Abstract
OBJECTIVE:
Anti-streptolysin O (ASO) and anti-DNase B (ADB) titers are used for the diagnosis of poststreptococcal complications. Ranges of normal values of ASO and ADB titers vary, depending on age, population and different time intervals. Although many studies have been performed for determination of the ASO titer in our country, only a few studies have been conducted for specification the upper limit of normal for (ULN) ADB. In our study we aimed to determine the upper limit of normal of ADB antibody titers in children aged 5–15.
METHODS:
One hundred and twenty one children aged from 5–15 who were admitted to our outpatient clinic of Haydarpaşa Numune Training and Research Hospital with noninfectious reasons between November 2013 and March 2014 were included in the study. Patients who met the following criteria were included in the study; absence of streptococcal infection in the last three months in physical examination and/or no growth of group A, C, and G of beta-haemolytic streptococci in throat culture, normal ranges of ASO and C reactive protein (CRP) levels. All serum samples were analyzed collectively by nephelometric method. The upper limit of normal value for anti-DNase B has been defined by separating the upper 20% from the lower 80% of all measurements.
RESULTS:
Anti-DNase B antibody levels were ranged between 50–576 IU/ml and its upper limit was 219.2 IU/ml. When analyzed according to age groups, anti-DNase B antibody levels in the group aged between 5–10, ranged between 50–576 IU/ml and its upper limit was 212.2 IU/ml, anti-DNase B antibody levels in the group aged 10–15, ranged between 50–408 IU/ml and its upper limit was 231.2 IU/ml (p=0.008).
CONCLUSION:
Based on our results, upper normal values ADB antibody showed variations with age in our results. Therefore national reference values should be detected by more comprehensive studies.
Keywords: Anti DNase B, serological tests, streptococcus pyogenes
Among infections caused by Streptococcus pyogenes (S. pyogenes) acute tonsillopharyngitis, and skin infections have particular importance. Acute rheumatoid fever (ARF), and poststreptococcal glomerulonephritis (PSGN) are two major suppurative complications developing after these infections. Acute glomerulonephritis develops after streptococcal pharyngitis or skin infection while ARA emerges after pharyngitis. In the diagnosis of these complications, clinical findings and results of the serologic tests is used, most frequently, measurements of antistreptolysin-O antibody (ASO), and anti-DNase B (ADB) titers have been used. Eighty-five percent of the cases are either carriers of group A beta hemolytic streptococci or they contract ASO during throat infection. Contrary to throat infections, in skin infections a weak ASO response is elicited while ADB levels increase.
Therefore, in the diagnosis of these infections combined use of ASO and ADB is recommended. Since antibody levels in ASO and ADB tests change depending on some factors as patient’s age, type of bacterial population (M serotype of S.pyogenes), epidemiology, and methods of measurements, each population should determine their own normal an-tibody titers at certain intervals [1]. In our country, despite numerous studies performed in various region and diverse age groups aiming at determination of normal ASO titers, limited number of studies investigating normal values of ADB titres have been conducted.
Therefore, in our study, we aimed to determine the upper limit of normal (ULN) value for ADB in children aged between 5–15 in our region.
MATERIALS AND METHODS
The study was performed in Microbiology Laboratory of Haydarpasa Numune Training and Research Hospital between November 2013 and March 2014. Before initiation of the investigation, approval was obtained from the Ethics Committee of Haydarpasa Numune Training and Research Hospital (KAEK 2013/KK/100).
A total of 121 children who were admitted to the outpatient clinics of pediatrics, for noninfectious reasons were included in the study. For the investigation, additional blood samples were not withdrawn from patients and the study was realized with blood samples obtained for routine tests. The children included in the study were questioned as for the presence of upper respiratory tract and skin infection experienced within the previous three months before initiation of the study. Children with a history of streptococcal infection or symptoms and signs of this infection were not included in the study.
Throat cultures were obtained from the children with suspect streptococcal infection contracted within the last three months. Thus, the patients with group A, C and G beta hemolytic streptococci colonization were not included in the study. Among infection markers, C-reactive protein (CRP) (Cardiophase®hs CRP system BNII/BN proSpec, Siemens) was analyzed, and patients with levels above 0.8 mg/dl were excluded from the study. Levels of ASO titers were measured collectively using automated nephelometric method (N latex ASL system BN2, Siemens Healthcare Diagnostics Inc. Newark USA). Children with higher serum ASO titers were excluded from the study.
Sera of patients without streptococcal infection or colonization and those with CRP (<0.8 mg/dl), and ASO titers (<200 IU/ml) within normal limits were separated, and kept at –70°C. Analysis of all serum samples were performed simultaneously. Levels of ADB were measured using automated nephelometric immunoassays in line with the recommendations of the manufacturing firm (Image 800, Nephelometry Array Systems, Beckman-Coulter Inc., USA). For statistical analysis NCSS (Number Cruncher Statistical System) 2007&PASS (Power Analysis and Sample Size) 2008 Statistical Software (Utah, USA) program was used.
RESULTS
Serum anti-DNase B levels of 121 patients who met all specified criteria were analyzed. Sixty-four male (52.9%), and 57 female (47.1%) pediatric patients with a mean age of 8.88±3.2 (range 5–15) were included in the study. Titers of these children ranged between 50 IU/ml, and 576 IU/ml (mean, 123.6±118.7 IU/ml) (Table 1). The distribution of ADB titers is graphically shown in Figure 1.
TABLE 1.
Mean ADB values among healthy children aged 5–15 years
| Min.-Max. | Mean±SD | |
| Age (year) | 5–15 | 8.88±3.2 |
| ADB (IU/mL) | 50–576 | 123.60±118.7 |
| n† | % | |
| Age (year) | ||
| 5–10 | 85 | 70.2 |
| 11–15 | 36 | 29.8 |
| Gender | ||
| Male | 64 | 52.9 |
| Female | 57 | 47.1 |
ADB: Anti-DNaseB; Min.: Minimum; Max.: Maximum; SD: Standard deviation;
Number of serum samples.
FIGURE 1.
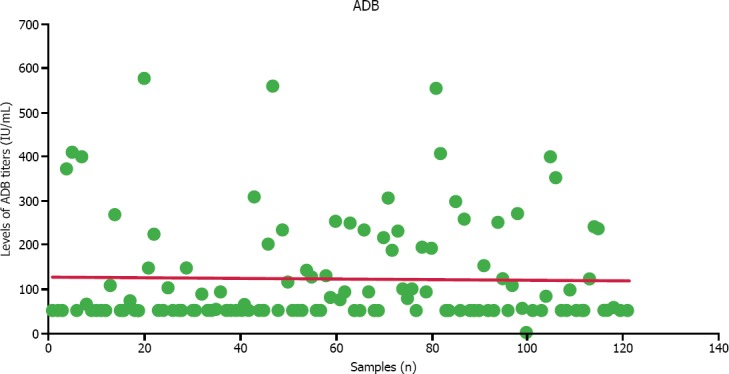
Graph demonstrating the distribution titers of ADB antibodies.
In our study, the children were evaluated based on their age groups as group 1 (5–10 yrs) and 2 (11–15 yrs). When ULN for titers of ADB were calculated based on measurements of 80% of the samples,[2] ULN values for ADB titers in Groups 1 and 2 were 212.2 IU/ml and 231.2 IU/ml, respectively (Table 2).
TABLE 2.
ADB titers according to age groups
| Age (year) | p | ||
|---|---|---|---|
| 5–10 years | 11–15 years | ||
| ADB (IU/ml) | |||
| Min.-Max. (Median) | 50–576 (50) | 50–408 (102.8) | |
| Mean±SD | 117±123.8 | 137.2±104.8 | |
| Geometric mean | 82.6 | 107.1 | |
| ULN (IU/ml) | 212.2 | 231.2 | 0.008** |
ADB: Anti-DNase B; Min.: Minimum; Max.: Maximum; SD: Standard deviation; ULN: Upper limit of normal value;
p<0.01; Mann Whitney U-test.
When both groups were combined, ULN value for ADB were detected as 219.2 IU/mL.
DISCUSSION
Antibodies developing against streptococci as ASO, and ADB reach at high levels nearly 2–3 weeks after the onset of acute infection.
Since streptococcal complications also emerge 2–3 weeks after the onset of acute infection, higher ASO and/or ADB titers will be detected in a single serum sample in most of the cases with ARA or PSGN.
Interpretation of antibody titers measured in a single serum sample depends on its upper limit of normal value. In the same community mean and ULN of ASO and ADB change with time.
The factors effecting recurrence rates of S.pyogenes infections also have an impact on ASO and ADB titers. Among these factors age of the patient, the incidence of GABHS in the patient’s environment, and GABHS M serotypes lead the way.
Therefore, conduction of healthy population in-vestigations is recommended to determine ULNs. Antistreptolysine–O and ADB titers should be evaluated based on upper limits of normal determined for the population in question, and these values should be updated periodically [1].
Since higher CRP titers are associated with many etiologies, they can also be related to streptococcal infections, so that groups of patients excluded from study. Levels of ASO titers increase in groups A, C, and G streptococcal infections and also it can rise in multiple myeloma, hypergammaglobinemia or higher levels of rheumatoid factor [3]. Considering the possible presence of a recently experienced streptococcal infection in our patient, we found it to be appropriate to exclude the patient with a higher ASO level from the study.
In our study, levels of ADB titers in children aged 5–15, ranged between 50 IU/ml, and 576 IU/ml (mean, 123.6 IU/ml, and median, 50 IU/ml). Upper limit of normal ADB value was calculated as 219.2 IU/ml. When the patients were evaluated based on age groups, ADB levels in age group of 11–15, were detected to be significantly higher when compared with the age group of 5–10 (p=0.008). In studies performed both in our country and abroad, different reference values were obtained as ULN values of ADB.
However in most of the studies, ULN of ADB has been determined as 200–240 IU/ml in compliance with our study results [4, 5, 6, 7]. In most of the studies upper limit of normal has been accepted as the value discriminating between the highest 20%, and the lowest 80% of the values after measurement of antibody titers.
In a study performed in USA, Klein et al. reported ULN values of ADB as 60 IU/mL, 170 IU/ml, and 85 IU/ml in the preschool period, school age and in adults, respectively [8].
In a study conducted by Kaplan et al. investigated levels of ADB titers in 1131 pediatric patients aged 2–12 in different region of the USA. In this study ULN value of ADB antibody titers was detected as 640 IU/ml, and increases in ADB titers were observed with aging [9].
In a study conducted by Johnson et al. [10] in USA, 160 children aged between 5–16 were followed up for 2 years. Many universities provided material for this study the authors detected potential increases in ASO and ADB titers without emergence of clinical manifestations of GABHS. In this study, ULN for ADB was indicated as 640 IU/ml. In a study performed by Lütticken et al. in Germany, ULN value of ADB was found to be 240 IU/ml [4].
Although poststreptocccal complications has been seen in almost every geographic region in the world, they are most frequently seen in African countries, Brasil, Central South Asia, and Sub-saharan African countries. Besides, aborigines in Australia, people living in New Zealand, and Pacific Islands have higher incidence rates. Therefore, in investigations performed in these regions higher ADB titers were encountered. In their study published in the year 2009, Steer et al. [11] investigated adult, and pediatric populations in Fiji islands in the Pacific Ocean. ADB titers were measured using nephelometric method in serum samples of children who had not contracted GABHS infection, and ULN value for ADB in the age group of 5–14 was detected as 499 IU/ml. In the same investigation, median value for ADB titer was determined as 265 IU/ml in the adult age group (>35 yrs), and a dramatic decrease in ADB titers with aging was indicated.
In India, Karmarkar et al. [5] investigated titers of ADB at 10 year intervals between 1991–1992, 2001–2002 and did not detect any change in ULN values of ADB titres. In this research upper limit of normal value of ADB was indicated as 200 IU/ml.
In a study conducted by Zaman et al. and published in the year 2002 [12] in Bangladesh ADB values were analyzed in 361 healthy children aged between 5–14 using a microtiter method. They found, geometric mean, and ULN values of ADB as 222 IU/ml, and 340 IU/ml, respectively.
In an investigation performed by Wang SH, and Huang ZD, upper limit of normal in school-aged children, and adults were found to be 240 IU/mL, and 160 IU/mL, respectively [6].
Two studies which investigated ADB titers in our country have striked our attention. In a study per-formed by Ince et al. in Ankara between August 1999, and August 2000, ADB titers were analyzed in the sera of 230 children whose throat cultures had not demonstrated, group A, C and G beta hemolytic streptococci using DNA enzymatic cleavage assays. In their study geometric means, and ULNs for ADB titers were detected as 72 and 200 todd units/ml, respectively. Their measured ULNs were compatible with ours. Still in the same study, increase in mean ADB titers were detected up to 12 years of age, and maximum levels were reported in children between 10–12 [7].
In a multicentered study conducted in Istanbul by Eren et al. [13] between December 2002, and March 2003, ADB was measured using a nephelometric method, ULN values of ADB were detected as 170 IU/ml in the age group of 0–4 and 480 IU/ml in the age group 5–19. ULN of ADB was 340 IU/ml in adults aged 20 years and over.
In conclusion, based on our data, we can say that upper limits of normal ADB values vary according to patient’s age and geographic region. Our study with a group of limited number of patients, admitted to our hospital with noninfectious reasons will not certainly represent all healthy population. Therefore, in order to determine our own reference values, we think that it will be appropriate to expand the study including healthy populations in the kindergartens, schools and different age groups in all regions of our homeland at regular intervals.
Footnotes
Conflict of Interest: Any conflict of interest was not declared between personal and/or legal entities and/or institutions.
Financial Disclosure: The budget of the research was met by Education, and Planning Board of Haydarpasa Numune Training and Research Hospital.
REFERENCES
- 1.Steven D. Douglas, Michele E. Paessler. Immunology, Section 11. In: Lynne S. Garcia., editor. Clinical Microbiology Procedures Hand book. Second Edition. Washington: ASM Pres; 2007. [Google Scholar]
- 2.Wannamaker Lw, Ayoub Em. Antibody titers in acute rheumatic fever. Circulation. 1960;21:598–614. doi: 10.1161/01.cir.21.4.598. [DOI] [PubMed] [Google Scholar]
- 3.Shet A, Kaplan EL. Clinical use and interpretation of group A streptococcal antibody tests: a practical approach for the pediatrician or primary care physician. Pediatr Infect Dis J. 2002;21:420–6. doi: 10.1097/00006454-200205000-00014. quiz 427–30. [DOI] [PubMed] [Google Scholar]
- 4.Lütticken R, Wannamaker LW, Kluitmann G, Neugebauer M, Pulverer G. Antibody response to group A streptococcal exoenzymes (author’s transl) [Article in German] Dtsch Med Wochenschr. 1976;101:958–63. doi: 10.1055/s-0028-1104196. [Abstract] [DOI] [PubMed] [Google Scholar]
- 5.Karmarkar MG, Venugopal V, Joshi L, Kamboj R. Evaluation &revaluation of upper limits of normal values of anti-streptolysin O & anti-deoxyribonuclease B in Mumbai. Indian J Med Res. 2004;119(Suppl):26–8. [PubMed] [Google Scholar]
- 6.Wang SH, Huang ZD. Clinical evaluation of anti-DNAse B test in rheumatic fever and rheumatic heart disease. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 1991;30:489–91. 521. [Abstract] [PubMed] [Google Scholar]
- 7.Ince E, Yalçınkaya F, Güriz H, Aysev A, Elhan A, Uçar T, Gökdemir R, et al. Antistreptolysin O and anti deoxyribonuclease B levels in healthy children Original Article. Türk Pediatri Arşivi. 2002;37:85–90. [Google Scholar]
- 8.Klein GC, Baker CN, Jones WL. “Upper limits of normal” antistreptolysin O and antideoxyribonuclease B titers. Appl Microbiol. 1971;21:999–1001. doi: 10.1128/am.21.6.999-1001.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Rothermel CD, Johnson DR. Antistreptolysin O and anti-deoxyribonuclease B titers: normal values for children ages 2 to 12 in the United States. Pediatrics. 1998;101(1 Pt 1):86–8. doi: 10.1542/peds.101.1.86. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DR, Kurlan R, Leckman J, Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis. 2010;50:481–90. doi: 10.1086/650167. [DOI] [PubMed] [Google Scholar]
- 11.Steer AC, Vidmar S, Ritika R, Kado J, Batzloff M, Jenney AW, et al. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin Vaccine Immunol. 2009;16:172–5. doi: 10.1128/CVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaman MM, Hassan MM, Ahmed J, Zareen S, Jalil MQ, Eshaque N, et al. Streptococcal antibodies among rural school children in Bangladesh. Bangladesh Med Res Counc Bull. 2002;28:1–6. [PubMed] [Google Scholar]
- 13.Aynur E. Değişik Yaş Gruplarında ASO ve anti-DN’az B Titrelerinin Karşılaştırılması. TMC Bülteni. 2003 Apr;2:3. [Google Scholar]


