom follow-up could be continued for 1 year, all thrombi remained, but were reduced and organized.
EHIT was observed in 2270 limbs (27.3%) among all patients who underwent surgery: C1, 22.0%; C2, 5.1%; C3, 0.2%; and C4, 0% (Table 3).
Table 3.
Endovenous heat-induced thrombosisi after endovenous ablation of the saphenous vein
| Group | Total | RFA | 1470R | 980B | P***** |
|---|---|---|---|---|---|
| Treated limbs | 8318 | 1026 | 1174 | 6118 | |
| Total EHIT | 2270 (27.3) | 570 (55.6) | 471 (40.1) | 1229 (20.1) | |
| Class 1 | 1826 (22.0) | 542 (52.8)* | 393 (33.5) | 891 (14.6) | 0.001 |
| Class 2 | 428 (5.1) | 27 (2.6) | 75 (6.4)** | 326 (5.3)** | 0.001 |
| Protrusion type | 323 (3.9) | 23 (2.2) | 64 (5.5) | 236 (3.9) | |
| Creeping type | 83 (1.0) | 2 (0.2) | 10 (0.9)*** | 71 (1.2)*** | 0.05 |
| Floating type | 22 (0.3) | 2 (0.2) | 1 (0.1) | 19 (0.3) | |
| Class 3**** | 16 (0.2) | 1 (0.1) | 3 (0.3) | 12 (0.2) | NS |
| Class 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
RFA: radiofrequency ablation; 1470R: endovenous laser ablation using 1470 nm diode lazer with radial 2rings fiber; 980B: endovenous laser ablation using 980 nm diode lazer with bare-tip fiber Value are number (%) of limbs. EHIT: endovenous heat-induced thrombus Class 2 protrusion type is defined as the project of the thrombus into the deep vein within 5mm in length Class 2 creeping type is defined as the prolong of the thrombus sticking the deep vein wall Class 2 floating type is defined as the floating thurombus into the deep vein over 5 mm in length
* P <0.001 vs 1470R and 980B ** and *** P <0.001 vs RFA **** Anticoagulant therapy was administer ***** χ2 test, NS: not significant
Overall, C1 was noted in 22.0%. However, when reviewing the incidence of C1 as a percentage of EHIT patients, those with C1 accounted for 80.4%, showing the highest percentage. There were differences in its incidence among the three groups. Briefly, C1 was observed in 52.8% of the patients in the RFA group, in 33.5% in the 1470R group, and in 14.6% in the 980B group; in the RFA group, the incidence was significantly higher (P <0.001). In the RFA group, 94.0% of EHIT patients had C1; in the RFA group, EHIT more frequently occurred in comparison with the other groups, but most patients were diagnosed with C1.
Overall, C2 was noted in 5.1%. The percentages in the RFA, 1470R, and 980B groups were 2.6%, 6.4%, and 5.3%, respectively; in the 980B and 1470R groups (after laser ablation), the values were significantly higher than in the RFA group (P <0.001). Morphologically, the protrusion type was primarily detected in all groups among C2 patients. The proportion of patients with floating type was the lowest, and there were no significant differences in its incidence among the three groups. On the other hand, there were no significant differences in the incidence of creeping type in C2 patients among the three groups. However, when comparing the incidence in surgically treated patients, the values in the 1470R and 980B groups were significantly higher than in the RFA group (P <0.05). Thus, in the 1470R and 980B groups, the incidences of C2 were higher than in the RFA group, and creeping type was more frequent.
The incidences of C3 in the RFA, 1470R, and 980B groups were 0.1%, 0.3%, and 0.2%, respectively; there were no ablation device-related differences, and there was no C4.
There were no differences in the follow-up of EHIT among the three groups. Some patients with C1 showed progression to C2/C3 (described below), but, in most patients, follow-up alone led to spontaneous disappearance on DUS 1 month after surgery.
With respect to the postoperative course in patients with protrusion type, spontaneous disappearance was achieved within 1 week to 1 month in most patients. In many patients with floating type, DUS after 1 week showed a spontaneous reduction or no change, leading to spontaneous disappearance or C1 within 1–3 months (C1 spontaneously disappeared within the subsequent 3 months). In the 980B group, one patient with floating type after surgery developed DVT with asymptomatic PE 1 month after surgery (described above). Four patients showed changes from a normal condition or C1 to floating type 1 month after surgery (n = 1 and 3, respectively). Creeping type was present on the anterior wall of the deep vein in GSV patients and on the posterior wall in SSV patients. It remained in 58 limbs (70%) on follow-up after 1 month. Of these, follow-up could be performed for 1 year in 26 patients (980B group alone). Of these nine (35%) patients, the thrombus was organized. In one patient, C1 progression to creeping type was noted 1 month after surgery.
For all patients with C3, heparin and following oral administration of an anticoagulant therapy (warfarin or edoxaban) was performed. In most patients, there was a reduction in 1 week after surgery, leading to disappearance or C1 within 1–3 months. Of the C1 patients, spontaneous disappearance was confirmed in those who could be followed-up for 1 year. In five patients, a normal condition, C1, or protrusion type C2 had changed/progressed to C3 1 month after surgery (n = 2, 1, and 2, respectively).
Discussion
Previous studies reported symptomatic DVT after venous stripping, but no study has performed postoperative follow-up of DVT patients, including those with asymptomatic DVT, using DUS in Japan. In 2004, Rij et al.3) examined the presence or absence of DVT using DUS after venous stripping and reported that distal-type DVT of the crural muscle vein was frequent, and that the incidence of proximal-type DVT was 0.5%. In this study, the incidence of proximal-type DVT after EVA was 0.05%, overall, and that of symptomatic DVT was 0.04%. With respect to devices, the incidences in the 980B, 1470R, and RFA groups were 0.05%, 0.09%, and 0%, respectively, suggesting that the incidence of DVT after EVA is not higher than after conventional surgery. Therefore, the incidences of DVT and PE after EVA without high ligation are not particularly higher than after saphenous vein removal. If indications are observed, EVA may be safe even in the absence of high ligation.
As a major characteristic, EVA does not require high ligation and crossectomy, which have been performed. On the other hand, initially, the risk of thrombus extension into the deep vein related to the absence of high ligation and/or crossectomy was deeply concerned. In 2005, Mozes et al.4) first reported three patients with thrombus extension into the deep vein after EVA. At that time, there was no expression of EHIT, and thrombus extension into the femoral vein was observed in 3 (7.7%) of 39 patients for whom postoperative DUS could be performed. For all patients, anticoagulant therapy was performed, and an inferior vena cava (IVC) filter was temporarily inserted in one patient. They indicated that there were no symptoms in any patient, and that thrombus had disappeared within 1 month in all patients. Kabnick et al.1) first presented an EHIT classification at the 18th meeting of the American Venous Forum. Subsequently, Frasier et al.5) reported the details of EHIT. They indicated that EHIT-related thrombus was characterized by reduced motility (adhered to the vein wall), relatively early disappearance, and high-level brightness on DUS, emphasizing that anticoagulant therapy is not necessary in many patients, differing from standard thrombus. Concerning the etiology of standard thrombus, including DVT, the normal venous endothelium has an antithrombotic activity, and a thrombus may develop at the site of blood flow congestion. It primarily consists of erythrocytes: a coagulation cascade by coagulation factors activated in the presence of congestion resulting in the excessive formation of a fibrin network, in which erythrocytes are captured. On the other hand, concerning the pathogenesis of EHIT, the vascular endothelium is destroyed by EVA-related heat, and, subsequently, macrophages, neutrophils, and eosinophils infiltrate the endothelium, leading to the formation of scar tissue through collagen secretion by fibroblasts migrating from the outer membrane. Therefore, EHIT is a relatively strong tissue, and may not cause PE. On DUS, it is visualized as high-level brightness. Therefore, the pathogenesis of EHIT markedly differs from that of spontaneous DVT.5) Actually, the results of this study also showed spontaneous disappearance in most patients with EHIT. Furthermore, PE occurred in one patient, but it is unclear whether or not PE was related to EHIT or DVT after surgery, and whether or not PE was present before surgery.
According to previous studies, risk factors for EHIT include age, sex, thrombotic predispositions, the severity of varicose veins of the lower limbs (large saphenous vein diameter, CEAP 4 or higher), and technical factors. On the other hand, some studies reported that EHIT was not related to large saphenous vein diameter, whereas others indicated that there was no relationship between EHIT and age/sex by adopting lasers differing in wavelength; risk factors for EHIT remain controversial.6–8) Based on the results of this study, there were no age- or sex-related differences. Basically, treatment was not performed for patients with thrombotic predispositions or a history of DVT; therefore, it was unclear whether or not EVA in such patients may become a risk factor. Concerning patients with a large mean saphenous vein diameter, EVA with 980B was basically indicated for patients with a mean saphenous vein diameter of ≤9 mm in our hospital, and EVA with 1470R or RFA was indicated even when the mean saphenous vein diameter was ≥9 mm, but, actually, the proportion of patients with a mean diameter of ≥9 mm was not high in Japan; in particular, there was no patient with a mean diameter of >12 mm. Therefore, it was unclear whether or not a large mean diameter is a risk factor. In the future, treatment with RFA or 1470R may be primarily selected; the proportion of patients with a large mean diameter may increase. Therefore, future examination may be necessary. Concerning patients with aneurysmal changes at near the deep vein junction, indication criteria for EVA in our hospital include a saccular venous aneurysm size of ≤15 mm or spindle venous aneurysm size of ≤20 mm for EVA with 980B and a saccular venous aneurysm size of ≤20 mm or spindle venous aneurysm size of ≤25 mm for EVA with 1470R or RFA. For patients with a larger diameter, removal is basically performed. Therefore, when performing EVA according to these criteria, aneurysmal changes at near the deep vein junction were not always considered to be a risk factor. Thus, in this study, we could not identify risk factors for EHIT. As preventive procedures, previous studies recommended that a starting point of ablation should be established on the distal side 1 to 2 cm from the deep vein–saphenous vein junction, that branches at the junction should be preserved to maintain venous blood flow from the branches, and that the proximal side should be compressed to prevent influx of steam bubbles (heated/boiled blood bubbles).9,10) The results of this study suggest the influence of steam bubbles on the development of C2 in patients who underwent EVLA. In particular, on EVLA, a large volume of steam bubbles is generated in comparison with RFA. Steam bubbles refer to water in blood boiled into steam, and the temperature is estimated to be approximately 100°C. Ablation induces heat denaturation of the venous wall, leading to stenosis of its lumen. However, when it is not sufficiently occluded or there is a large stump on the proximal side, slightly heated steam flows in the remnant saphenous vein stump on the proximal side. At the stump, the blood flow volume is small, suggesting that influx of high-temperature steam influences blood and vascular endothelium, leading to thrombus formation through heat coagulation of blood or endothelial disorder. Subsequently, steam bubbles are cooled to a large volume of blood through heat exchange and influx into the femoral vein, and the temperature of steam bubbles may reduce; therefore, this may markedly influence the area adjacent to the starting point of ablation. This suggests that steam bubbles are involved in the pathogenesis of creeping type,11) because the incidence of creeping type, involving extension to the deep vein, is high in EVLA patients, and because the length is ≤2 to 3 cm. Hirokawa et al.12) performed EVA with 980B for a specific period after its start, assuming that a dilated, incomplete ablated vein may remain when a starting point of ablation is insufficient, leading to EHIT, and reported that this method decreased the incidence of EHIT. Furthermore, another study suggested that the stump length from the deep vein junction is involved in thrombus formation after ablation. Labropoulos et al.13) reported that the incidences of thrombus after saphenous vein stripping, EVLA, and GSV harvesting were 2.7%, 9.3%, and 15.3%, respectively. The stump length from the deep vein junction may be the longest after GSV harvesting, followed by that after EVLA and that after stripping, suggesting a long stump is a risk factor for EHIT or thrombus enlargement.
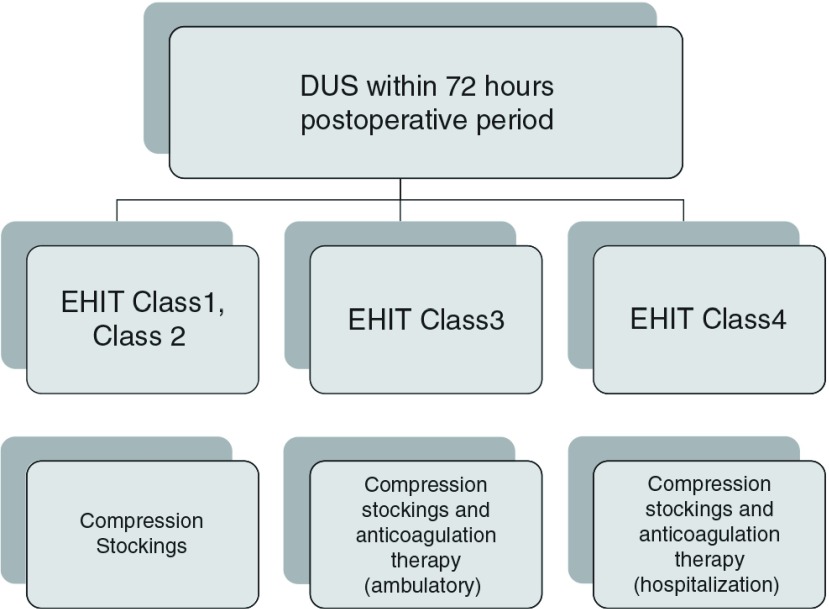
To investigate the presence or absence of thrombotic complications, including EHIT, it is recommended that DUS should be performed within 72 hours after surgery and within 1–3 months in the Guidelines for Endovascular Treatment for Varicose Veins of the Lower Limbs.2) Internationally, EHIT, especially C2 or higher, is observed in 0.2% to 7.7% of patients after EVA.4,14–16) According to our previous studies involving EVLA patients, the incidence of EHIT was 3.8% to 8.9%.17–19) The results of this study showed that the incidence ranged from 2.7% to 6.6%. Initially, Kabnick et al.1) reported follow-up for C1 and follow-up by DUS for C2 with the use of low-molecular-weight heparin until C1 achievement (2011). For C3/C4 patients, treatment was performed in accordance with DVT treatment, and anticoagulant therapy was performed for C1 or higher patients.20) However, in 2014, Kabnick1) estimated that the incidence of symptomatic PE directly caused by EHIT was ≤0.01%, as there had been no such case report. He indicated that periodic DUS was not necessary for patients without symptoms from the medico-economical viewpoint.21) After the introduction of EVA, we also initially continued follow-up based on the strategies described in section “Methods” when detecting EHIT. However, in this study, most patients with C2 were classified as protrusion type, and C2 spontaneously disappeared within 1 week to 1 month through a spontaneous course; therefore, currently, DUS at 1 week is omitted in patients with C2 (Fig. 2). However, in four patients with DVT, its onset could not be predicted using DUS; therefore, in the future, adequate points of DUS and patients to be examined must be reviewed.
Fig. 2.
Treatment algorithm of endovenous heat-induced thrombus in our hospital. DUS: duplex ultrasonography; EHIT: endovenous heat-induced thrombus
In 10 patients, EHIT on DUS, that is, asymptomatic after 1 month was more advanced than within 48 hours. They consisted of four patients who underwent SSV surgery and six patients who underwent GSV surgery: one patient with C2 or two patients with C3 despite the absence of EHIT within 48 hours, four patients with a change from C1 to C2, one patient with a change from C1 to C3, and two patients with a change from C2 to C3. Of these 10 patients, 7 in the 980B group, 1 in the 1470R group, and 2 in the RFA group. There was no characteristic in the background of these patients. In some patients, EHIT became more advanced between DUS within 72 hours and after 1 month, but such cases are rare.
With respect to the association between EHIT and proximal DVT, proximal DVT occurred in four patients. In one of these with asymptomatic DVT, a solitary, minor thrombus of the popliteal vein was detected in the absence of EHIT after GSV ablation; there may have been no association with EHIT. Of the other three patients with symptomatic DVT, ultrasonography within 72 hours showed normal findings in two patients, but revealed EHIT in one patient. Morphologically, this patient had floating type C2 involving a relatively small size. It was not evaluated as C3, which may be more advanced, and the extent of peripheral thrombus-related occlusion at the onset of DVT involved the popliteal vein. DVT accounted for 0.01% of all surgically treated limbs, 0.04% of limbs with EHIT, and 0.2% of C2 patients; there was no relationship between EHIT and proximal DVT. Furthermore, it is unclear whether or not the onset of DVT could have been prevented if early anticoagulant therapy had been performed when detecting floating type on DUS early after surgery, and whether or not the absence of patients with a change from C3 to DVT resulted from anticoagulant therapy; the relationship between EHIT and DVT remains to be clarified. As future development, preventive anticoagulant therapy may not be necessary for the following reasons: the incidences of floating type and C3 or higher are low; EVLA is not indicated for patients with a high Caprini score; it is difficult to predict risk factors for EHIT; and there is no relationship between EHIT and venous thromboembolism (VTE). For VTE prevention, the incidence of VTE is lower than after conventional saphenous vein stripping, and there is no specific preventive method after EVA. Briefly, EVA should be conducted under local anesthesia so that patients can walk early after surgery, and intraoperative sclerotherapy should be avoided. Basically, DUS may be necessary, as its purpose is not to diagnose thrombosis alone. In particular, it is useful for evaluating postoperative curative effects. Therefore, the timing of DUS may differ among purposes. Conventional DUS within 72 hours is not essential, considering that the interval from surgery until detection was 1 month in all patients with proximal DVT in this study. However, it may become necessary if patients with DVT within 72 hours after surgery are reported from other institutions, as the mechanism of EHIT has not been completely clarified, and if anticoagulant therapy becomes essential for C3 patients in the future. However, currently, it is described that DUS within 72 hours is necessary in the guidelines for endovascular treatment; it must be performed. DUS after 1 month may be essential, because it is necessary to evaluate the presence or absence of early recanalization, hematoma formation, ablated vein contractility, and therapeutic effects on arteriovenous fistulae in addition to VTE investigation. Concerning EHIT treatment, follow-up may be appropriate for C2 or lower patients, but anticoagulant therapy may be necessary for C3 patients for the time being, as this has not been sufficiently examined due to the limited number of patients. In the future, a multicenter, randomized controlled trial (RCT) should be conducted to investigate the presence or absence of anticoagulant therapy.
Conclusion
We examined postoperative thrombotic complications. Although the incidence of C2 or higher was significantly lower in the RFA group, neither the relationship between EHIT and proximal DVT nor risk factors for EHIT were clarified in this study. It was impossible to predict the onset of DVT using DUS within 72 hours after surgery, as recommended, or after 1 week for patients with C2 or higher, which had been performed in our hospital. In the future, the timing and frequency of postoperative DUS, patients to be examined, and its necessity for patients with asymptomatic conditions must be sufficiently reviewed. Furthermore, EHIT differs from standard thrombus; therefore, whether or not anticoagulant therapy is necessary after the onset of EHIT, and, if necessary, the type of patients to be examined must be examined.
Disclosure Statement
There are no conflicts of interest for Nobuhisa Kurihara and Takashi Yamamoto. Masayuki Hirokawa received a retaining fee from Integral Corporation and a lecture’s fee from Japan Covidien Co., Ltd.
Footnotes
This report was presented as ‘Complications related to endovascular laser treatment for varicose veins of the lower limbs and preventive strategies’ in Panel Discussion 1 of the 55th Annual Meeting of Japanese College of Angiology (October 2014, Kurashiki).
This is a translation of J Jpn Coll Angiol 2015; 55: 153–161.
References
- 1).Kabnick LS, Ombrellino M, Agis H, et al. Endovenous heat induced thrombus (EHIT) at the superficial-deep venous junction: a new post-treatment clinical entity, classification and potential treatment strategies. 18th Annual Meeting of the American Venous Forum, Miami, Florida, 2006. [Google Scholar]
- 2).Satokawa H, Sugiyama S, Hirokawa M, et al. Guidelines on endovascular therapy for varicose veins. 2009–2010 report by the society subcommittee. Jpn J Phlebol 2010; 4: 289-309. (in Japanese) [Google Scholar]
- 3).van Rij AM, Chai J, Hill GB, et al. Incidence of deep vein thrombosis after varicose vein surgery. Br J Surg 2004; 91: 1582-5. [DOI] [PubMed] [Google Scholar]
- 4).Mozes G, Kalra M, Carmo M, et al. Extension of saphenous thrombus into the femoral vein: a potential complication of new endovenous ablation techniques. J Vasc Surg 2005; 41: 130-5. [DOI] [PubMed] [Google Scholar]
- 5).Frasier K, Latessa V. Minimally invasive vein therapy and treatment options for endovenous heat-induced thrombus. J Vasc Nurs 2008; 26: 53-7. [DOI] [PubMed] [Google Scholar]
- 6).Rhee SJ, Cantelmo NL, Conrad MF, et al. Factors influencing the incidence of endovenous heat-induced thrombosis (EHIT). Vasc Endovascular Surg 2013; 47: 207-12. [DOI] [PubMed] [Google Scholar]
- 7).Sufian S, Arnez A, Labropoulos N, et al. Endovenous heat-induced thrombosis after ablation with 1470 nm laser: Incidence, progression, and risk factors. Phlebology 2015; 30: 325-30. [DOI] [PubMed] [Google Scholar]
- 8).Shutze WP, Kane K, Fisher T, et al. The effect of wavelength on endothermal heat-induced thrombosis incidence after endovenous laser ablation. J Vasc Surg Venous Lymphat Disord 2016; 4: 36-43. [DOI] [PubMed] [Google Scholar]
- 9).Sadek M, Kabnick LS, Rockman CB, et al. Increasing ablation distance peripheral to the saphenofemoral junction may result in a diminished rate of endothermal heat-induced thrombosis. J Vasc Surg Venous Lymphat Disord 2013; 1: 257-62. [DOI] [PubMed] [Google Scholar]
- 10).Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg 2002; 28: 596-600. [DOI] [PubMed] [Google Scholar]
- 11).van der Geld CW, van den Bos RR, van Ruijven PW, et al. The heat-pipe resembling action of boiling bubbles in endovenous laser ablation. Lasers Med Sci 2010; 25: 907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hirokawa M. Textbook of Endovascular Laser Ablation. In: Hirokawa M, ed. Japan Medical Journal, Tokyo, 2011. (in Japanese) [Google Scholar]
- 13).Labropoulos N, Bishawi M, Gasparis A, et al. Great saphenous vein stump thrombosis after harvesting for coronary artery bypass graft surgery. Phlebology 2014; 29: 215-9. [DOI] [PubMed] [Google Scholar]
- 14).Marsh P, Price BA, Holdstock J, et al. Deep vein thrombosis (DVT) after venous thermoablation techniques: rates of endovenous heat-induced thrombosis (EHIT) and classical DVT after radiofrequency and endovenous laser ablation in a single centre. Eur J Vasc Endovasc Surg 2010; 40: 521-7. [DOI] [PubMed] [Google Scholar]
- 15).Dexter D, Kabnick L, Berland T, et al. Complications of endovenous lasers. Phlebology 2012; 27: 40-5. [DOI] [PubMed] [Google Scholar]
- 16).Kane K, Fisher T, Bennett M, et al. The incidence and outcome of endothermal heat-induced thrombosis after endovenous laser ablation. Ann Vasc Surg 2014; 28: 1744-50. [DOI] [PubMed] [Google Scholar]
- 17).Hirokawa M, Kurihara N. Standard procedure for endovenous laser ablation with 980 nm diode laser. Jpn J Vasc Surg 2012; 21: 583-8. (in Japanese) [Google Scholar]
- 18).Hirokawa M, Kurihara N. Comparison of bare-tip and radial fiber in endovenous laser ablation with 1470 nm diode laser. Ann Vasc Dis 2014; 7: 239-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kurihara N, Hirokawa M. Endovenous laser ablation using 1470 nm diode laser and a radial 2Ring fiber. Jpn J Phlebol 2014; 26: 34-40. (in Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kabnick LS, Berland TL. Endothermal heat induced thrombosis (EHIT). Presented at the 38th Annual Vascular and Endovascular Issues, Techniques and Horizons (VEITHsymposium), November 16–20, 2011, New York City. [Google Scholar]
- 21).Jones RT, Kabnick LS. Perioperative duplex ultrasound following endothermal ablation of the saphenous vein: is it worthless? J Invasive Cardiol 2014; 26: 548-50. [PubMed] [Google Scholar]