Abstract
Objective: The purpose of this study was to observe the direct effects of oral bacteria, such as Porphyromonas gingivalis (Pg) and Treponema denticola (Td), on the peripheral vasculature.
Materials and Methods: Beagles were directly injected (at various doses) with Pg or Td. Each leg vein was exposed, ligated at proximal and distal sites, and then injected with bacteria diluted with sterile saline. The collected vascular tissue was examined microscopically, and samples of the vascular tissue and blood were cultured and then subjected to the polymerase chain reaction (PCR) in order to detect the bacterial deoxyribonucleic acid (DNA).
Results: No genes of the injected bacteria were detected in the Td-inoculated blood or vascular tissue samples collected 2 weeks after the injection. The Pg gene was also not detected in the blood samples collected 4 weeks after the injection although it was detected in the vascular tissue using PCR. Microscopic examinations showed that the inflammatory reactions in the perivascular tissue increased in a bacterial dose-dependent manner, as expected.
Conclusion: We observed the direct effects of oral bacteria on vascular tissue. Further studies are needed to investigate the correlations between oral bacteria and systemic diseases. (This article is a translation of Jpn J Phlebol 2015; 26: 41-6.)
Keywords: oral bacteria, peripheral vascular disease, periodontitis
Introduction
Many previous studies have examined the association between intraoral infections, periodontal disease, and systemic illnesses.1) In particular, numerous studies have investigated the association between cardiovascular disease and periodontal disease, in which the so-called periodontal disease bacteria, that is, the causative microorganisms of periodontal disease, have been detected in atheromatous lesions in coronary arteries with coronary artery disease,2) clots in lump walls associated with aortic aneurysms,3,4) blocked blood vessels associated with Buerger disease,5) and blocked blood vessel walls associated with arteriosclerosis obliterans.6) In these clinical conditions, periodontal disease bacteria are said to enter the systemic circulation from the oral cavity by changing to a bacteremic state, before they damage the target tissues directly or via lipopolysaccharides originating from the bacteria and/or inflammatory mediators.7,8) However, the idea that periodontal disease bacteria directly damage target tissues remains a hypothesis,9) and the actual effects of administering periodontal disease bacteria to a target tissue, particularly blood vessels, remain unknown. The purpose of this study was to examine whether periodontal disease bacteria directly damage blood vessels by producing a model of periodontal disease bacteria-induced peripheral vascular disorder.
Materials and Methods
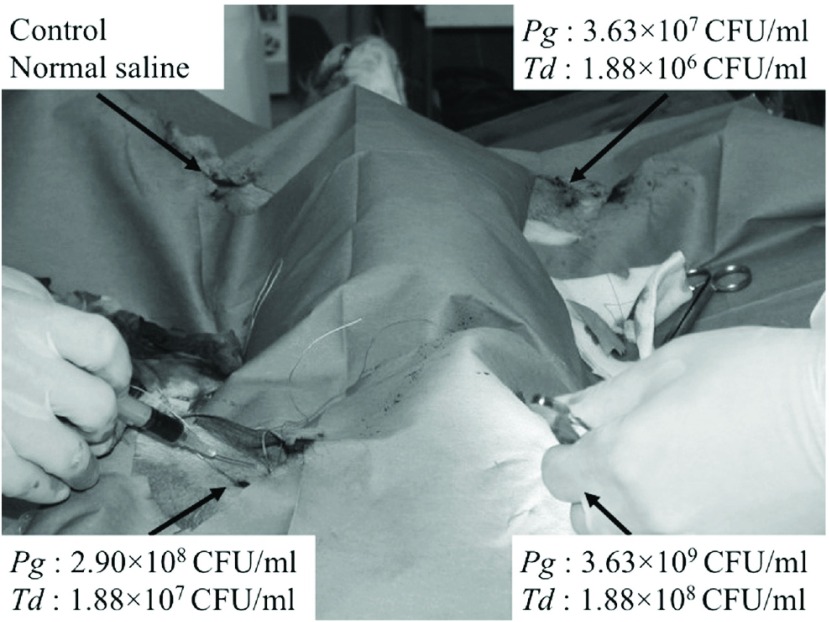
In this study, 3-year-old beagles (males, approximately 8 kg) were used. As Porphyromonas gingivalis (Pg) and Treponema denticola (Td) were the strains of periodontal bacteria that were most commonly detected in the artery wall in cases of arteriosclerosis obliterans treated at our department,6) we decided to administer Pg and Td in this study. The bacteria were isolated and cultured for the study. We exposed veins in the limbs of the beagles under general anesthesia and ligated them after securing them at central and peripheral sites. Then, we injected 1 ml of the chosen substance into the ligated veins using a 23G needle (Fig. 1). One dog was injected with various concentrations of Pg (3.63 × 107 colony-forming units (CFU)/ml, 2.90 × 108 CFU/ml, and 3.63 × 109 CFU/ml), and the saline was injected into another vein as a control. Another dog was injected with various concentrations of Td (1.88 × 106 CFU/ml, 1.88 × 108 CFU/ml, and 1.88 × 109 CFU/ml), and the saline was injected into another vein as a control.
Fig. 1.
An experimental image taken during the bacterial inoculation procedure. We injected the oral bacteria into three leg veins, with each vein receiving a different dose of bacteria, and one control leg vein was injected with normal saline. Pg: Porphyromonas gingivalis; Td: Treponema denticola; CFU: colony-forming units
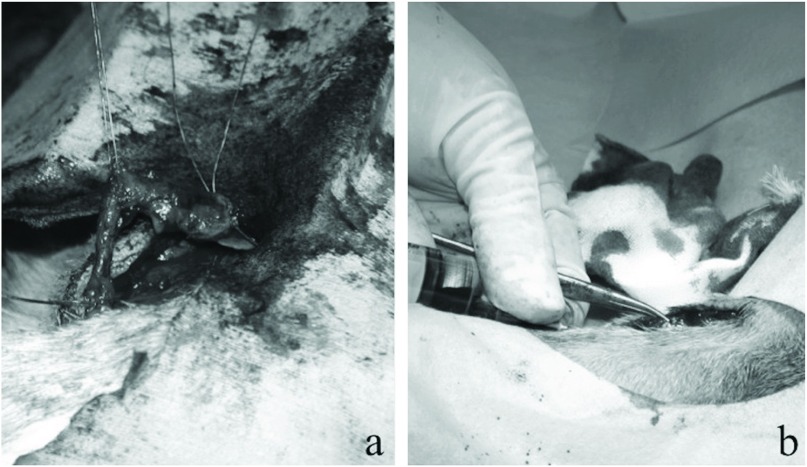
At 2 weeks after the injection in the Td-administered group and 4 weeks after the injection in the Pg-administered group, we collected the veins in which bacteria from the oral cavity had been injected as well as samples of the circulating blood (Fig. 2). The veins were collected after 4 weeks in the Pg-administered group because in previous experiments involving rats performed at our department, the changes induced in the blood vessels by the administration of Pg were more prominent after 4 weeks than after 2 weeks.10) We subjected the collected blood and veins to examine the deoxyribonucleic acid (DNA) of each bacterial strain based on the polymerase chain reaction (PCR) using a high-pure PCR template preparation kit (Roche Corporation, Germany).4) In addition, we conducted a histopathological examination using an optical microscope and hematoxylin and eosin (H&E) staining. We determined the degree of inflammation of the veins as follows: mild–inflammatory cells had infiltrated less than one-third of the overall circumference of the vein wall; moderate–inflammatory cells had infiltrated from one-third to two-thirds of the overall circumference of the vein wall; and severe–inflammatory cells had infiltrated more than two-thirds of the overall circumference of the vein wall.
Fig. 2.
An experimental image obtained during the harvesting procedure. Leg veins (a) and blood samples (b) were collected 2 or 4 weeks after the injection. The leg veins were examined microscopically, and the PCR was used to detect bacterial DNA. We also subjected blood samples to DNA detection. PCR: polymerase chain reaction; DNA: deoxyribonucleic acid
Results
Although it was easy to collect the target veins from the control limbs, which were injected with saline, it was difficult to collect them from the limbs that were injected with bacteria from the oral cavity, due to significant inflammatory changes in the vicinity.
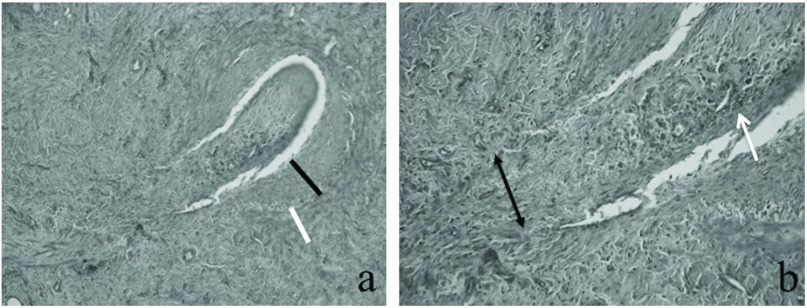
In the beagles-administered Td, we were unable to detect the bacteria in either the control or bacterial-injected limbs using PCR. Furthermore, we were also unable to detect the bacteria in the collected blood using PCR. As for the beagles that were injected with Pg, we were unable to detect the bacteria in the collected blood samples using PCR. While we were unable to detect the bacteria in the veins that were injected with saline, we were able to detect the bacteria in the veins injected with Pg (Table 1). Furthermore, H&E staining revealed inflammatory changes in the veins into which bacteria from the oral cavity had been injected, whereas no inflammatory changes were seen in the veins into which the saline was injected. This suggested that the inflammatory changes had been caused by the periodontal disease bacteria. The vessel wall, particularly the intima, was thickened; moreover, the formation of clots due to the deposition of hemosiderin was observed in parts of the vein lumen (Fig. 3).
Table 1.
The inoculations of bacteria and the results of the detection of DNA and microscopic findings
| No. | Location of leg | Bacteria, concentration (CFU/ml) | Detection of DNA by PCR | Microscopic reactions | |
|---|---|---|---|---|---|
| Blood | Leg vein | ||||
| 1-1 | U, Rt | Normal saline, control | – | – | – |
| 1-2 | U, Lt | Td, 1.88 × 106 | – | – | Mild |
| 1-3 | L, Rt | Td, 1.88 × 108 | – | – | Mild |
| 1-4 | L, Lt | Td, 1.88 × 109 | – | – | Moderate |
| 2-1 | U, Rt | Normal saline, control | – | – | – |
| 2-2 | U, Lt | Pg, 3.63 × 107 | – | + | Moderate |
| 2-3 | L, Rt | Pg, 2.90 × 108 | – | + | Moderate |
| 2-4 | L, Lt | Pg, 3.63 × 109 | – | + | Severe |
DNA: deoxyribonucleic acid; PCR: polymerase chain reaction; U: upper; L: lower; Rt: right; Lt: left; CFU: colony-forming unit; Pg: Porphyromonas gingivalis; Td: Treponema denticola
Fig. 3.
The microscopic findings of the leg veins injected with Porphyromonas gingivalis solution. (a) The intima (black bar), media, and adventitia (white bar) were thick due to inflammatory reactions, and the perivascular tissue had become fibrotic (hematoxylin and eosin stain, 20×). (b) In the lumen of the injected leg vein, organized thrombi with hemosiderin (white arrow) were recognized, and the disruption of smooth muscle cells (black arrow) was seen at the injection site (hematoxylin and eosin stain, 40×).
Discussion
Many studies have examined the involvement of periodontal disease in peripheral vascular disease. For example, periodontal disease exhibits similar associations with arteriosclerosis obliterans to hypertension and dyslipidemia according to an epidemiological survey, that is, patients with periodontal disease have a relative risk (1.77) of developing arteriosclerosis obliterans.11) On the other hand, while there have been few epidemiological surveys of Buerger disease, which is a representative of peripheral artery disease, it has been examined in other types of studies.12) The mechanism responsible for arteriosclerosis obliterans condition has been reported to be as follows: periodontal disease bacteria coaggregate in atherosclerosis lesions, adhere to vascular endothelial cells, and activate coagulation factor X, resulting in a tendency to clot; furthermore, Hgp44 adhesion on the surfaces of the bacteria gives rise to FcγRIIa production in platelets and platelet aggregation via IgG.13) In other words, in arteriosclerosis obliterans, intimal injuries, such as the formation of atheroma, occur first, and the clinical condition develops due to the presence of periodontal disease bacteria. In addition, animal studies have obtained similar findings. For instance, Zhang et al.14) reported that administering Pg to rabbits and injuring the arterial intima using a balloon resulted in a significant intimal thickening in comparison with the Pg non-administration group.
Regarding Buerger disease, because it is generally characterized by the maintenance of the membrane structure of the arteries without any atherosclerosis-related changes in the intima, it has been suggested that clot formation due to periodontal disease bacteria might be involved in the development of the clinical condition.5) Previously, during a mixing test involving Pg in which we divided blood components into leukocytes and platelets, we found that Pg was taken up relatively easily by the leukocytes, whereas Pg attached to the surfaces of the platelets was taken up into intraplatelet vacuoles. Moreover, the adhesion and uptake of Pg by the platelets were increased. As a result, we were able to show that Pg caused platelet activation and cohesion, which might have led to clot formation.15) This was also proven via experiments involving rats. Specifically, we embedded subcutaneous pumps into the jugular veins of rats, which continuously infused Pg. We then attempted to detect Pg DNA in the rats’ limb arteries at 2 or 4 weeks after 2 weeks’ sustained infusion and conducted a histopathological examination of the limb arteries. As a result, we detected Pg DNA using PCR. Furthermore, intra-arterial clot formation was observed in arteries whose wall structures had been maintained.10)
In this study, we observed the direct effects of periodontal disease bacteria on blood vessels by administering the bacteria into blood vessels, as described previously. As it is difficult to identify periodontal disease bacteria using normal culture methods,16) in this study, we detected the bacteria using PCR, as has been described in previous studies.17,18) Our method differed from those used in previous studies in that the periodontal disease bacteria arrived at the target blood vessel via the systemic circulation, whereas we were previously unable to distinguish whether the observed changes were due to the direct effects of the periodontal disease bacteria on blood vessel walls or systemic reactions that occurred via inflammatory mediators that travelled through the systemic circulation. Thus, in this study, in order to observe changes that did not involve the systemic circulation, we ligated the blood vessels into which periodontal disease bacteria were injected at central and peripheral sites, that is., we isolated them from the systemic circulation. As a result, we were able to histopathologically examine intravenous clots and confirmed that periodontal disease bacteria induced intimal thickening.
Furthermore, in this study, differences in the detection rates of Pg and Td and the reactions they induced were observed. First, regarding the detection rates of the bacteria, the detection rate of Pg is said to be high in arteriosclerotic disease,4) whereas the detection rate of Td is high in Buerger disease.5) Such inter-disease variations in the detection rates of periodontal disease bacteria might explain why Pg was detected but Td was not detected in this study. In addition, regarding the point that Pg produced more marked changes than Td, it is possible that this was partly due to the abovementioned differences in the detection rates of these bacteria. As the detection rate of Pg was high, whereas Td was not detected, we consider that it is possible that Pg induced more sustained reactions in the veins. As for the fact that Pg displayed stronger reactions with platelets than Td, this was considered to be related to differences between the strains.
Conclusion
By intravenously administering periodontal disease bacteria into beagles, we were able to confirm that periodontal disease bacteria have direct effects on blood vessels, which has merely been a hypothesis until now. Going forward, by selectively administering periodontal disease bacteria into other tissues, we will attempt to observe the direct effects of periodontal disease bacteria on organs other than blood vessels, which might help to elucidate the associations between periodontal disease bacteria and systemic illnesses.
Disclosure Statement
None of the authors has any conflicts of interest.
Authors’ Contributions
Study conception: KI and TI
Data collection: YI and TI
Analysis: KI and TI
Writing: critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Footnotes
This article is a translation of Jpn J Phlebol 2015; 26: 41-6.
References
- 1).Igari K, Kudo T, Toyofuku T, et al. Association between periodontitis and the development of systemic diseases. Oral Biol Dent doi.org/10.7243/2053-5775-2-4, 2014. [Google Scholar]
- 2).Ishihara K, Nabuchi A, Ito R, et al. Correlation between detection rates of periodontopathic bacterial DNA in coronary stenotic artery plaque [corrected] and in dental plaque samples. J Clin Microbiol 2004; 42: 1313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol 2009; 24: 64-8. [DOI] [PubMed] [Google Scholar]
- 4).Kurihara N, Inoue Y, Iwai T, et al. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2004; 28: 553-8. [DOI] [PubMed] [Google Scholar]
- 5).Iwai T, Inoue Y, Umeda M, et al. Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg 2005; 42: 107-15. [DOI] [PubMed] [Google Scholar]
- 6).Chen YW, Umeda M, Nagasawa T, et al. Periodontitis may increase the risk of peripheral arterial disease. Eur J Vasc Endovasc Surg 2008; 35: 153-8. [DOI] [PubMed] [Google Scholar]
- 7).Chen YW, Nagasawa T, Wara-Aswapati N, et al. Association between periodontitis and anti-cardiolipin antibodies in Buerger disease. J Clin Periodontol 2009; 36: 830-5. [DOI] [PubMed] [Google Scholar]
- 8).Chen YW, Iwai T, Umeda M, et al. Elevated IgG titers to periodontal pathogens related to Buerger disease. Int J Cardiol 2007; 122: 79-81. [DOI] [PubMed] [Google Scholar]
- 9).Iwai T, Umeda M, Inoue Y. Are there any objections against our hypothesis that buerger disease is an infectious disease? Ann Vasc Dis 2012; 5: 300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Kubota T, Inoue Y, Iwai T, et al. Arterial thrombosis after intravenous infusion of oral bacterium in a rat model. Ann Vasc Surg 2008; 22: 412-6. [DOI] [PubMed] [Google Scholar]
- 11).Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med 2004; 15: 403-13. [DOI] [PubMed] [Google Scholar]
- 12).Igari K, Inoue Y, Iwai T. The epidemiologic and clinical findings of patients with buerger disease. Ann Vasc Surg 2016; 30: 263-9. [DOI] [PubMed] [Google Scholar]
- 13).Naito M, Sakai E, Shi Y, et al. Porphyromonas gingivalis-induced platelet aggregation in plasma depends on Hgp44 adhesin but not Rgp proteinase. Mol Microbiol 2006; 59: 152-67. [DOI] [PubMed] [Google Scholar]
- 14).Zhang MZ, Li CL, Jiang YT, et al. Porphyromonas gingivalis infection accelerates intimal thickening in iliac arteries in a balloon-injured rabbit model. J Periodontol 2008; 79: 1192-9. [DOI] [PubMed] [Google Scholar]
- 15).Li X, Iwai T, Nakamura H, et al. An ultrastructural study of Porphyromonas gingivalis-induced platelet aggregation. Thromb Res 2008; 122: 810-9. [DOI] [PubMed] [Google Scholar]
- 16).Iwai T. Periodontal bacteremia and various vascular diseases. J Periodont Res 2009; 44: 689-94. [DOI] [PubMed] [Google Scholar]
- 17).Ashimoto A, Chen C, Bakker I, et al. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 1996; 11: 266-73. [DOI] [PubMed] [Google Scholar]
- 18).Toyofuku T, Inoue Y, Kurihara N, et al. Differential detection rate of periodontopathic bacteria in atherosclerosis. Surg Today 2011; 41: 1395-400. [DOI] [PubMed] [Google Scholar]