Abstract
OBJECTIVE:
Antagonism of the central nervous system inhibitor neurotransmitter gamma-Aminobutyric acid (GABA) or serotonergic system activation is an important factor in the pathogenesis of intrathecal morphine-induced pruritus. This study tested the hypothesis that preoperative use of ondansetron, gabapentin or mirtazapine can prevent morphine-induced pruritus.
METHODS:
We randomly allocated 80 patients of American Society of Anesthesiology (ASA) classification I and II physical status who were to undergo unilateral inguinal hernia or pilonidal sinus operations under spinal anesthesia into 4 equal groups. The first 3 groups received oral doses of 30 mg mirtazapine, 8 mg ondansetron, and 1200 mg gabapentin at 2 hours, 10 minutes, and 1 hour before surgery, respectively, and the fourth group was given a placebo. All patients received intrathecal injection of 15 mg of 0.5% hyperbaric bupivacaine and 0.2 mg morphine. Pruritus was evaluated at 0, 3, 6, 9, 12, and 24 hours after intrathecal morphine administration, and details of presence, onset time, duration, localization, and severity of pruritus were recorded.
RESULTS:
Incidence of pruritus was significantly more frequent in the placebo group compared to ondansetron, gabapentin, and mirtazapine groups (70%, 55%, 35%, and 35%, respectively). In general, onset of pruritus was between 2 and 6 hours after intrathecal morphine injection; however, onset in the gabapentin group (mean±SD: 4.75±2.7 hours; p=0.019) was delayed compared to other groups. It was observed that pruritus persisted relatively longer in the ondansetron and placebo groups (mean±SD: 6±3.08; 5.82±2.96 hours, respectively; p=0.047). No statistical determination was made regarding location of pruritus. Severity of pruritus was greater in the placebo group (p=0.0001). Necessity for antipruritic treatment was not statistically significantly different between groups.
CONCLUSION:
Incidence and severity of intrathecal morphine-induced pruritus decreased with use of each of all 3 drugs compared to placebo.
Keywords: Gabapentin, intrathecal, mirtazapine, morphine, ondansetron, pruritus
Intrathecal use of morphine provides intense, prolonged analgesic effect, and it is frequently used in the relief of postoperative pain [1]. However, the side effects of opioids, which can affect patient comfort and safety, restrict the therapeutic effects. Itching is the most frequently seen side effect of intrathecal morphine, with an incidence ranging 62–94% [2, 3]. Itching continues to be a challenging and problematic issue that is difficult for anesthetists to treat [4, 5]. Pharmacological agents, such as antihistamines, 5-HT3 (serotonin) receptor antagonists, opioid antagonists, opioid agonist-antagonists, propofol, and non-steroidal anti-inflammatory drugs (NSAID) have been used to fight this challenging side effect [1, 6]. Interaction between intrathecal morphine and 5-hydroxytryptamine subtype 3 (5-HT3), which affects the central nervous system (CNS), is thought to play a role in the pathogenesis of itching [5]. Therefore, ondansetron, as a 5-HT3 receptor antagonist, and mirtazapine, a presynaptic a-2 antagonist that increases central serotoninergic effect by blocking 5-HT2 and 5-HT3 receptors, may be effective in controlling itching. Though its mechanism has not been explained, itching may be associated with opioids that behave as antagonists to central inhibitor neurotransmitters (GABA and glycine) [7]. The anticonvulsant agent gabapentin, which is a structural analogue of GABA, may be effective in the management of itching. To this end, the present prospective, randomized, placebo-controlled study was conducted to determine the efficacy of ondansetron, gabapentin, and mirtazapine in the prevention of the itching that frequently develops after spinal anesthesia using bupivacaine and morphine on patients undergoing unilateral inguinal hernia or pilonidal sinus surgery, and to assess the superiority of one drug over another.
MATERIALS AND METHODS
The present study was performed on a total of 80 patients with American Society of Anesthesiology (ASA) classification I and II physical status aged 18–65 years who underwent unilateral inguinal hernia or pilonidal sinus surgery. Approval was obtained from the ethics committee of Istanbul University Faculty of Medicine.
During preoperative visit, all patients were informed about the procedure and their written consent was obtained. Patients for whom regional anesthesia was contraindicated; those who declined to undergo the procedure; individuals allergic to drugs used; patients with any systemic disease that causes itching; patients with history of locomotor diseases or postoperative nausea and vomiting; those suffering from preoperative pruritus; cases treated with opioids or antiemetics; mentally retarded persons; and antidepressant, antipsychotic, and antiepileptic drug users were not included in the study. In addition, patients in whom dural puncture could not be achieved, and those using opioids perioperatively as pain relievers were excluded from the study. None of the patients received premedication. Patients were randomized equally into groups of 20 subjects. As prophylaxis, Group 1 received oral dose 8 mg ondansetron 10 minutes before the operation, and Group 2 was given oral dose 1200 mg gabapentin 1 hour preoperatively. Group 3 received oral dose 30 mg mirtazapine 2 hours before surgery, and Group 4 was given sugar pills as placebo 10 minutes before the operation.
Venous access was provided using a 20-gauge cannula 30 minutes preoperatively, and patients were hydrated with 500 ml 0.9% NaCl intravenous (IV) infusion. Patients were monitored, and noninvasive measurements of systolic arterial pressure (SAP), mean arterial pressure (MAP), heart rate (HR), and blood oxygen saturation (SpO2) level were taken. Patients were seated, and site of the spinal anesthesia was disinfected with sterile solution. L3-4 or L4-5 intervertebral space was located and 26-gauge spinal needle was inserted through midline approach to enter subarachnoidal space. After clear drainage of cerebral spinal fluid (CSF) was observed, spinal anesthesia was applied using 0.5% hyperbaric bupivacaine 15 mg (3 mL), and 0.2 mg (0.1 mL) morphine. Patients were placed in supine position and head was elevated 30 degrees. Level of sensory block was evaluated using pinprick test for dermatome level, and grade of motor block was assessed using Bromage Scale: 0=lack of paralysis, the patient is able to freely move hip, feet, knee, and ankle; 1=patient is able to move knee, and feet, but cannot elevate straight leg; 2=patient cannot bend knee, can only move feet; 3=complete paralysis.
At the time of surgical team incision, patients were given midazolam (Demizolam®) at a dose of 0.05 mg/kg. Degree of sedation was evaluated using Ramsay scale: 1=patient is agitated and restless; 2=patient is cooperative, oriented, and tranquil; 3=patient is sleeping, but responds to verbal stimuli; 4=patient is sleeping, moderate response to painful stimuli; 5=patient is sleeping, slow response to painful stimuli; 6=patient is sleeping, lack of any response to painful stimuli.
SAP and MAP levels below 80 and 60 mmHg, respectively, were accepted as hypotension. In the event of persistent hypotension, IV fluid replacement, then 5–10 mg IV ephedrine was administered. Heart rate below 50 bpm was considered bradycardia and treated with 0.5 mg IV atropine. In addition, it was predetermined that midazolam would be re-instituted during operation if needed to maintain Ramsay score between 2–4 points and that oxygen support via facemask would be kept ready in case of need.
At postoperative 0, 3, 6, 9, 12, and 24 hours, patients were questioned about the presence of itching, and if present, further questioned about time of onset, duration, location, and severity (none, mild, moderate or severe). If itching was severe, then 10 mg IV diphendydramine was administered, followed by an opioid antagonist, if necessary. At the same time intervals, patients were questioned about severity of pain using visual analogue scale (VAS) in which 0 indicated patient was asymptomatic and 10 represented the most severe pain. Intramuscular diclofenac sodium at a dose of 75 mg was administered when VAS score was >5. At the same time intervals, inquiries were also made about presence of nausea or vomiting (absent, mild, moderate or severe). In case of severe nausea or vomiting, metoclopramide (10 mg IV) was administered. Other side effects related to intrathecal morphine application, including urinary retention, constipation, and respiratory depression, were also evaluated.
While evaluating study data, for statistical analysis Number Cruncher Statistical System software (2007 package; NCSS, LLC; Kaysville, Utah, USA) was used. In addition to descriptive statistical methods (mean, standard deviation), in repetitive measurements of multiple groups, Friedman test was used, and for comparisons between groups, Kruskal-Wallis test was used. Subgroups were compared employing Dunn’s multiple comparison test, and for qualitative data, chi-square and McNemar’s tests were used. Results were evaluated at a level of p<0.05.
RESULTS
None of the eligible patients was excluded from the study for any reason; a total of 80 patients were allocated equally into 4 groups that were compared based on demographic data. No significant difference was seen between groups with regard to age, gender, body weight, operative time, and type of surgery (p>0.05) (Table 1).
TABLE 1.
Comparison of demographic data of the patient groups
| Group 1 (n=20) | Group 2 (n=20) | Group 3 (n=20) | Group 4 (n=20) | |
|---|---|---|---|---|
| Age (years) | 41.6±10.92 | 41.4±10 | 41.75±9.94 | 41.55±9.84 |
| Gender (M/F) | 16/4 | 15/5 | 16/4 | 17/3 |
| Height (cm) | 174±6.97 | 171.9±6.27 | 173.95±6.64 | 173.15±6.64 |
| Body weight (kg) | 76.95±7.91 | 76.65±6.83 | 76.4±7.98 | 78.45±8.09 |
| Operative time (min) | 62±11.85 | 60.25±11.64 | 61.25±7.41 | 61.25±9.72 |
| Type of surgery (n; %) | ||||
| İnguinal hernia repair | 13; 53.84% | 12; 60% | 12; 60% | 12; 60% |
| Pilonidal sinus excision | 7; 46.16% | 8; 40% | 8; 40% | 8; 40% |
During 24 hours of follow-up, a statistically significant difference was observed between incidence of itching at postoperative 3 and 6 hours (p=0.01 and p=.008, respectively). At these time points, incidence of itching was higher in Group 4 compared to Groups 1, 2, and 3. However at other time points, a significant difference was not detected among the 4 groups (Table 2).
TABLE 2.
Comparison of incidence of itching in patient groups
| Group 1 | Group 2 | Group 3 | Group 4 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| 0 hr | 1 | 5 | 0 | 0 | 1 | 5 | 3 | 15 | =0.256 |
| 3 hr | 7 | 35 | 4 | 20 | 7 | 35 | 14 | 70 | =0.01 |
| 6 hr | 11 | 55 | 7 | 35 | 4 | 20 | 14 | 70 | =0.008 |
| 9 hr | 5 | 25 | 7 | 35 | 2 | 10 | 7 | 35 | =0.228 |
| 12 hr | 1 | 5 | 3 | 15 | 0 | 0 | 3 | 15 | =0.238 |
| 24 hr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
A statistically significant difference was observed between onset and duration of itching in all patient groups. In Group 2, onset of itching was statistically significantly delayed compared to Group 3 and Group 4 (p=0.019). Duration of itching in Group 1 and Group 4 was found to be statistically significantly longer versus Groups 2 and 3 (p=0.047) (Figure 1).
FIGURE 1.
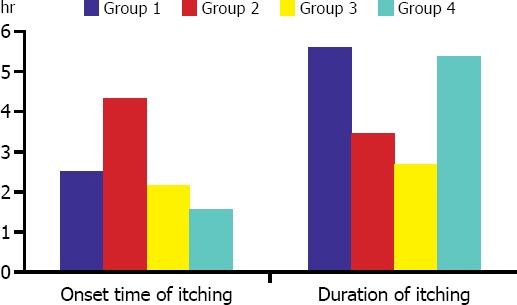
Comparison of patient groups regarding onset time and duration of itching.
Itching was observed on face, trunk, lower, and upper extremities, and no significant intergroup difference was detected with regard to location of itching. In Group 4, severity of itching observed at postoperative third and sixth hours was higher relative to other groups. (p=0.006, and p=0.009, respectively). However, a significant difference was not found between groups as for necessity of treatment for itching (Figure 2).
FIGURE 2.
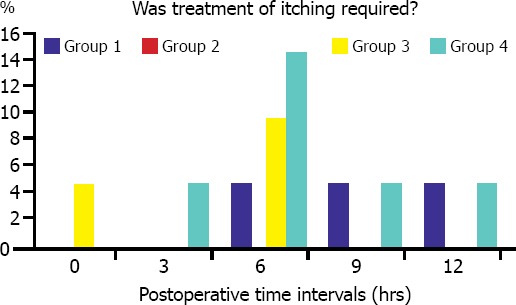
Comparison of the patient groups regarding treatment of itching.
In Group 4, mean VAS scores detected at postoperative hour 4 and 9 were higher compared to other groups. However, need for pain treatment did not differ among groups. No difference in presence of adverse effects of opioids, such as nausea, vomiting, and urinary retention was observed. While no difference was detected between intraoperative sedation doses, mean Ramsay score at post treatment 60 minutes in Group 2 was found to be statistically significantly higher when compared to mean values of Groups 1, 3, and 4 (p=0.004). Ramsay sedation scores did not exceed 5 points in any of the patients, nor was respiratory depression seen in any patient.
DISCUSSION
Intrathecal morphine is an attractive alternative in the management of postoperative pain in that it provides intense and long-term analgesia without motor block and marked central system depression [1]. Itching is the most frequently seen side effect of intrathecal opioids, with a reported incidence of 62–94% [2, 3, 4, 5, 6, 7, 8]. Numerous theories have been proposed to explain the mechanism of itching caused by intrathecal opioids, however underlying etiology is still unknown. This unwanted side effect can be very disturbing to the patient, patient may be resistant to traditional antipruritic treatments, itching may adversely affect patient satisfaction, and may hinder postoperative management of pain.
In the present study evaluating itching related to the use of intrathecal morphine, itching of relatively higher incidence (70%) and severity was observed in the placebo group. In all groups, itching was generally observed to begin between postoperative hours 2 and 6; in the gabapentin group, time of onset was greater than in other groups (6±3.08 hours vs 5.82±2.96 hours). Scratch marks were observed on face, trunk, upper, and lower extremities; however, in none of these locations was the itching statistically significantly more frequent. Excluding the mirtazapine group, in all other groups itching was more severe at 3, 6, and 9 hours post surgery. Treatment for itching was required in 1 ondansetron, 2 mirtazapine, and 3 placebo users, while none of the patients in the gabapentin group required antipruritic treatment. Yet a significant difference in need for treatment was not observed between groups.
Sheen et al. administered prophylactic treatment with 1200 mg oral gabapentin or placebo to prevent itching developed after orthopedic surgery of lower extremities in 2 groups of 43 patients who had received 0.2 mg intrathecal morphine, and they found higher incidence rates of itching in placebo group when compared with gabapentin group (77.5% vs 47.5%) [9]. Similar to the present study, itching manifested later in the gabapentin group relative to the placebo group (gabapentin: 6.2±1.8 hours). In the current study location sites of itching did not differ between groups; however, in the Sheen et al. study, itching was most frequently (68%) observed in the trigeminal region. In addition, in the present study, antipruritic treatment was not required in the gabapentin group; however, 3% of patients in the gabapentin group in their study required antipruritic treatment.
In another study, Sheen et al. compared prophylactic use of mirtazapine with placebo on 110 patients, and observed higher incidence of itching in the placebo group (52% and 75%, respectively). Duration of itching was not different between groups, but onset of itching was delayed with mirtazapine premedication (3.2±7.2 hours) [10]. In the current study, time to onset of itching did not differ between mirtazapine and placebo groups, but onset of itching was delayed in the gabapentin group (4.75±2.7 hours). Though itching was more frequently localized on face, in the mirtazapine group, itching was most frequently localized on the trunk. The face was also the most common location for itching in the placebo group. Itching was more severe in the placebo group, and 19% of the patients required treatment. In the mirtazapine group, only 4 patients needed treatment. In the current study, treatment was required for 10% of the patients in the mirtazapine group, and 15% in the placebo group, without any significant intergroup difference in the severity of itching.
Koju et al. used prophylactic doses of 4 mg IV ondansetron, and also 4 mL physiologic saline as placebo in 50 patients who were to undergo cesarean section in order to prevent itching caused by intrathecal morphine, and observed itching 16–88% more frequently in the placebo group. Ondansetron also demonstrated effectiveness reducing postoperative nausea and vomiting, two side effects that were seen more frequently in the placebo group (8% and 56%, respectively) [11]. In another study investigating intrathecal morphine-induced itching, Kung et al. prophylactically used 8 mg IV ondansetron during umbilical cord clamping or postoperatively in the postanesthesia recovery room as a therapeutic dose in 82 patients who had undergone cesarean section, while the placebo group received only physiologic saline. When compared to the placebo group, prophylactic or therapeutic use of ondansetron did not decrease severity of itching at all [12]. In the present study, decrease in the severity of itching at third and sixth hours after administration of ondansetron was observed, but without any significant difference when compared to placebo group.
In a prospective randomized, double-blind placebo-controlled study performed by Chiravanich et al. on 180 cases of orthopedic surgery using intrathecal 0.5% isobaric bupivacaine and 0.2 mg morphine, the authors compared preoperative prophylactic use of 600 mg oral gabapentin and placebo in the prevention of itching induced by intrathecal morphine [13]. They observed only 21.4–41.7% decrease in the severity of itching at postoperative 4 hours without serious drop in the degree of itching with gabapentin [14]. In the current study, gabapentin decreased severity of itching at postoperative 3 and 6 hours, without a significant difference compared to placebo group.
Szarvas et al. compared higher doses of intrathecal morphine (dose of 0.01 mg/kg up to 0.7 mg) with ondansetron and placebo in the prevention of postoperative itching, and apart from above-mentioned studies, superiority of ondansetron over placebo was not reported [1]. Yazigi et al. combined highly lipophilic sufentanyl (2.5 mcg) to intrathecal 0.1 mg morphine, and compared this combination with 8 mg ondansetron and placebo, and couldn’t observe any difference between groups in prevention of postoperative itching [15]. This finding was explained by preferential binding of sufentanyl to serotonin receptors in the spinal cord relative to ondansetron because of its rapid onset of action.
Pirat et al. compared ondansetron and placebo in young male patients, and Somrat Charuluxananan et al. compared nalbufin, ondansetron, and placebo in 240 women who had undergone cesarean section. As in the present study, a significant difference was not detected between groups regarding postoperative nausea and vomiting [16, 5]. The effect of morphine on the chemoemetic trigger zone in the area postrema is dependent on size of dose, and Yazigi et al. found ondansetron to be superior to placebo in the prevention of nausea and vomiting with smaller dose of morphine [15]. A significant intergroup difference was not detected with respect to pain score, urinary retention, gastrointestinal side effects or sedation. Respiratory depression was not seen in any patient. In a study performed by Chinachoti et al. on patients receiving intrathecal morphine, as in the Sheen et al. study where mirtazapine and placebo were compared, mirtazapine provided significantly higher (50%) levels of sedation when compared with the placebo [10, 14].
The present study had some limitations. The perception of itching manifests with individual variation; however, efforts were made to evaluate and measure its effects with care. Second, use of prophylactic drugs with similar taste and appearance in all groups may be preferable. In addition, precise appropriate doses to prevent itching were unknown. Finally, antipruritic drugs used in the clinic for prophylaxis had different degrees of effectiveness. Lack of statistical difference was attributed to small number of patients in population group; therefore, studies with larger patient series will be more appropriate.
In conclusion, the present study compared antipruritic effectiveness of prophylactic oral dose of 8 mg ondansetron, 1200 mg gabapentin, 30 mg mirtazapine, and sugar pills given as oral placebo administered to a total of 80 patients allocated equally into 4 groups before application of intrathecal 15 mg (3 mL) 0.5% hyperbaric bupivacaine plus 0.2 mg (0.1 mL) morphine for spinal anesthesia for unilateral hernia repair or pilonidal sinus surgery. All 3 drugs decreased incidence, duration, and severity of itching when compared with placebo. No intergroup difference was observed with regard to requirement for antipruritic treatment. The authors recommend studies with larger patient series to further examine this subject.
Footnotes
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship contributions: Concept - A.A.; Design - F.D.S.; Supervision - O.E.; Materials - A.A.; Data collection and/or processing - S.B., H.P., G.B., G.T.; Analysis and/or interpretation - A.A., F.D.S., Literature search - A.A., R.Y.A.; Writing - A.A., R.Y.A.; Critical review - O.E.
REFERENCES
- 1.Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15:234–9. doi: 10.1016/s0952-8180(02)00501-9. [DOI] [PubMed] [Google Scholar]
- 2.Carr D, Cousins M. Spinal route of analgesia, opioids and future options. In: Bridenbaugh P, editor. Neural blockade in clinical anesthesia and management of pain. 3rd ed. Philadelphia: Lippincott-Raven; 1998. pp. 115–83. [Google Scholar]
- 3.Charuluxananan S, Somboonviboon W, Kyokong O, Nimcharoendee K. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25:535–9. doi: 10.1053/rapm.2000.7809. [DOI] [PubMed] [Google Scholar]
- 4.Szarvas S, Chellapuri RS, Harmon DC, Owens J, Murphy D, Shorten GD. A comparison of dexamethasone, ondansetron, and dexamethasone plus ondansetron as prophylactic antiemetic and antipruritic therapy in patients receiving intrathecal morphine for major orthopedic surgery. Anesth Analg. 2003;97:259–63. doi: 10.1213/01.ane.0000066310.49139.2a. [DOI] [PubMed] [Google Scholar]
- 5.Charuluxananan S, Kyokong O, Somboonviboon W, Narasethakamol A, Promlok P. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2003;96:1789–93. doi: 10.1213/01.ANE.0000066015.21364.7D. [DOI] [PubMed] [Google Scholar]
- 6.Yeh HM, Chen LK, Lin CJ, Chan WH, Chen YP, Lin CS, et al. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2000;91:172–5. doi: 10.1097/00000539-200007000-00032. [DOI] [PubMed] [Google Scholar]
- 7.Gürkan Y, Toker K. Prophylactic ondansetron reduces the incidence of intrathecal fentanyl-induced pruritus. Anesth Analg. 2002;95:1763–6. doi: 10.1097/00000539-200212000-00054. [DOI] [PubMed] [Google Scholar]
- 8.Korhonen AM, Valanne JV, Jokela RM, Ravaska P, Korttila K. Ondansetron does not prevent pruritus induced by low-dose intrathecal fentanyl. Acta Anaesthesiol Scand. 2003;47:1292–7. doi: 10.1046/j.1399-6576.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 9.Sheen MJ, Ho ST, Lee CH, Tsung YC, Chang FL. Preoperative gabapentin prevents intrathecal morphine-induced pruritus after orthopedic surgery. Anesth Analg. 2008;106:1868–72. doi: 10.1213/ane.0b013e3181730130. [DOI] [PubMed] [Google Scholar]
- 10.Sheen MJ, Ho ST, Lee CH, Tsung YC, Chang FL, Huang ST. Prophylactic mirtazapine reduces intrathecal morphine-induced pruritus. Br J Anaesth. 2008;101:711–5. doi: 10.1093/bja/aen241. [DOI] [PubMed] [Google Scholar]
- 11.Koju RB, Gurung BS, Dongol Y. Prophylactic administration of ondansetron in prevention of intrathecal morphine-induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. BMC Anesthesiol. 2015;15:18. doi: 10.1186/1471-2253-15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kung AT, Yang X, Li Y, Vasudevan A, Pratt S, Hess P. Prevention versus treatment of intrathecal morphine-induced pruritus with ondansetron. Int J Obstet Anesth. 2014;23:222–6. doi: 10.1016/j.ijoa.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Chiravanich W, Oofuvong M, Kovitwanawong N. Single dose of gabapentin for prophylaxis intrathecal morphine-induced pruritus in orthopedic surgery: a randomized controlled trial. J Med Assoc Thai. 2012;95:186–90. [PubMed] [Google Scholar]
- 14.Chinachoti T, Nilrat P, Samarnpiboonphol P. Nausea, vomiting and pruritus induced by intrathecal morphine. J Med Assoc Thai. 2013;96:589–94. [PubMed] [Google Scholar]
- 15.Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F, Hayek G. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritus after cesarean delivery with intrathecal sufentanil-morphine. J Clin Anesth. 2002;14:183–6. doi: 10.1016/s0952-8180(01)00381-6. [DOI] [PubMed] [Google Scholar]
- 16.Pirat A, Tuncay SF, Torgay A, Candan S, Arslan G. Ondansetron orally disintegrating tablets versus intravenous injection for prevention of intrathecal morphine-induced nausea, vomiting, and pruritus in young males. Anesth Analg. 2005;101:1330–6. doi: 10.1213/01.ANE.0000180830.12355.D9. [DOI] [PubMed] [Google Scholar]


