Abstract
Dipeptidyl peptidase-4 (DPP4) is an important regulator of incretins and inflammation, and it is involved in the pathophysiological process of myocardial infarction (MI). This study investigated the role of plasma DPP4 activity (DPP4a) in patients with ST-segment elevation myocardial infarction (STEMI) who had undergone percutaneous coronary intervention (PCI). We recruited 747 consecutive PCI-treated STEMI patients from a tertiary referral center from January 2014 to October 2015. The outcomes of interest were the rates of no-reflow, in-hospital major adverse cardiac or cerebrovascular events (iMACCE), in-hospital complications (IHC) and in-hospital major bleeding. The DPP4a was lower in STEMI patients compared with the controls (p < 0.0001). Multivariate logistic-regression analyses (adjusted for confounding variables) showed that a 1 U/L increase in DPP4a was associated with an increased rate of no-reflow events (odds ratio [OR]: 1.07; 95% CI: 1.02–1.11; p < 0.01) and a decreased rate of major bleeding events (OR: 0.90; 95% CI: 0.82–0.98; p = 0.02). There were no associations between DPP4a and either iMACCE or IHC. In conclusions, high levels of DPP4a are independently associated with an increased rate of no-reflow events and a decreased rate of major bleeding events in PCT-treated STEMI patients.
ST-segment elevation myocardial infarction (STEMI) is an acute manifestation of coronary heart disease, and it is a frequent cause of death1. A better understanding of the risk factors and pathogenic mechanisms underlying STEMI may help to improve the patients’ prognosis and quality of life.
Dipeptidyl peptidase 4 (DPP4) is an exopeptidase that is expressed on the surface of a diverse range of cells. It is a protease that cleaves off amino-terminal dipeptides that have L-proline, L-alanine or serine in the penultimate position2. As a cell surface protein, DPP4 is involved in regulation of the immune system, signal transduction and apoptosis3. A soluble form of DPP4 circulates in the plasma. Soluble DPP4 comes from clearance of membrane-type DPP4 or it is secreted by cells (such as endothelial cells). Soluble DPP4 also has membrane-type DPP4 enzymatic activity4. The levels of plasma DPP4 activity (DPP4a) are elevated in several diseases, including type 2 diabetes mellitus (T2DM), obesity5, atherosclerosis6, and osteoporosis7.
Research has shown that treatment with DPP4 inhibiters before cardiac ischemia-reperfusion (I/R) injuries leads to improved survival rates and better heart function in rats, which is partly due to the activation of the phospho-Ak mouse strain thymoma (pAkt), phospho-glycogen synthase kinase 3 (pGSK3), and atrial natriuretic peptide (ANP) pathways8. In addition, the inhibition of DPP4 can alleviate atherosclerosis9 and heart failure10. Accordingly, one could hypothesize that high levels of DPP4a may worsen myocardial I/R injuries, causing poorer cardiovascular outcomes. However, to the best of our knowledge, no study has evaluated whether DPP4a is associated with adverse clinical outcomes in STEMI patients. The aim of this study was to investigate whether plasma DPP4a is associated with adverse in-hospital cardiovascular events in these patients.
Results
The study included 747 patients with STEMI. Most of the subjects were men (82.7%) and the mean age was 57.8 years. Among the included patients, 165 had diabetes; 123 of these diabetic patients were on oral hypoglycemic agents and 114 were using insulin. The median levels of hemoglobin A1c (HbA1c) in the diabetic patients was 7.0% (inter-quartile range, 6.3–8.1%).
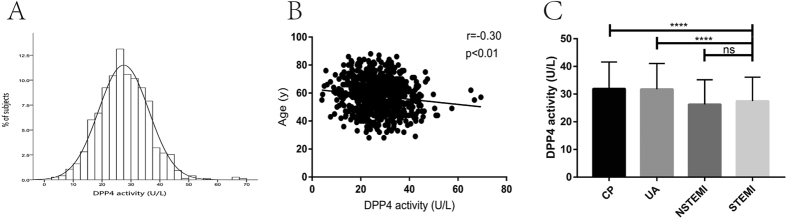
The levels of DPP4a in the participants were normally distributed (Fig. 1A), and DPP4a was negatively associated with age (Pearson’s r = −0.30, p < 0.01; Fig. 1B) but not with fasting plasma glucose (Pearson’s r = −0.05, p = 0.20), and there was no significant difference between male and female participants (p = 0.80). The DPP4a in the STEMI patients was significantly lower than that of the chest pain (CP) and unstable angina (UA) controls, but it was not significantly different to that of the non-STEMI (NSTEMI) controls (STEMI patients: 27.49 ± 0.31 U/L, n = 747; CP controls: 31.96 ± 0.83 U/L, n = 134, p < 0.0001; UA controls: 31.76 ± 0.67 U/L, n = 190, p < 0.0001; NSTEMI controls: 26.26 ± 0.74 U/L, n = 146, p = 0.12; Fig. 1C).
Figure 1. Characteristics of DPP4a at baseline in STEMI patients.
(A) DPP4a was normally distributed. (B) DPP4a was negatively associated with age. (C) DPP4a was lower in the STEMI group compared with the CP and UA control groups. ****p < 0.0001 CP, chest pains; DPP4, dipeptidyl peptidase-4; ns, non-significant; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina.
The STEMI patients were divided into quartiles according to their DPP4a measurements. The individuals in the highest quartile were younger and less likely to have diabetes and had higher levels of total and LDL cholesterol and GGT, and lower level of fibrinogen (Tables 1 and 2).
Table 1. Baseline demographic, clinical, and angiographic characteristics by DPP4a quartiles.
| U/L | Total | Q1 | Q2 | Q3 | Q4 | P |
|---|---|---|---|---|---|---|
| ≤21.53 | 21.54–27.14 | 27.15–32.92 | ≥32.96 | |||
| N | 747 | 187 | 187 | 186 | 187 | — |
| Age (y) | 57.84 ± 11.67 | 59.84 ± 11.50 | 58.18 ± 11.98 | 58.01 ± 12.01 | 55.35 ± 10.78 | <0.01 |
| Male, n (%) | 618 (82.7) | 154 (82.4) | 152 (81.3) | 158 (84.9) | 154 (82.4) | 0.81 |
| BMI | 25.79 ± 3.40 | 25.56 ± 3.19 | 25.76 ± 3.63 | 25.65 ± 3.22 | 26.17 ± 3.54 | 0.32 |
| Hypertension, n (%) | 414 (55.4) | 115 (61.5) | 106 (56.7) | 97 (52.2) | 96 (51.3) | 0.17 |
| Hyperlipidaemia, n (%) | 83 (11.1) | 25 (13.4) | 17 (9.1) | 27 (14.6) | 14 (7.5) | 0.09 |
| Diabetes mellitus, n (%) | 165 (22.1) | 30 (29.9) | 17 (20.3) | 27 (21.1) | 14 (17.1) | 0.02 |
| Current smoker, n (%) | 339 (45.4) | 77 (48.4) | 73 (44.5) | 97 (58.4) | 92 (54.1) | 0.06 |
| Ex-smoker, n (%) | 88 (11.8) | 28 (25.5) | 23 (20.2) | 20 (22.5) | 17 (17.9) | 0.59 |
| Previous MI, n (%) | 107 (14.3) | 24 (12.8) | 21 (11.2) | 28 (15.1) | 34 (18.2) | 0.25 |
| Previous PCI, n (%) | 206 (27.6) | 53 (28.3) | 42 (22.5) | 62 (33.5) | 49 (26.2) | 0.11 |
| Previous CABG, n (%) | 7 (0.9) | 4 (2.1) | 2 (1.2) | 0 (0.0) | 1 (0.5) | 0.17 |
| Medications, n (%) | ||||||
| Aspirin | 742 (99.3) | 187 (100.0) | 185 (98.9) | 184 (98.9) | 186 (99.5) | 0.53 |
| ACEI/ARB | 685 (91.7) | 171 (91.4) | 173 (92.5) | 168 (90.3) | 173 (92.5) | 0.85 |
| β-blocker | 658 (88.1) | 166 (88.8) | 168 (89.8) | 161 (86.6) | 163 (87.2) | 0.75 |
| Clopidogrel | 728 (97.5) | 184 (98.4) | 185 (98.9) | 177 (95.2) | 182 (97.3) | 0.10 |
| Statin | 741 (99.2) | 187 (100.0) | 185 (98.9) | 184 (98.9) | 185 (98.9) | 0.57 |
| Nitrate | 670 (89.7) | 172 (92.0) | 170 (90.9) | 160 (86.0) | 168 (89.8) | 0.25 |
| Aldosterone antagonist | 114 (15.3) | 33 (17.6) | 26 (13.9) | 28 (15.1) | 27 (14.4) | 0.76 |
| Infarcted location, n (%) | ||||||
| Anterior | 356 (47.7) | 89 (47.6) | 88 (47.1) | 96 (51.6) | 83 (44.4) | 0.57 |
| Inferior | 336 (50.0) | 87 (46.6) | 83 (44.4) | 80 (43.0) | 86 (46.0) | 0.90 |
| Posterior | 32 (4.3) | 5 (2.6) | 10 (5.3) | 7 (3.8) | 10 (5.3) | 0.51 |
| Lateral | 23 (3.1) | 6 (3.2) | 6 (3.2) | 3 (1.6) | 8 (4.3) | 0.52 |
| Number of disease vessels, n (%) | ||||||
| Single vessel disease | 185 (24.8) | 42 (22.5) | 51 (27.3) | 53 (28.5) | 39 (20.9) | 0.25 |
| Double vessel disease | 147 (19.7) | 34 (18.2) | 30 (16.0) | 38 (20.4) | 45 (24.1) | 0.24 |
| Triple vessel disease | 415 (55.6) | 111 (59.3) | 106 (56.7) | 95 (51.1) | 103 (55.0) | 0.44 |
| Pre TIMI grade flow, n (%) | ||||||
| 0 | 457 (61.2) | 112 (59.8) | 114 (60.9) | 112 (60.1) | 117 (62.6) | 0.95 |
| 1 | 147 (19.7) | 42 (22.5) | 36 (19.3) | 31 (16.7) | 38 (20.3) | 0.56 |
| 2 | 96 (12.9) | 20 (10.7) | 26 (13.9) | 28 (15.1) | 22 (11.8) | 0.58 |
| 3 | 49 (6.6) | 13 (7) | 11 (5.9) | 15 (8.1) | 10 (5.3) | 0.72 |
| Final TIMI grade flow, n (%) | ||||||
| 0 | 2 (0.3) | 0 (0) | 0 (0) | 1 (0.5) | 1 (0.5) | 0.57 |
| 1 | 11 (1.5) | 1 (0.5) | 2 (1.1) | 3 (1.6) | 5 (2.7) | 0.36 |
| 2 | 40 (5.4) | 7 (3.7) | 9 (4.8) | 10 (5.4) | 14 (7.5) | 0.43 |
| 3 | 694 (92.9) | 179 (95.8) | 176 (94.1) | 172 (92.5) | 167 (89.3) | 0.09 |
| Myocardial blush grade, n (%) | ||||||
| 0/1 | 45 (6.0) | 6(3.2) | 10(5.3) | 12(6.5) | 17(9.1) | 0.12 |
| 2 | 55 (7.4) | 9(4.8) | 12(6.4) | 15(8.1) | 19(10.2) | 0.11 |
| 3 | 647 (86.6) | 172(92.0) | 165(88.3) | 159(85.4) | 151(80.7) | 0.01 |
| No reflow phenomenon, n (%) | 45 (6.0) | 6 (3.2) | 10 (5.3) | 12 (6.5) | 17 (9.1) | 0.12 |
| Malignant reperfusion arrhythmia, | 37 (5.0) | 10 (5.3) | 9 (4.6) | 7 (3.7) | 11 (5.9) | 0.81 |
| No. of stent per patients | 1.88 ± 1.12 | 1.50 ± 0.81 | 1.91 ± 1.22 | 1.73 ± 1.01 | 2.36 ± 1.29 | 0.30 |
| Maximal inflation pressure (atm) | 14.28 ± 2.78 | 14.40 ± 2.27 | 14.73 ± 3.93 | 13.82 ± 1.89 | 14.18 ± 2.89 | 0.82 |
| Percent diameter stenosis (%) | 97.2 ± 3.6 | 98.1 ± 3.5 | 94.9 ± 4.4 | 97.6 ± 3.4 | 98.0 ± 3.3 | 0.81 |
| Post percent diameter stenosis (%) | 2 ± 1 | 2 ± 1 | 2 ± 2 | 2 ± 1 | 2 ± 1 | 0.93 |
| Stent diameter (mm) | 3.17 ± 0.41 | 3.23 ± 0.30 | 3.25 ± 0.43 | 3.30 ± 0.49 | 2.93 ± 0.34 | 0.14 |
| Total stent length (mm) | 50.92 ± 36.09 | 39.30 ± 18.41 | 55.27 ± 42.87 | 43.18 ± 30.82 | 65.91 ± 43.69 | 0.36 |
| Thrombus aspiration, n (%) | 120 (16.1) | 30 (16.0) | 26 (13.9) | 36 (19.4) | 28 (15.0) | 0.51 |
| IABP, n (%) | 39 (5.2) | 9 (4.8) | 8 (4.3) | 12 (6.5) | 10 (5.3) | 0.81 |
| CTO, n (%) | 19 (2.5) | 4 (2.1) | 7 (3.7) | 3 (1.6) | 5 (2.7) | 0.60 |
| iMACCE, n (%) | 5 (0.7) | 1 (0.5) | 2 (1.1) | 1 (0.5) | 1 (0.5) | 0.88 |
| IHC, n (%) | 91 (12.2) | 26 (13.9) | 20 (10.7) | 23 (12.4) | 22 (11.8) | 0.82 |
| Major bleeding, n (%) | 16 (2.1) | 7 (3.7) | 4 (2.1) | 5 (2.7) | 0 (0) | 0.08 |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blckers; BMI, body mass index; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; DPP4a, plasma dipeptidyl peptidase-4 activity; MI, myocardial infarction; IABP, intra-aortic balloon pumping; IHC, in-hospital complications; iMACCE, in-hospital major adverse cardiac or cerebrovascular events; PCI, percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction.
Table 2. Baseline laboratory factors by DPP4a quartiles and their correlations with DPP4a.
| U/L | Total | Q1 | Q2 | Q3 | Q4 | P | Correlation |
|
|---|---|---|---|---|---|---|---|---|
| ≤21.53 | 21.54–27.14 | 27.15–32.92 | ≥32.96 | r | p | |||
| Lipid profile | ||||||||
| Total cholesterol (mg/dL) | 4.11 ± 1.04 | 3.97 ± 1.03 | 4.06 ± 1.03 | 4.07 ± 1.08 | 4.32 ± 1.01 | 0.01 | 0.13a | <0.01 |
| Triglyceride (mg/dL) | 1.56 ± 0.84 | 1.53 ± 0.71 | 1.55 ± 0.78 | 1.45 ± 0.78 | 1.68 ± 0.85 | 0.07 | 0.06a | 0.11 |
| HDL cholesterol (mg/dL) | 1.05 ± 0.29 | 1.01 ± 0.28 | 1.03 ± 0.28 | 1.07 ± 0.29 | 1.09 ± 0.31 | 0.06 | 0.11a | 0.01 |
| LDL cholesterol (mg/dL) | 2.53 ± 0.89 | 2.42 ± 0.85 | 2.50 ± 0.85 | 2.53 ± 0.86 | 2.67 ± 0.86 | 0.03 | 0.11a | <0.01 |
| Coagulation profile | ||||||||
| Thrombin time (sec) | 16.60 (15.80–18.53) | 16.30 (15.60–17.80) | 16.70 (15.80–18.20) | 16.70 (15.90–19.63) | 16.90 (16.20–19.48) | <0.01 | 0.16b | <0.01 |
| Prothrombin time (sec) | 13.64 ± 1.40 | 13.74 ± 1.41 | 13.62 ± 1.23 | 13.66 ± 1.08 | 13.55 ± 1.80 | 0.67 | −0.01a | 0.83 |
| APTT (sec) | 37.85 (34.90–43.33) | 38.10 (34.10–41.90) | 38.00 (35.28–43.65) | 38.05 (35.30–44.43) | 37.60 (35.10–42.55) | 0.79 | 0.02b | 0.76 |
| Fibrinogen (g/L) | 3.75 ± 1.16 | 4.07 ± 1.35 | 3.91 ± 1.22 | 3.58 ± 1.01 | 3.45 ± 0.94 | <0.01 | −0.21a | <0.01 |
| FPB (mmol/l) | 6.97 ± 2.71 | 7.21 ± 2.86 | 7.00 ± 2.72 | 6.94 ± 2.90 | 6.72 ± 2.28 | 0.53 | −0.05a | 0.20 |
| HbA1C (%)* | 6.40 ± 1.38 | 6.61 ± 1.62 | 6.20 ± 1.16 | 6.49 ± 1.47 | 6.26 ± 1.14 | 0.13 | −0.06a | 0.27 |
| peak CK-MB (ng/mL) | 6.83 (1.83–131.76) | 4.52 (1.74–76.99) | 7.42 (1.86–162.03) | 8.85 (1.88–154.60) | 9.85 (1.82–215.43) | 0.11 | 0.10b | 0.01 |
| peak cTNT (ng/mL) | 0.65 (0.45–4.22) | 0.62 (0.05–3.21) | 0.53 (0.06–3.95) | 0.56 (0.30–4.70) | 1.08 (0.50–5.26) | 0.64 | 0.05b | 0.19 |
| peak myoglobin (ng/mL) | 49.84 (28.43–384.70) | 44.19 (28.03–191.68) | 38.09 (29.20–356.58) | 49.84 (28.34–618.10) | 127.60 (28.00–691.60) | 0.12 | 0.14b | <0.01 |
| pro-BNP (pg/mL) | 653.90 (250.00–1581.00) | 855.70 (250.15–1960.50) | 659.20 (241.10–1486.00) | 687.90 (282.70–1611.00) | 553.70 (234.23–1396.00) | 0.21 | −0.07b | 0.06 |
| Creatinine (umol/L) | 78.30 (68.20–90.18) | 79.70 (65.30–95.80) | 79.90 (66.45–91.75) | 78.50 (70.28–91.65) | 76.35 (67.45–85.50) | 0.09 | −0.07b | 0.049 |
| GGT (U/L) | 32.80 (21.70–53.90) | 30.40 (21.33–45.78) | 30.60 (21.20–54.30) | 31.00 (22.60–48.95) | 38.00 (23.60–73.10) | <0.01 | 0.14b | <0.01 |
APTT, activated partial thromboplastin time; BNP, brain natriuretic peptide; CK-MB, MB isoenzyme of creatine kinase; cTNT, cardiac troponin T; DPP4a, plasma dipeptidyl peptidase-4 activity; FPG, fasting plasma glucose; GGT, gamma-glutamyltransferase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein;
aPearson correlation coefficients; bSpearman correlation coefficients.
*HbA1c was only detected in 373 patients; Number of patients detected were 107 in Q1, 88 in Q2, 88 in Q3 and 89 in Q4.
The analysis of correlation demonstrated that DPP4a was positively associated with total, LDL and HDL cholesterol, thrombin time, peak CK-MB, peak myoglobin, and GGT in STEMI patients, while it was negatively associated with fibrinogen and creatinine (all p < 0.05) (Table 2). However, in the NSTEMI control group, DPP4a was not associated with fibrinogen, peak CK-MB, peak myoglobin, creatinine, thrombin time, or GGT (Supp. Table 1). The multiple linear regression analysis of the STEMI patient data showed that age, fibrinogen, peak CK-MB, and GGT (all p < 0.05) were independently associated with DPP4a in STEMI patients (Table 3).
Table 3. Associations between various demographic and laboratory factors and DPP4a.
| Β | 95% CI | p | |
|---|---|---|---|
| Age | −0.10 | (−0.18, −0.02) | 0.01 |
| Fibrinogen | −1.27 | (−2.12, −0.43) | <0.01 |
| Peak CK-MB | 0.01 | (0.006, 0.02) | <0.01 |
| GGT | 0.04 | (0.02, 0.06) | <0.01 |
B-coefficients and 95% CI meant the unstandardized coefficients and their 95% confidence interval. The following variables were removed from the model: gender, body mass index (BMI), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, peak myoglobin, peak cardiac troponin (cTNT), fasting plasma glucose, thrombin time, prothrombin time, activated partial thromboplastin time (APTT), D-dimers, pro-brain natriuretic peptide (pro-BNP), creatinine, history of hypertension, previous myocardial infarction (MI), smoking status, use of an angiotensin-converting-enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB), statin use, aspirin use, β-blocker use, and aldosterone antagonist use.
CK-MB, MB isoenzyme of creatine kinase; DPP4a, plasma dipeptidyl peptidase-4 activity; GGT, gamma-glutamyltransferase.
The respective prevalences of no-reflow events, iMACCE, IHC, and in-hospital major bleeding events were 6.0%, 0.7%, 12.2%, and 2.1%, respectively. In the unadjusted analysis, the analysis that adjusted for demographic factors and the fully adjusted analysis, a higher level of DPP4a was associated with greater odds of no-reflow events (adjusted OR [aOR] 1.07 [1.02–1.11] per 1 U/L DPP4a; p < 0.01) and a lower odds of major bleeding events (aOR, 0.90 [0.82–0.98] per 1 U/L DPP4a; p = 0.02). However, DPP4a was neither significantly associated with iMACCE or IHC (Table 4) nor with any of their components. The following aORs per 1 U/L DPP4a are the aORs for each component after adjusting for age and sex: in-hospital death: 1.24 [0.99–1.56], p = 0.06; nonfatal MI: 0.95 [0.84–1.06], p = 0.34; stroke: none; acute heart failure: 1.00 [0.96–1.04], p = 0.94; atrial fibrillation: 1.00 [0.92–1.09], p = 0.99; CP: 1.02 [0.98–1.06], p = 0.45; complete atrioventricular block: 0.97 [0.88–1.07], p = 0.56; cerebrovascular disease: none; and ventricular fibrillation or ventricular tachycardia: 1.03 [0.98–1.09], p = 0.30). In the NSTEMI control group, after adjusting for age and sex, a 1 U/L increase in DPP4a was not associated with no-reflow events (aOR 1.00 [0.93–1.07], p = 0.98), iMACCE (aOR 1.33 [0.78–2.27], p = 0.30), IHC (aOR 1.18 [0.95–1.46], p = 0.13) or major bleeding events (aOR 1.05 [0.98–1.12], p = 0.17).
Table 4. Associations between DPP4a and no-reflow events, iMACCE, IHC, and in-hospital major bleeding events.
| Unadjusted | p Value | Model 1* | p Value | Model 2† | p | |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| No reflow phenomenon | ||||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q2 | 1.70 (0.61–4.79) | 0.3 | 1.71 (0.61–4.82) | 0.31 | 1.38 (0.41–4.63) | 0.61 |
| Q3 | 2.08 (0.76–5.67) | 0.12 | 2.16 (0.79–5.91) | 0.13 | 2.81 (0.89–8.85) | 0.08 |
| Q4 | 3.02 (1.16–7.83) | 0.02 | 3.16 (1.20–8.29) | 0.02 | 3.39 (1.08–10.61) | 0.04 |
| per 1 U/L | 1.05 (1.02–1.09) | <0.01 | 1.06 (1.02–1.09) | <0.01 | 1.07 (1.02–1.11) | <0.01 |
| iMACCE | ||||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q2 | 1.00 (0.14–7.17) | 1.00 | 1.00 (0.14–7.28) | 1.00 | 0.88 (0.11–7.23) | 0.91 |
| Q3 | 0.50 (0.04–5.56) | 0.57 | 0.48 (0.04–5.38) | 0.55 | 0.50 (0.04–6.13) | 0.59 |
| Q4 | 0.50 (0.05–5.53) | 0.57 | 0.49 (0.04–5.58) | 0.56 | 0.45 (0.04–5.91) | 0.55 |
| per 1 U/L | 0.97 (0.88–1.07) | 0.52 | 0.97 (0.88–1.07) | 0.51 | 0.97 (0.88–1.07) | 0.57 |
| IHC | ||||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q2 | 0.74 (0.40–1.38) | 0.35 | 0.78 (0.41–1.45) | 0.43 | 0.99 (0.47–2.09) | 0.98 |
| Q3 | 0.87 (0.48–1.60) | 0.66 | 0.92 (0.50–1.68) | 0.78 | 1.03 (0.48–2.18) | 0.95 |
| Q4 | 0.83 (0.45–1.52) | 0.54 | 0.95 (0.51–1.76) | 0.87 | 0.90(0.40–2.03) | 0.81 |
| per 1 U/L | 1.00 (0.98–1.03) | 0.86 | 1.01 (0.98–1.04) | 0.53 | 1.01 (0.98–1.04) | 0.40 |
| Major bleeding | ||||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q2 | 0.56 (0.16–1.95) | 0.36 | 0.57 (0.17–2.00) | 0.38 | 0.61 (0.10–3.75) | 0.54 |
| Q3 | 0.71 (0.22–2.28) | 0.57 | 0.74 (0.23–2.39) | 0.62 | 1.04 (0.22–5.01) | 0.95 |
| Q4 | — | — | — | |||
| per 1 U/L | 0.92 (0.86–0.98) | 0.01 | 0.92 (0.86–0.98) | 0.01 | 0.90 (0.82–0.98) | 0.02 |
IHC, in-hospital complications; iMACCE, in-hospital major adverse cardiac or cerebrovascular events; DPP4a, plasma dipeptidyl peptidase-4 activity; OR, odds ratio; CI, confidence interval.
*Adjusted for age and sex.
†Adjusted for age, sex, BMI, creatinine, GGT, total cholesterol, fibrinogen, peak CK-MB, pro-BNP, fasting plasma glucose, hypertension, previous MI, smoking status, ACEI/ARB use, and β-blocker use.
Discussion
We showed that DPP4a was lower in the STEMI patients than in the CP and UA controls. Moreover, in the STEMI patients, DPP4a was positively associated with no-reflow events and negatively associated with major bleeding events. Adjusting for baseline variables did not affect these results.
A previous study reported that coronary artery disease (CAD) patients with myocardial infarction (MI) have a decreased concentration of plasma DPP4 than CAD patients without MI11, and a second study reported that DPP4a decreased over the 4 months after patients experienced MI12. However, circulating DPP4 can become inactive in certain conditions and, in these conditions, the concentration of plasma DPP4 is no longer associated with DPP4a. We showed that DPP4a also decreased after MI. Our results indicated that elevated DPP4a could be harmful to the heart and blood vessels. This concurs with previous research, that found that DPP4a increased in patients with heart failure and it was associated with poorer heart function compared with healthy subjects13. In addition, research has shown that higher levels of DPP4a are associated with left ventricular systolic and diastolic dysfunction14 and larger carotid artery intima-media thickness6 in T2DM patients. These results are consistent with research on animal models, in which DPP4 inhibitors were shown to improve functional recovery, decrease passive left ventricular compliance, increase endothelial cell density and decrease cardiomyocyte hypertrophy after I/R injury4.
However, three large randomized clinical trials failed to show that DPP4 inhibitors can decrease cardiovascular events, and one of them indicated an unexpected increase in the risk of heart failure in T2DM patients15. Moreover, a meta-analysis showed that DPP4 inhibitors do not decrease the risk of all-cause and cardiovascular mortality16. Our study also found that DPP4a was not associated with either iMACCE or IHC. These results suggest that DPP4 may play several roles in humans, which could lead to it having a neutral effect on cardiovascular events.
The occurrence of post-PCI no-reflow attenuates the benefit of reperfusion therapy and it is associated with poor clinical outcomes17. The pathophysiology of no-reflow is complex, as it involves cell edema, leukocyte infiltration, vasoconstriction, inflammation, and distal embolization18. DPP4 may affect no-reflow in both GLP-1-dependent and independent ways.
GLP-1 is an incretin that was originally found to regulate plasma glucose levels. It is rapidly inactivated by DPP4, so it has a short half-life (less than 2 min)19. The receptor for GLP-1 (GLP-1R) is expressed on cardiomyocytes, endothelial cells, and coronary smooth muscle cells20. In humans and in animal models, GLP-1R stimulation has beneficial effects on cardiac contractility, blood pressure and cardiac output, independent of its hypoglycemic effect21. Research has shown that GLP-1 increases the expression of p38 mitogen-activated protein kinases (MAPK), nitric oxide (NO) and glucose transporter 1 (GLUT1) and thus it has been shown to increase myocardial glucose uptake in an experimental heart I/R model22. Our research team previously found that GLP-1 is able to protect stem cells from heart I/R injuries23,24, and liraglutide (a GLP1 analog) can prevent no-reflow and improve heart function in STEMI patients25,26.
However, DPP4 may also act in a GLP-1R independent way. DPP4 is widely expressed on inflammatory cells and it can upregulate T-cell activation. The addition of soluble recombinant DPP4 promotes the recall antigen-induced proliferation of peripheral blood lymphocytes, while soluble mutant (inactive) DPP4 does not have this effect27.
We found that higher levels of DPP4a are negatively associated with major bleeding events; in fact, there were no major bleeding events in the highest DPP4a quartile. The fact that circulating DPP4a is negatively correlated with major bleeding events and positively correlated with no-reflow events indicates that DPP4a may be associated with an enhanced pro-coagulation state. A previous study found that, after AMI, DPP4 activity on the endothelial cell membranes of intramyocardial blood vessels is significantly decreased, and the loss of membrane-bound DPP4 causes the endothelial cells to change from a normal state to an enhanced pro-coagulation state28. It is hypothesized that soluble DPP4 is produced from membrane-bound DPP4 when it is lost from vascular endothelial cells. Thus, higher levels of DPP4a in the plasma of STEMI patients is associated with an enhanced pro-coagulation state. This hypothesis is based on the fact that soluble DPP4 could be cleaved from the membrane of human adipocytes, smooth muscle cells and liver cells by the involvement of matrix metalloproteases, a phenonemnon called “sheddases”29,30. Interestingly, we also found that DPP4a is negatively correlated with fibrinogen in STEMI patients. The decreased fibrinogen is usually associated with decreased coagulation31 but not with post-PCI bleeding32. It has been reported that the membrane-bound and soluble forms of DPP4 may have different levels of efficiency regarding their ability to cleave substrates33,34. As fibrin is a DPP4 substrate35, the soluble form of DPP4 may truncate fibrin more easily than the membrane-bound form, leading to a negative correlation between DPP4a and fibrinogen. Further basic research is needed to explore these issues.
Methods
Enrollment
The People’s Liberation Army General Hospital (PLAGH) is a large national tertiary referral center in Beijing, China. Between January 2014 and October 2015, we recruited consecutive Chinese patients with STEMI aged from 28 to 88 years who had undergone PCI at PLAGH. STEMI was defined as having typical chest pains for >30 min and elevated levels of troponin T, together with a clear ST-segment elevation of >0.1 mm in at least two contiguous electrocardiographic leads. We excluded those who were unsuitable for PCI according to the current guidelines, which included those who needed coronary artery bypass grafting (CABG), those who could be treated with thrombolytic therapy alone, those with significant hepatic or renal dysfunction and those with severe infections36,37.
Blood samples were collected at admission, and the plasma was frozen at −80 °C until further analysis. Initially, we recruited 851 patients and subsequently excluded: patients with cancer (n = 27; as cancer can affect both DPP4a and the outcomes of interest), patients who did not provide blood samples (n = 32), and patients who were taking a DPP4 inhibitor (n = 28) or a glucagon-like peptide-1 (GLP-1) analogue (n = 17). In total, 747 STEMI patients were included.
In addition, three sets of patients who attended the PLAGH during the study recruitment period were recruited for three control groups: the chest pains (CP) control group, the unstable angina (UA) control group and the non-ST segment elevation myocardial infarction (NSTEMI) control group. For the CP control group, we recruited 134 patients who had come to the hospital with CP but whose angiographic results and markers of myocardial necrosis were normal. For the UA control group, 190 patients with UA who had undergone PCI were recruited. Lastly, 146 patients with NSTEMI who had undergone PCI were recruited for the NSTEMI control group. The diagnoses of UA and NSTEMI were based on current guidelines38.
The study protocol was prepared in accordance with the Helsinki Declaration and it was approved by the ethics committee of the PLAGH and the Beijing Ethics Association. All the participants gave written consent to take part in the study. The PCI procedures were performed according to the current standard guidelines36. Each patient was required to take aspirin indefinitely after the procedure and to take clopidogrel for at least 12 months. The trial was registered with ClinicalTrials.gov (registration number: NCT02849691).
Variable definitions
We collected clinical data on each patient, including details of their PCI procedure, in-hospital outcomes and plasma factors. In addition, the Thrombolysis In Myocardial Infarction (TIMI) and the Myocardial Blush Grade (MBG) scoring systems were used to evaluate the anterograde flow in the infarct-related coronary artery39,40. Angiographic no-reflow was defined as a TIMI grade of <3 with a MBG of 0–141.
Other outcomes of interest included in-hospital major adverse cardiac or cerebrovascular events (iMACCE), which is a composite events based on death, nonfatal MIs and strokes, and in-hospital complications (IHC), which comprised acute heart failure, atrial fibrillation, chest pain, re-acute MI, complete atrioventricular block, cerebrovascular disease, ventricular fibrillation and ventricular tachycardia. In addition, we collected data on in-hospital major bleeding events. This was defined as having an decrease in hemoglobin levels (from baseline to nadir) of ≥4 g/dL, an intracranial hemorrhage, a retroperitoneal hemorrhage, a red blood cell transfusion along with a baseline hemoglobin level of ≥9.0 g/dL, or a red blood cell transfusion along with a witnessed bleeding event and a baseline hemoglobin level of < 9.0 g/dl42,43. Hypertension was defined as a blood pressure of ≥140/90 mmHg or the use of an antihypertensive agent. Diabetes was defined as a fasting plasma glucose level of ≥7 mmol/L, a 75-g oral glucose tolerance test that resulted in a 2-h plasma glucose level of ≥11.1 mmol/L, or the use of anti-diabetic agents.
Biochemical measurements
Plasma DPP4a was determined by measuring the quantity of p-nitroaniline (pNa) cleaved by DPP4 from the substrate H-Gly–Pro-pNa (L1880, Bachem, Switzerland), with slight changes44. In brief, for each participant, 5-μl blood sample was added to 150-μl 50 mM tris-HCl that contained 1 mM H-Gly–Pro-pNa. The absorbance at 405 nm was measured at 0 min and 60 min. The activity was expressed in units of U/L, with 1 U representing the production of 1 mmol of pNa every minute at 37 °C. We also obtained additional biochemical blood measurements including levels of fast plasma glucose, HbA1c, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, pro-brain natriuretic peptide (pro-BNP), creatinine, MB isoenzyme of creatine kinase (CK-MB), thrombin time, prothrombin time, activated partial thromboplastin time (APTT), fibrinogen, and gamma-glutamyltransferase (GGT) using standard clinical analytical methods (AU5800, Beckman, USA; CA-7000, Sysmex, Japan).
Statistical analysis
The PP and QQ plots were used to test the normality of the data. Where the data associated with continuous variables were normally distributed, the results were presented as means ± SDs. Some of the plasma factors were not normally distributed, and these results were presented as medians (inter-quartile range). The data associated with dichotomous variables were presented as frequencies (with percentages). We divided the participants into four categories according to the DPP4a quartile cut-off values (which were 21.54 U/L, 27.15 U/L and 32.96 U/L) and we used these categories in the subsequent analyses.
The clinical and biochemical characteristics that were normally distributed were compared using analyses of variance (ANOVAs), while the non-normal variables were compared using Kruskal–Wallis tests and the dichotomous variables were compared using chi-square tests. Spearman’s rank correlation coefficient was used to analyze the correlation between DPP4a and plasma factors that were not normally distributed, and Pearson’s correlation coefficient was used if they were normally distributed.
In addition, the associations between DPP4a and the plasma or clinical factors were assessed using a multivariate linear regression model with a forward selection approach. The variables considered for inclusion in the model included age, gender, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, creatinine, peak CK-MB, peak myoglobin, peak cardiac troponin T (cTNT), fasting plasma glucose, thrombin time, prothrombin time, APTT, fibrinogen, D-dimers, GGT, pro-BNP, history of hypertension, previous MI, smoking status, use of an angiotensin-converting-enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB), statin use, aspirin use, β-blocker use, and aldosterone antagonist use. The normality of the residuals was tested by plotting a histogram and a P-P plot of the regression standardized residuals, which indicated that the residuals had a normal distribution (data not shown). The multicollinearity of the variables used in the linear regression model was tested by calculating the tolerance and the variance inflation factor (VIF). To avoid multicollinearity, we included GGT in the multiple linear regression but not aspartate transaminase (AST) or alanine transaminase (ALT). The tolerance values of age, GGT, peak CK-MB and fibrinogen were greater than 0.94 and VIF values were less than 1.07, which indicates nonsignificant multicollinearity.
Subsequently, multivariate logistic regression models were estimated to identify potential independent relationships between DPP4a and no-reflow events, iMACCE, IHC, and major bleeding events. A forward selection approach was used to select the variables to include in the models. The variables adjusted in the regression models were those that were found to be statistically significant during forward-selection approach or biologically relevant (Model 3), which included adjusting for age, sex, BMI, creatinine, AST, total cholesterol, fibrinogen, peak CK-MB, pro-BNP, fasting plasma glucose, history of hypertension, previous MI, smoking status, ACEI/ARB use, and β-blocker use. In each of the logistic regression models, DPP4a was analyzed in quartiles and as a continuous variable. The odds ratios (ORs) and 95% CIs were reported. Hosmer-Lemeshow goodness-of-fit statistic test were used in all logistic regression analysis, with all P > 0.1. All the statistical analyses were performed using SPSS 13.0 software (SPSS, IL, USA). A two-sided p value of < 0.05 was considered to represent statistical significance.
Additional Information
How to cite this article: Li, J. W. et al. Plasma DPP4 activity is associated with no-reflow and major bleeding events in Chinese PCI-treated STEMI patients. Sci. Rep. 6, 39412; doi: 10.1038/srep39412 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank Dr. Tongzhi Wu MD, PhD (Royal Adelaide Hospital, Adelaide, Australia) for his assistance in devising the methodological approach used to detect DPP4a. This study was supported by the Pilot Foundation of the Beijing Lisheng Cardiovascular Health Foundation, China (LHJJ201611827) and China Postdoctoral Science Foundation (2016M603025).
Footnotes
Author Contributions J.W.L. was responsible for the conception and design of the study. J.L. analyzed the data. W.R.C wrote the manuscript. J.J. contributed to the discussion and reviewed/edited the manuscript. Y.Q.Y. analyzed the data and contributed to the discussion. Y.D.C. reviewed/edited the manuscript and gave the final approval for the manuscript.
References
- Windecker S., Bax J. J., Myat A., Stone G. W. & Marber M. S. Future treatment strategies in ST-segment elevation myocardial infarction. Lancet 382, 644–657, doi: 10.1016/S0140-6736(13)61452-X (2013). [DOI] [PubMed] [Google Scholar]
- Zhong J. & Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Frontiers in immunology 6, 477, doi: 10.3389/fimmu.2015.00477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Junior W. S., Godoy-Matos A. F. & Kraemer-Aguiar L. G. Dipeptidyl Peptidase 4: A New Link between Diabetes Mellitus and Atherosclerosis? BioMed research international 2015, 816164, doi: 10.1155/2015/816164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Maiseyeu A., Davis S. N. & Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circulation research 116, 1491–1504, doi: 10.1161/CIRCRESAHA.116.305665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y., Sato Y., Kamimoto T., Kawazoe K. & Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. American journal of physiology. Endocrinology and metabolism 300, E372–379, doi: 10.1152/ajpendo.00319.2010 (2011). [DOI] [PubMed] [Google Scholar]
- Zheng T. P., Liu Y. H., Yang L. X., Qin S. H. & Liu H. B. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of subclinical atherosclerosis in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Atherosclerosis 242, 580–588, doi: 10.1016/j.atherosclerosis.2015.07.042 (2015). [DOI] [PubMed] [Google Scholar]
- Zheng T. et al. Plasma DPP4 Activities Are Associated With Osteoporosis in Postmenopausal Women With Normal Glucose Tolerance. The Journal of clinical endocrinology and metabolism 100, 3862–3870, doi: 10.1210/jc.2015-2233 (2015). [DOI] [PubMed] [Google Scholar]
- Connelly K. A. et al. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovascular therapeutics 31, 259–267, doi: 10.1111/1755-5922.12005 (2013). [DOI] [PubMed] [Google Scholar]
- Shah Z. et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124, 2338–2349, doi: 10.1161/CIRCULATIONAHA.111.041418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta T. et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation 126, 1838–1851, doi: 10.1161/CIRCULATIONAHA.112.096479 (2012). [DOI] [PubMed] [Google Scholar]
- Aghili N. et al. Polymorphisms in dipeptidyl peptidase IV gene are associated with the risk of myocardial infarction in patients with atherosclerosis. Neuropeptides 46, 367–371, doi: 10.1016/j.npep.2012.10.001 (2012). [DOI] [PubMed] [Google Scholar]
- Moro P. J. et al. Mononuclear cell adenosine deaminase and CD26/dipeptidylpeptidase-IV activities are sensitive markers of reperfusion during percutaneous transluminal angioplasty. International journal of cardiology 166, 225–229, doi: 10.1016/j.ijcard.2011.10.090 (2013). [DOI] [PubMed] [Google Scholar]
- dos Santos L. et al. Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circulation. Heart failure 6, 1029–1038, doi: 10.1161/CIRCHEARTFAILURE.112.000057 (2013). [DOI] [PubMed] [Google Scholar]
- Ravassa S. et al. The activity of circulating dipeptidyl peptidase-4 is associated with subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus. Cardiovascular diabetology 12, 143, doi: 10.1186/1475-2840-12-143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J. W. & Kim S. Dipeptidyl Peptidase 4 Inhibitors and the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Tale of Three Studies. Diabetes & metabolism journal 39, 373–383, doi: 10.4093/dmj.2015.39.5.373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Hopper I., Skiba M. & Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovascular therapeutics 32, 147–158, doi: 10.1111/1755-5922.12075 (2014). [DOI] [PubMed] [Google Scholar]
- Resnic F. S. et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. American heart journal 145, 42–46, doi: 10.1067/mhj.2003.36 (2003). [DOI] [PubMed] [Google Scholar]
- Bouleti C., Mewton N. & Germain S. The no-reflow phenomenon: State of the art. Archives of cardiovascular diseases 108, 661–674, doi: 10.1016/j.acvd.2015.09.006 (2015). [DOI] [PubMed] [Google Scholar]
- De Caterina R., Madonna R., Sourij H. & Wascher T. Glycaemic control in acute coronary syndromes: prognostic value and therapeutic options. European heart journal 31, 1557–1564, doi: 10.1093/eurheartj/ehq162 (2010). [DOI] [PubMed] [Google Scholar]
- Zhong J., Gong Q., Goud A., Srinivasamaharaj S. & Rajagopalan S. Recent Advances in Dipeptidyl-Peptidase-4 Inhibition Therapy: Lessons from the Bench and Clinical Trials. Journal of diabetes research 2015, 606031, doi: 10.1155/2015/606031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher B., Reichetzeder C. & Alter M. L. Renal and cardiac effects of DPP4 inhibitors–from preclinical development to clinical research. Kidney & blood pressure research 36, 65–84, doi: 10.1159/000339028 (2012). [DOI] [PubMed] [Google Scholar]
- Zhao T. et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. The Journal of pharmacology and experimental therapeutics 317, 1106–1113, doi: 10.1124/jpet.106.100982 (2006). [DOI] [PubMed] [Google Scholar]
- Zhou H. et al. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Scientific reports 5, 12898, doi: 10.1038/srep12898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. et al. Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free radical biology & medicine 77, 363–375, doi: 10.1016/j.freeradbiomed.2014.09.033 (2014). [DOI] [PubMed] [Google Scholar]
- Chen W. R. et al. Effects of liraglutide on no-reflow in patients with acute ST-segment elevation myocardial infarction. International journal of cardiology 208, 109–114, doi: 10.1016/j.ijcard.2015.12.009 (2016). [DOI] [PubMed] [Google Scholar]
- Chen W. R. et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. American heart journal 170, 845–854, doi: 10.1016/j.ahj.2015.07.014 (2015). [DOI] [PubMed] [Google Scholar]
- Tanaka T. et al. Enhancement of antigen-induced T-cell proliferation by soluble CD26/dipeptidyl peptidase IV. Proceedings of the National Academy of Sciences of the United States of America 91, 3082–3086 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnen P. A. et al. Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic research in cardiology 107, 233, doi: 10.1007/s00395-011-0233-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu T. et al. Similar increased serum dipeptidyl peptidase IV activity in chronic hepatitis C and other viral infections. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 27, 59–68 (2003). [DOI] [PubMed] [Google Scholar]
- Rohrborn D., Eckel J. & Sell H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS letters 588, 3870–3877, doi: 10.1016/j.febslet.2014.08.029 (2014). [DOI] [PubMed] [Google Scholar]
- Kell D. B. & Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integrative biology : quantitative biosciences from nano to macro 7, 24–52, doi: 10.1039/c4ib00173g (2015). [DOI] [PubMed] [Google Scholar]
- Mahmud E. et al. Effect of Serum Fibrinogen, Total Stent Length, and Type of Acute Coronary Syndrome on 6-Month Major Adverse Cardiovascular Events and Bleeding After Percutaneous Coronary Intervention. The American journal of cardiology 117, 1575–1581, doi: 10.1016/j.amjcard.2016.02.032 (2016). [DOI] [PubMed] [Google Scholar]
- Ansorge S. et al. Novel aspects of cellular action of dipeptidyl peptidase IV/CD26. Biological chemistry 392, 153–168, doi: 10.1515/BC.2011.008 (2011). [DOI] [PubMed] [Google Scholar]
- Wagner L. et al. Identifying neuropeptide Y (NPY) as the main stress-related substrate of dipeptidyl peptidase 4 (DPP4) in blood circulation. Neuropeptides 57, 21–34, doi: 10.1016/j.npep.2016.02.007 (2016). [DOI] [PubMed] [Google Scholar]
- Mentlein R. & Heymann E. Dipeptidyl peptidase IV inhibits the polymerization of fibrin monomers. Archives of biochemistry and biophysics 217, 748–750 (1982). [DOI] [PubMed] [Google Scholar]
- Levine G. N. et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124, 2574–2609, doi: 10.1161/CIR.0b013e31823a5596 (2011). [DOI] [PubMed] [Google Scholar]
- American College of Emergency, P. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 61, 485–510, doi: 10.1016/j.jacc.2012.11.018 (2013). [DOI] [PubMed] [Google Scholar]
- Levine G. N. et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation, doi: 10.1161/CIR.0000000000000404 (2016). [DOI] [Google Scholar]
- Ganz W. The thrombolysis in myocardial infarction (TIMI) trial. The New England journal of medicine 313, 1018, doi: 10.1056/nejm198510173131611 (1985). [DOI] [PubMed] [Google Scholar]
- van ‘t Hof A. W. et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 97, 2302–2306 (1998). [DOI] [PubMed] [Google Scholar]
- Niccoli G., Burzotta F., Galiuto L. & Crea F. Myocardial no-reflow in humans. Journal of the American College of Cardiology 54, 281–292, doi: 10.1016/j.jacc.2009.03.054 (2009). [DOI] [PubMed] [Google Scholar]
- Gruberg L. et al. The Association of Previous Revascularization With In-Hospital Outcomes in Acute Myocardial Infarction Patients: Results From the National Cardiovascular Data Registry. JACC. Cardiovascular interventions 8, 1954–1962, doi: 10.1016/j.jcin.2015.08.030 (2015). [DOI] [PubMed] [Google Scholar]
- Consuegra-Sanchez L. et al. Impact of previous vascular burden on in-hospital and long-term mortality in patients with ST-segment elevation myocardial infarction. Revista espanola de cardiologia 67, 471–478, doi: 10.1016/j.rec.2013.10.017 (2014). [DOI] [PubMed] [Google Scholar]
- Wu T. et al. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes research and clinical practice 106, e3–6, doi: 10.1016/j.diabres.2014.08.004 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.