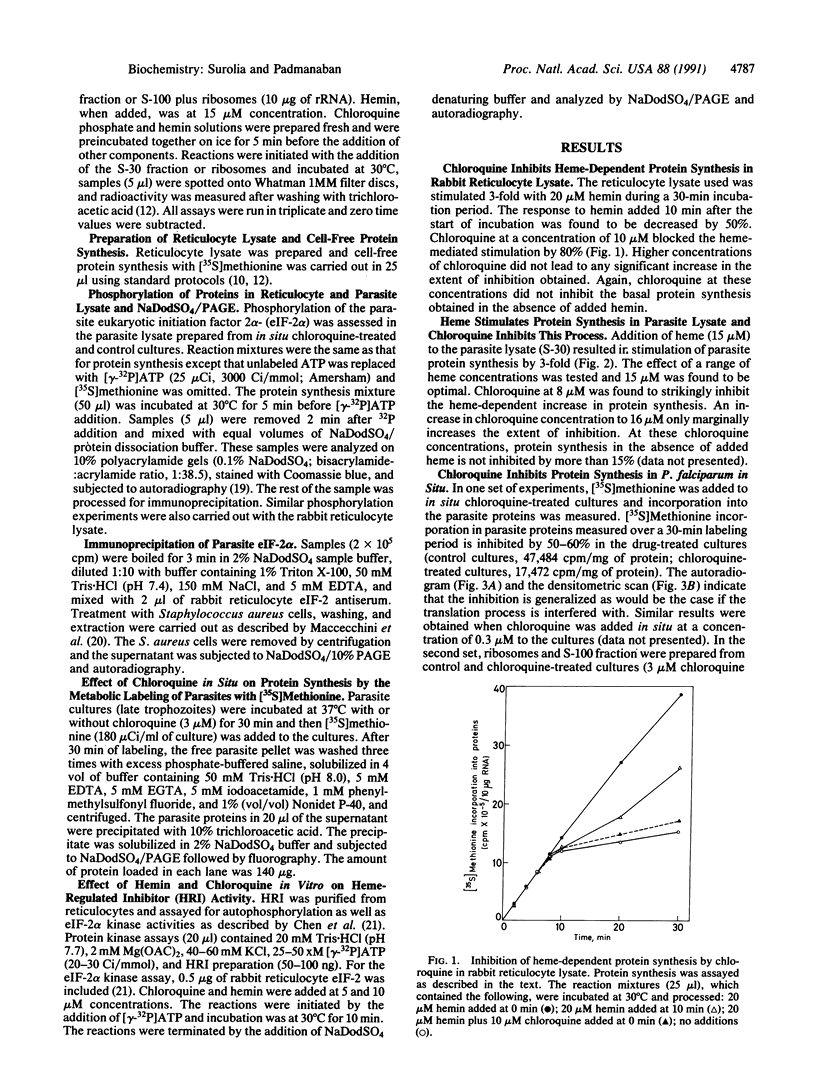
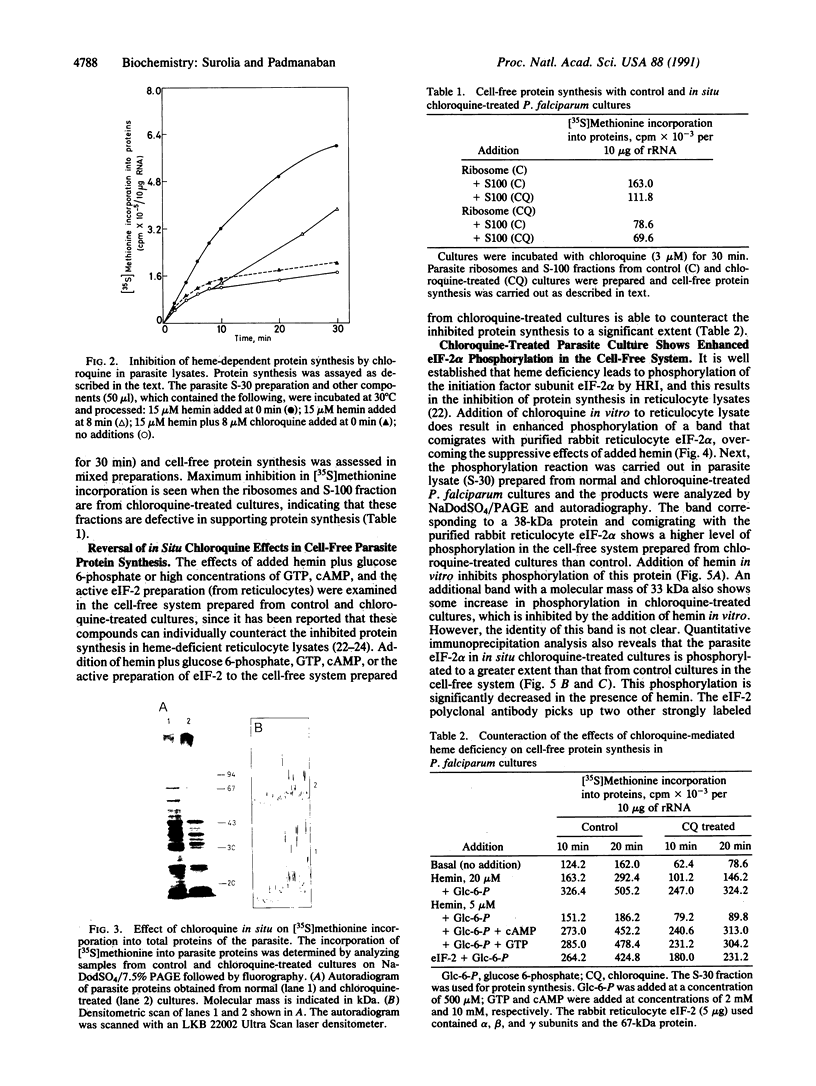
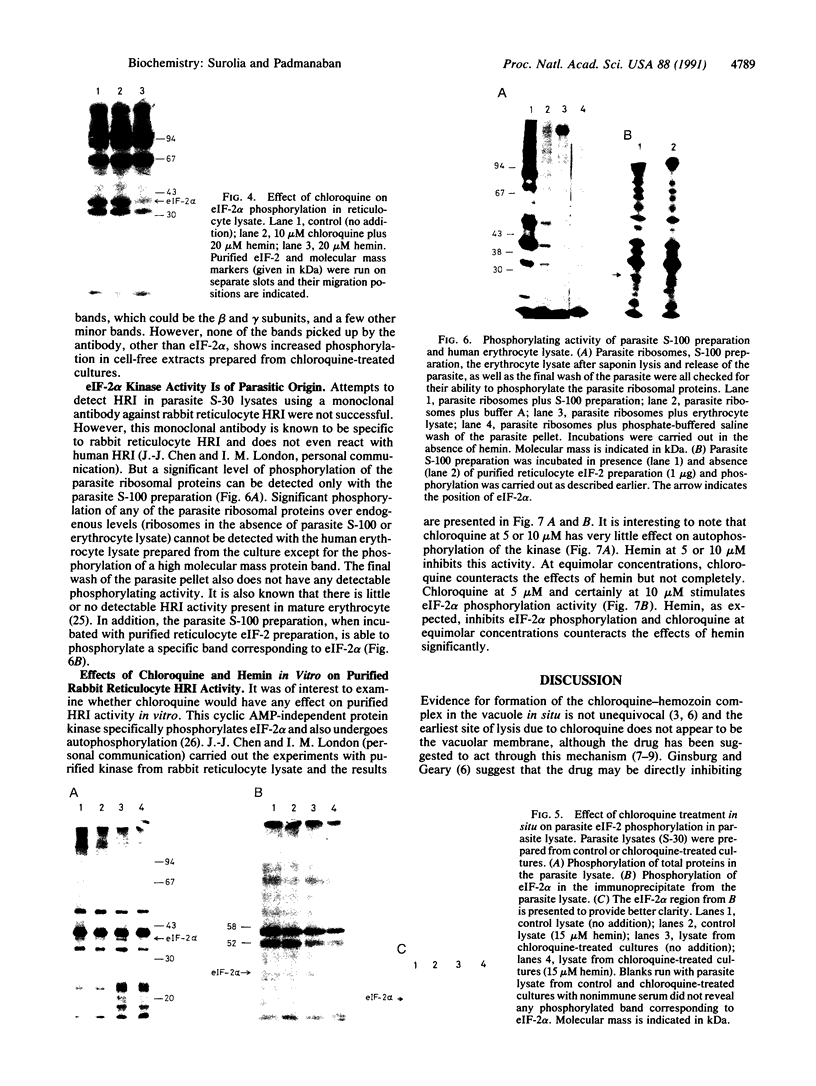
Abstract
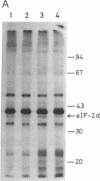
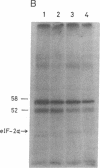
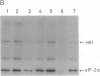
A cell-free protein-synthesizing system has been reconstituted using the S-30 fraction or ribosomes and the S-100 fraction from Plasmodium falciparum. Addition of heme in vitro stimulates cell-free protein synthesis strikingly. Chloroquine inhibits the heme-dependent protein synthesis in the parasite lysate. The drug has also been found to inhibit parasite protein synthesis in situ at therapeutic concentrations soon after addition to parasite cultures. Ribosomes as well as the S-100 fraction isolated from such chloroquine-treated cultures are defective in protein synthesis. Addition of hemin plus glucose 6-phosphate or high concentrations of GTP, cAMP, and an active preparation of eIF-2 to the parasite cell-free system restores protein synthesis to a significant extent in chloroquine-treated cultures. Under conditions of inhibition of protein synthesis in situ by chloroquine in the culture, the parasite eukaryotic initiation factor 2 alpha- (eIF-2 alpha) is phosphorylated in the parasite lysate to a greater extent than that observed in the control culture. Addition of hemin in vitro suppresses this phosphorylation. eIF-2 alpha kinase activity is present in the parasite lysate and is not a contaminant derived from the human erythrocytes used to culture the parasite. The heme-chloroquine interactive effects can also be demonstrated with purified eIF-2 alpha kinase from rabbit reticulocyte lysate. It is proposed that chloroquine inhibits heme-dependent protein synthesis in the parasite and this is an early event mediating the growth-inhibitory effects of the drug.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. L., O'Brien R. L., Hahn F. E. DNA: reaction with chloroquine. Science. 1965 Sep 3;149(3688):1111–1113. doi: 10.1126/science.149.3688.1111. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D., Mohan Rao C., Panijpan B. The malaria parasite monitored by photoacoustic spectroscopy. Science. 1984 Feb 24;223(4638):828–830. doi: 10.1126/science.6695185. [DOI] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Yang J. M., Petryshyn R., Kosower N., London I. M. Disulfide bond formation in the regulation of eIF-2 alpha kinase by heme. J Biol Chem. 1989 Jun 5;264(16):9559–9564. [PubMed] [Google Scholar]
- Cohen S. N., Yielding K. L. Inhibition of DNA and RNA polymerase reactions by chloroquine. Proc Natl Acad Sci U S A. 1965 Aug;54(2):521–527. doi: 10.1073/pnas.54.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta B., Chakrabarti D., Roy A. L., Gupta N. K. Roles of a 67-kDa polypeptide in reversal of protein synthesis inhibition in heme-deficient reticulocyte lysate. Proc Natl Acad Sci U S A. 1988 May;85(10):3324–3328. doi: 10.1073/pnas.85.10.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R., London I. M. Relationship between phosphorylation and activity of heme-regulated eukaryotic initiation factor 2 alpha kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):866–870. doi: 10.1073/pnas.78.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Banyal H. S., Phillips G., Pfaller M. A., Krogstad D. J. Lysis of Plasmodium falciparum by ferriprotoporphyrin IX and a chloroquine-ferriprotoporphyrin IX complex. Antimicrob Agents Chemother. 1982 May;21(5):819–822. doi: 10.1128/aac.21.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J Biol Chem. 1987 Nov 15;262(32):15552–15555. [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Geary T. G. Current concepts and new ideas on the mechanism of action of quinoline-containing antimalarials. Biochem Pharmacol. 1987 May 15;36(10):1567–1576. doi: 10.1016/0006-2952(87)90038-4. [DOI] [PubMed] [Google Scholar]
- Gross M., Rubino M. S., Starn T. K. Regulation of protein synthesis in rabbit reticulocyte lysate. Glucose 6-phosphate is required to maintain the activity of eukaryotic initiation factor (eIF)-2B by a mechanism that is independent of the phosphorylation of eIF-2 alpha. J Biol Chem. 1988 Sep 5;263(25):12486–12492. [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. Acid-vesicle function, intracellular pathogens, and the action of chloroquine against Plasmodium falciparum. N Engl J Med. 1987 Aug 27;317(9):542–549. doi: 10.1056/NEJM198708273170905. [DOI] [PubMed] [Google Scholar]
- Kwakye-Berko F., Meshnick S. R. Binding of chloroquine to DNA. Mol Biochem Parasitol. 1989 Jun 1;35(1):51–55. doi: 10.1016/0166-6851(89)90141-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick S. R. Chloroquine as intercalator: a hypothesis revived. Parasitol Today. 1990 Mar;6(3):77–79. doi: 10.1016/0169-4758(90)90215-p. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Petryshyn R., Rosa F., Fagard R., Levin D., London I. M. Control of protein synthesis in human reticulocytes by heme-regulated and double-stranded RNA dependent eIF-2 alpha kinases. Biochem Biophys Res Commun. 1984 Mar 30;119(3):891–899. doi: 10.1016/0006-291x(84)90857-x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Cox R. A., Higginson B., McLaren D. J., Williamson J. The ribosomes of the simian malaria, Plasmodium knowlesi. I. Isolation and characterization. J Protozool. 1975 Nov;22(4):568–572. doi: 10.1111/j.1550-7408.1975.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. The ribosomes of the simian malaria Plasmodium knowlesi--II. A cell-free protein synthesizing system. Comp Biochem Physiol B. 1976;53(4):447–450. doi: 10.1016/0305-0491(76)90196-6. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]