Abstract
OBJECTIVE:
The term chronic inflammatory disease (CID) refers to a category of inflammatory diseases that includes Ankylosing spondylitis (AS) and familial Mediterranean fever (FMF). The incidence of adverse cardiovascular events is greater among patients with CID, though they may not have conventional atherosclerotic risk factors. Endothelial dysfunction is one of the underlying fundamental mechanisms that trigger development of atherosclerotic alterations in arteries, and flow-mediated dilatation (FMD) is a noninvasive method to determine endothelial dysfunction. Recent studies have shown a relationship between high triglyceride high-density lipoprotein cholesterol (TG/HDL-C) ratio and coronary atherosclerosis. Many studies have demonstrated that patients with CID have lower FMD values compared to healthy population, indicating endothelial dysfunction. However TG/HDL ratio and its relationship to FMD in patients with CID has not been investigated. The present study investigated whether TG/HDL ratio in CID patients differs from that of healthy population, and its relationship to FMD in patients with CID.
METHODS:
A total of 58 patients with CID and a group of 58 healthy volunteer individuals were enrolled in the study. FMD measurements were taken with high resolution ultrasound (US), and TG/HDL ratios were calculated.
RESULTS:
Patients with CID had significantly higher TG/HDL-C ratio (2.5 [2.2–2.8] vs 2.3 [2.1–2.5]; p=0.03) and lower FMD values (5.2 [4.2–6.3] vs 6.7 [6.3–9.7]; p<0.001), compared to healthy group, and a negative correlation was found between FMD levels and TG/HDL ratio of the study population.
CONCLUSION:
Higher TG/HDL ratio and lower FMD values found in CID patients may reflect increased atherosclerotic risk.
Keywords: Chronic inflammatory disease, flow-mediated dilatation, triglyceride/high-density lipoprotein-cholesterol ratio
The term chronic inflammatory disease (CID) refers to a category of diseases, including Ankylosing spondylitis (AS) and familial Mediterranean fever (FMF). Although they may not have traditional risk factors of atherosclerosis, incidence of cardiovascular events is increased in this group of patients [1]. Harmful effects of chronic inflammation on vascular system play a fundamental role in the increase of cardiovascular events in patients with CID. Both experimental and clinical studies have demonstrated the role of inflammation on the development of atherosclerosis, having found it to be associated with all stages and acute complications of atherosclerosis [2, 3]. In the development of vascular pathology that triggers atherosclerosis, active inflammatory processes involving leucocytes and soluble substances play important roles [4].
Endothelial dysfunction is the basic mechanism that triggers development of atherosclerotic changes, and flow-mediated dilatation (FMD) in brachial artery is a noninvasive method to determine endothelial dysfunction [5].
Many clinical studies have demonstrated significant decreases in FMD, indicating endothelial dysfunction, in patients with CID relative to normal population, and derangement in FMD has been identified as predictor of atherosclerosis [6].
Recently, use of triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C) ratio has been recommended as a simple method to determine insulin resistance and cardiometabolic risk in healthy individuals [7, 8, 9, 10, 11]. A case-control study revealed that higher TG/HDL-C ratio can strongly predict risk of myocardial infarction [12].
The reliability of TG/HDL-C ratio in prediction of risk of atherosclerosis in patients with CID has not been investigated thus far. In this study, we tested whether TG/HDL-C ratio is increased in patients with CID relative to healthy population, and examined the relationship between FMD, an indicator of endothelial functions of arteries, and TG/HDL-C ratio. Based on the relationship between TG/HDL-C ratio and FMD, another objective of the study was to test whether combined use of these two markers would be a stronger predictor of atherosclerotic risk.
MATERIALS AND METHODS
Study population
A total of 58 CID patients, consisting of both AS and FMD patients, and a group of 58 healthy volunteers were included in the study. All participants were evaluated for major cardiovascular risk factors such as diabetes mellitus (DM), history of coronary artery disease (CAD), and use of cigarettes or alcohol. Exclusion criteria were history of stroke; congestive heart failure (CHF); CAD; hypertension; obstructive sleep apnea (OSA); impaired glucose tolerance; familial dyslipidemia; hepatic, henolytic, and renal diseases; excess alcohol intake (>120g/d); morbid obesity (body mass index [BMI]>35 kg/m2); and vasoactive drug users. Patients with Q wave, left bundle block, ST segment, and T wave changes specific to myocardial ischemia on electrocardiogram (ECG) were also excluded. The study was conducted in compliance with the World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Approval of the ethics committee was granted, and written informed consent was obtained from all study participants.
Biochemical analysis
Study groups were advised to abstain from intense physical activity and alcohol intake for 3 days before biochemical analysis. Venous blood samples were drawn from each participant after 24 hours of fasting to measure biochemical parameters. For the measurement of serum glucose, spectrophotometry was performed using Aeroset automated analyzer (Abbott Laboratories, Abbott Park, IL, USA). Total serum cholesterol, HDL-C, and low-density lipoprotein cholesterol (LDL-C) levels were measured using enzymatic methods. Plasma high-sensitivity-C-reactive protein (hs-CRP) levels were calculated using enzyme-linked immunosorbent assay (ELISA) test.
Echocardiographic study
All echocardiographic measurements were made using a GE Vivid 7 cardiac ultrasound (US) machine (GE Healthcare, Horton, Norway). Standard pulse wave, tissue Doppler, M-mode, and 2-D echocardiographic measurements were recorded [8]. Measurements of left ventricular end diastolic diameter (LVEDD) and left ventricular end systolic diameter (LVESD, interventricular septal (IVS), and posterior wall (PD) thickness were performed using M-mode modality from parasternal long-axis view.
E wave and E wave deceleration time (DT), and early (E) and late (L) diastolic peak flow velocities were retrieved from transmitral Doppler images. All tissue Doppler measurements were made from apical 4-chamber view with 5 mm-sample volume positioned lateral to the edge of mitral annulus [13]. During Doppler screening, 5–10 cycles with flow velocity of 100 mm/sec were recorded. Tissue Doppler measurements included myocardial early (E’), and atrial (A’) peak velocities (m/sec). Doppler US measurements were recorded during normal respiration. For the evaluation of LV diastolic function, E/A, E/E’, and E’/A’ values were calculated. Average of 3 measurements of diastolic parameters made during 3 successive cycles was computed. Measurements were performed by a researcher blind to patient data and analyzed by two researchers who were also blind to the study results.
Vascular evaluation
FMD of brachial artery was evaluated with measurement of response to transient ischemia using high-resolution ultrasonography [14]. Measurements were made while all participants were in supine position and rested for 10 minutes. Right arm of participants was free and brought to extension. FMD measurements of brachial artery were made 2–5 cm above antecubital fossa. Entire length of brachial artery was scanned using GE Vivid 7 17–5 MHz linear array transducer. B-mode and pulse Doppler spectral curves were recorded. Basal diameter of brachial artery was measured, and cuff of sphygmomanometer was wrapped proximal to artery visualized on forearm. Pressure cuff of sphygmomanometer was inflated to 30 mm Hg above systolic blood pressure of the patient and left in place for 5 minutes before being deflated. The maximum diameter of brachial artery was measured in this manner 6 times. All measurements were made at onset of end-diastolic R wave on ECG. FMD percentages of maximum diameter of brachial artery during hyperemia triggered by basal and transient ischemia were estimated based on the formula FMD=(maximum brachial artery diameter-basal brachial artery diameter/basal brachial artery diameter)x100.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). Normality of distribution of variables was evaluated using Shapiro-Wilk test. Descriptive statistics were expressed as medians (25–75 percentiles) for continuous variables and frequencies, and as percentages for categorical variables. In intergroup comparisons of continuous variables, Mann-Whitney U test was used, and for categorical variables chi-square test was used. Correlation coefficient and its significance were calculated using Spearman’s rank correlation test, and p<0.05 was accepted as the level of significance.
RESULTS
Study population
Median age of the patient (CID) and control group was 37 (31–47) and 39 (37–41) years, respectively, without any intergroup difference. Systolic and diastolic blood pressure and body mass indices of groups did not differ significantly (Table 1).
TABLE 1.
Comparison of demographic characteristics and biochemical parameters of study groups
| CID Group (n=58) | Control Group (n=58) | p | |
|---|---|---|---|
| Age, years | 37 (31–47) | 39 (37–41) | 0.16 |
| Gender, F/M | 19/39 | 25/33 | 0.34 |
| SBP, mmHg | 120 (110–125) | 120 (110–130) | 0.50 |
| DBP, mmHg | 80 (75–81) | 80 (76–80) | 0.72 |
| BMI, kg/m2 | 25.8±3.9 | 26.9±2.3 | 0.07 |
| FBG mg/dL | 94 (90–97) | 90 (87–96) | 0.08 |
| hs-CRP | 4.8 (1.8–12.0) | 1.3 (0.8–2.2) | <0.001 |
| LDL-C, mg/dL | 111±28 | 112±30 | 0.85 |
| HDL-C, mg/dL | 53 (45–55) | 53 (49–55) | 0.72 |
| Triglyceride, mg/dL | 124 (117–145) | 121 (110–132) | 0.06 |
| TG/HDL-C | 2.5 (2.2–2.8) | 2.3 (2.1–2.5) | 0.03 |
BMI: Body mass index; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; HDL-C: High-density lipoprotein cholesterol; hs-CRP: High-sensitivity C-reactive protein; LDL-C: Low-density lipoprotein cholesterol; SBP: Systolic blood pressure; TG: Triglyceride.
Biochemical evaluation
Hs-CRP levels of CID patients were significantly higher (4.8 [1.8–12.0] vs 1.3 [0.8–2.2]; p<0.001) (Table 1). TG/HDL-C levels of the patients with CID were significantly higher when compared to control group (2.5 [2.2–2.8] vs 2.3 [2.1–2.5]; p=0.03) (Figure 1).
FIGURE 1.
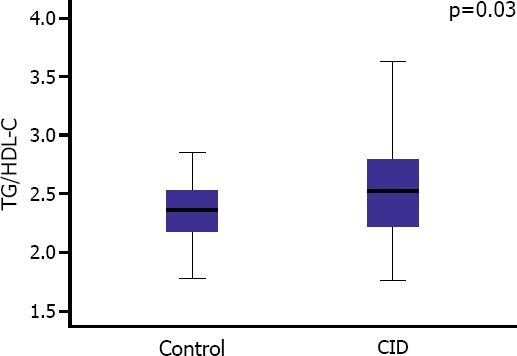
Comparison of TG/HDL-C ratio between study groups.
Echocardiographic examination
LVEDD, LVESD, ejection fraction (EF), thickness of IVS, and PD were similar between groups (Table 2). E/E’ ratios of patients with CID were lower when compared to control group with a p-value close to level of significance (3.8±0.9 vs 4.2±1.1; p=0.06) (Table 2).
TABLE 2.
Comparison of echocardiographic parameters and FMD values of study groups
| CID Group (n=58) | Control Group (n=58) | P | |
|---|---|---|---|
| E, m/s | 0.77±0.17 | 0.76±0.16 | 0.68 |
| A, m/s | 0.66±0.12 | 0.62±0.13 | 0.07 |
| E/A | 1.18±0.28 | 1.25±0.28 | 0.15 |
| DT, msec | 197 (177–221) | 189 (177–201) | 0.06 |
| IVRT, msec | 109±17 | 106±19 | 0.62 |
| E’, m/s | 0.21±0.06 | 0.19±0.04 | 0.05 |
| A’, m/s | 0.16±0.04 | 0.14±0.03 | <0.001 |
| E’/A’ | 1.31±0.42 | 1.35±0.31 | 0.57 |
| E/E’ | 3.8±0.9 | 4.2±1.1 | 0.06 |
| LVEDD mm | 46 (42–49) | 45 (42–48) | 0.57 |
| LVESD mm | 29 (27–32) | 29 (27–30) | 0.98 |
| IVS, mm | 9 (9–10) | 10 (9–11) | 0.12 |
| PD, mm | 9 (8–9) | 9 (8–10) | 0.13 |
| EF % | 68 (65–70) | 67 (65–70) | 0.35 |
| FMD % | 5.2 (4.2–6.3) | 6.7 (6.3–9.7) | <0.001 |
A: Atrial; DT: Deceleration time; E: Early; EF: Ejection fraction, FMD: Flow-mediated dilatation; IVRT: Isovolumic relaxation time; IVS: Interventricular septum; LVEDD: Left ventricular end-diastolic diameter; LVESD: Left ventricular end-systolic diameter; PPD: Posterior wall.
Vascular evaluation
FMD values of the patients with CID were significantly lower when compared to CID patients (5.2 [4.2–6.3] vs 6.7 [6.3–9.7]; p<0.001) (Figure 2). In addition, negative correlation was detected between elevated TG/HDL-C ratio and FMD values (Figure 3).
FIGURE 2.
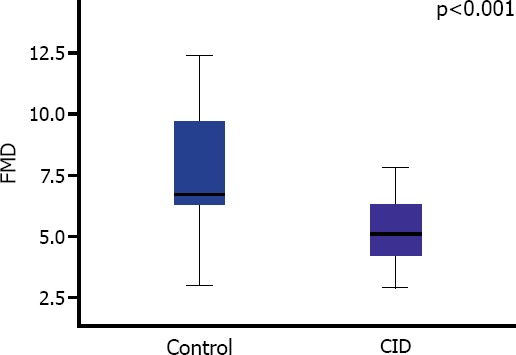
Comparison of FMD values of study groups.
FIGURE 3.
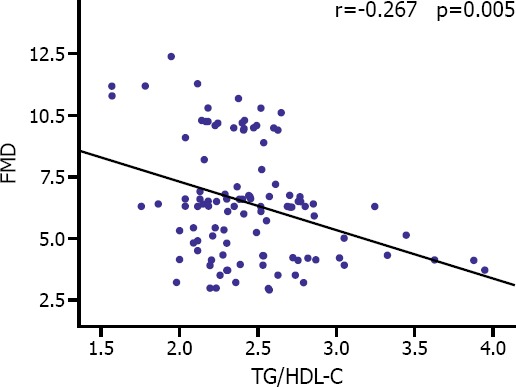
Correlation curve for comparison between TG/HDL-C ratio and FMD values of study population.
DISCUSSION
Studies have demonstrated that inflammation plays a fundamental role at all stages of atherosclerosis from beginning through development of thrombotic complications [2, 3].
FMF is an autoinflammatory disease caused by mutation of FMF gene, and is characterized by periodic hyperfebrile episodes and polyserositis. Asymptomatic periods exist between episodes of FMF, but during these intervals subclinical inflammation continues and it has been established that in cases of chronic inflammatory disease such as FMF, risk of atherosclerosis remains [15, 16].
Anxylosing spondylitis is a prototype of spondyloarthropathies, and in these patients 1.5–2-fold increase in mortality rates is seen due to cardiovascular complications compared to overall population. As is the case with other chronic inflammatory rheumatologic diseases, accelerated atherosclerotic processes are responsible for increase in cardiovascular mortality. Inflammation plays a fundamental role in accelerated atherogenesis in this patient group [17, 18, 19, 20, 21]. In spondyloarthropathic patients with chronic inflammatory polyarthritis, a significant correlation was observed between levels of C-reactive protein, erythrocyte sedimentation rate, and presence of endothelial dysfunction [2]. This observation reveals the role of inflammation in development of atherosclerosis in spondyloarthropathies.
Endothelial dysfunction was determined with a noninvasive method, while response of brachial artery diameter to reactive hyperemia was measured with the aid of high-resolution US device. Reactive hyperemia induces increase in blood flow, wall strain, nitric oxide release, and FMD. FMD can be measured as an index of vasomotor function [22, 23]. Close relationship between forearm and coronary arteries reflects systemic nature of atherosclerosis [24]. Çalışkan et al. revealed presence of endothelial dysfunction in patients with FMF [25]. Akdoğan et al. detected significantly lower FMD in FMF patients compared to healthy population [26]. Bodnár et al. demonstrated presence of endothelial dysfunction in patients with AS based on significant decrease in FMD values compared to healthy population [27], and Sarı et al. found significantly lower FMD values in AS patients when compared to healthy population [28].
In the present study, FMD values in a group of CID patients consisting of cases of AS, and FMF, were compared to those of healthy population and, similar to previous studies, significantly lower FMD levels were detected in the CID patient group.
FMD indirectly demonstrates endothelial dysfunction of coronary arteries, and TG/HDL-C ratio is an easily calculated and reproducible predictor of atherosclerosis in daily practice [29]. Close relationship between TG/HDL-C ratio and insulin resistance has been noted [30]. In addition, TG/HDL-C has been demonstrated to be a strong predictor independent of important prognostic variables, including total mortality, incidence of coronary artery disease, cardiovascular mortality, age, cigarette use, hypertension, and diabetes [31, 32, 33, 34].
Acay et al. detected significantly higher TG/HDL-C ratio in patients with FMF versus healthy population [35]. However, the correlation between TG/HDL-C ratio and endothelial dysfunction in CID cases has not been fully clarified.
In this study, correlation between TG/HDL-C ratio, defined as an atherogenic index, and FMD was investigated. In parallel with findings in previous studies, this study found TG/HDL-C ratio in patients with CID was significantly higher when compared to control group. A negative correlation was detected between FMD and TG/HDL-C ratio.
The findings of this study indicate that in patients with CID, increased TG/HDL-C ratio is correlated with decreased FMD value, an indicator of endothelial dysfunction that is precursor of atherosclerosis. Thus, higher TG/HDL-C ratio was found to be associated with presence of endothelial dysfunction and atherogenesis. In conclusion, this study determined that TG/HDL-C ratio in CID patients may be used as an easily evaluated, reliable predictor of atherosclerosis.
Footnotes
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship contributions: Consept - N.K., M.C.; Design - N.K., M.C., F.A., G.A., Y.Y.; Supervision - N.K., M.C.; Funding - N.K., M.C., Materials - N.K., M.C.; Data Collection and processing - N.K., M.C., F.A., G.A., Y.Y.; Analysis and interpretation - N.K., M.C., K.D., O.K., M.E.C, M.K.; Literature search N.K., M.C., K.D., O.K., M.E.C., M.K.; Writing - N.K., M.C., K.D., O.K., M.E.C., M.K.; Critical review - N.K., M.C., K.D., O.K., M.E.C., M.K.
REFERENCES
- 1.Del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Juanatey C, Llorca J, Miranda-Filloy JA, Amigo-Diaz E, Testa A, Garcia-Porrua C, et al. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:287–93. doi: 10.1002/art.22530. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Allahverdian S, Pannu PS, Francis GA. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc Res. 2012;95:165–72. doi: 10.1093/cvr/cvs094. [DOI] [PubMed] [Google Scholar]
- 5.Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79:93–100. doi: 10.1161/01.cir.79.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Sandoo A, Veldhuijzen van Zanten JJ, Metsios GS, Carroll D, Kitas GD. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2011;50:2125–39. doi: 10.1093/rheumatology/ker275. [DOI] [PubMed] [Google Scholar]
- 7.Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC, Espinoza-Camacho MA, Jiménez-Morales M, Piña E, et al. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res. 2013;54:2795–9. doi: 10.1194/jlr.M040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34:583–8. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 9.Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219–22. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Jia L, Long S, Fu M, Yan B, Tian Y, Xu Y, et al. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism. 2006;55:1141–8. doi: 10.1016/j.metabol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–5. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 13.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 14.Harris LM, Faggioli GL, Shah R, Koerner N, Lillis L, Dandona P, et al. Vascular reactivity in patients with peripheral vascular disease. Am J Cardiol. 1995;76:207–12. doi: 10.1016/s0002-9149(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 15.Drenth JP, van der Meer JW. Hereditary periodic fever. N Engl J Med. 2001;345:1748–57. doi: 10.1056/NEJMra010200. [DOI] [PubMed] [Google Scholar]
- 16.Holmes AH, Booth DR, Hawkins PN. Familial Mediterranean fever gene. N Engl J Med. 1998;338:992–3. doi: 10.1056/NEJM199804023381413. [DOI] [PubMed] [Google Scholar]
- 17.Lehtinen K. Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis. 1993;52:174–6. doi: 10.1136/ard.52.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by B-mode ultrasound: the Atherosclerosis Risk in Communities Study. Ultrasound Med Biol. 1996;22:791–9. doi: 10.1016/0301-5629(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu S, Joly H, Baron G, Tournadre A, Dubost JJ, Ristori JM, et al. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology (Oxford) 2008;47:1203–7. doi: 10.1093/rheumatology/ken198. [DOI] [PubMed] [Google Scholar]
- 20.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 21.Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34:585–92. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, et al. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–53. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, et al. Low Serum Level of Klotho Is an Early Predictor of Atherosclerosis. Tohoku J Exp Med. 2015;237:17–23. doi: 10.1620/tjem.237.17. [DOI] [PubMed] [Google Scholar]
- 25.Caliskan M, Gullu H, Yilmaz S, Erdogan D, Unler GK, Ciftci O, et al. Impaired coronary microvascular function in familial Mediterranean fever. Atherosclerosis. 2007;195:161–7. doi: 10.1016/j.atherosclerosis.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Akdogan A, Calguneri M, Yavuz B, Arslan EB, Kalyoncu U, Sahiner L, et al. Are familial Mediterranean fever (FMF) patients at increased risk for atherosclerosis? Impaired endothelial function and increased intima media thickness are found in FMF. J Am Coll Cardiol. 2006;48:2351–3. doi: 10.1016/j.jacc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Bodnár N, Kerekes G, Seres I, Paragh G, Kappelmayer J, Némethné ZG, et al. Assessment of subclinical vascular disease associated with ankylosing spondylitis. J Rheumatol. 2011;38:723–9. doi: 10.3899/jrheum.100668. [DOI] [PubMed] [Google Scholar]
- 28.Sari I, Okan T, Akar S, Cece H, Altay C, Secil M, et al. Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology (Oxford) 2006;45:283–6. doi: 10.1093/rheumatology/kei145. [DOI] [PubMed] [Google Scholar]
- 29.Sonmez A, Yilmaz MI, Saglam M, Unal HU, Gok M, Cetinkaya H, et al. The role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict cardiovascular outcomes in chronic kidney disease. Lipids Health Dis. 2015;14:29. doi: 10.1186/s12944-015-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Chávez A, Simental-Mendía LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79:126–31. [PubMed] [Google Scholar]
- 31.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2009;157:548–55. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drexel H, Aczel S, Marte T, Benzer W, Langer P, Moll W, et al. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care. 2005;28:101–7. doi: 10.2337/diacare.28.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Shishehbor MH, Hoogwerf BJ, Lauer MS. Association of triglyceride-to-HDL cholesterol ratio with heart rate recovery. Diabetes Care. 2004;27:936–41. doi: 10.2337/diacare.27.4.936. [DOI] [PubMed] [Google Scholar]
- 34.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med. 2001;161:361–6. doi: 10.1001/archinte.161.3.361. [DOI] [PubMed] [Google Scholar]
- 35.Acay A, Ulu MS, Ahsen A, Ozkececi G, Demir K, Ozuguz U, et al. Atherogenic index as a predictor of atherosclerosis in subjects with familial Mediterranean fever. Medicina (Kaunas) 2014;50:329–33. doi: 10.1016/j.medici.2014.11.009. [DOI] [PubMed] [Google Scholar]


