Abstract
Several considerations suggest that levels of the two major modes of double-strand break (DSB) repair, homologous recombination (HR), and nonhomologous end joining (NHEJ), are regulated through the cell cycle. However, this idea has not been explicitly tested. In the absence of the telomere-binding protein Taz1, fission yeast undergo lethal telomere fusions via NHEJ. These fusions occur only during periods of nitrogen starvation and fail to accumulate during logarithmic growth, when the majority of cells are in G2. We show that G1 arrest is the specific nitrogen starvation-induced event that promotes NHEJ between taz1- telomeres. Furthermore, the general levels of NHEJ and HR are reciprocally regulated through the cell cycle, so that NHEJ is 10-fold higher in early G1 than in other cell cycle stages; the reverse is true for HR. Whereas NHEJ is known to be dispensable for survival of DSBs in cycling cells, we find that it is critical for repair and survival of DSBs arising during G1.
Keywords: Cell cycle, DNA repair, HR, NHEJ, Taz1, telomere
DNA double-strand breaks (DSBs) are among the most deleterious types of damage with which cellular DNA repair systems must contend. If a DSB is left unrepaired in a dividing cell, the portion of the chromosome that is left unconnected to the centromere is unable to segregate to the daughter cell, giving rise to chromosome deletions. If incorrectly repaired, DSBs may lead to chromosome translocations and other aberrations (Pierce et al. 2001b).
Two major pathways repair DSBs, nonhomologous end joining (NHEJ), and homologous recombination (HR; van Gent et al. 2001). NHEJ joins two DNA ends irrespective of their sequence, thereby generating errors if the two ends are unrelated or inaccurately processed. Alternatively, DSBs can be joined via HR processes that use homologous DNA sequences (usually in the sister chromatid) as templates for repairing broken ends, thus providing error-free repair. HR is the pathway of choice in budding and fission yeast, as NHEJ mutants are resistant to γ-radiation, whereas HR mutations severely compromise survival of γ-radiation (Siede et al. 1996; Manolis et al. 2001).
Remarkably, although cells can detect and respond to a single DSB generated by DNA damage (Sandell and Zakian 1993), they are perfectly capable of recognizing the numerous ends of chromosomes (e.g., 184 in a G2 human somatic cell) as nondeleterious structures. To conserve genome stability, telomeres have developed the property of being refractory to DNA repair processes. Recent studies have uncovered some of the components underlying this property (Ferreira et al. 2004). In humans, the TRF2 protein binds telomeres and protects them from inappropriate repair reactions; interfering with TRF2 function leads to chromosome end fusions, cell cycle arrest, and apoptosis (Karlseder et al. 1999).
Taz1 is the fission yeast ortholog of both TRF2 and the other human telomeric DNA-binding protein, TRF1 (Cooper et al. 1997; Li et al. 2000; Ferreira and Cooper 2001). In the absence of Taz1, several telomere functions are disrupted, but cells are still viable in unperturbed cell cycles (Cooper et al. 1997, 1998). We have shown that Taz1 loss renders telomeres vulnerable to the two DSB repair pathways, but that end fusions, which form via NHEJ, only occur as cells arrest in G1 during nitrogen starvation (Ferreira and Cooper 2001). During logarithmic growth, fission yeast are mainly in G2 and taz1- telomere fusions are absent. However, NHEJ-mediated taz1- telomere fusions arise during logarithmic growth in rad22- HR-deficient cells, suggesting that HR protects dysfunctional (taz1-) telomeres from NHEJ. These results led us to propose that NHEJ becomes prominent in G1-arrested cells, whereas HR dominates during logarithmic (mainly postreplicative) growth.
Several other lines of evidence support the idea that the two major modes of DSB repair are cell cycle regulated. From a teleological standpoint, cells might prefer error-free HR whenever possible, that is, when template copies, preferably sister chromatids, are available. Conversely, during G1, error-prone NHEJ would be necessary, as sister chromatid templates are unavailable. Although diploid cells possess homologous chromosomes during G1, mechanisms exist to disfavor mitotic recombination between homologous chromosomes and the potential ensuing loss of heterozygosity in somatic mammalian cells (Moynahan and Jasin 1997). In chicken DT40 lymphocytes, NHEJ mutants (ku70-/- and DNAPK-/-) are particularly sensitive to ionizing radiation during G1, whereas Rad54-/- HR mutants are more sensitive during S and G2 phases (Takata et al. 1998). Furthermore, levels of plasmid recircularization via NHEJ increase during G1 in budding yeast (Karathanasis and Wilson 2002), whereas the formation of DSB-induced Rad52 HR foci is reduced in G1 cells (Lisby et al. 2001).
Here, we demonstrate that HR and NHEJ are reciprocally regulated through the cell cycle. By changing cell cycle profiles, we can modulate the appearance of NHEJ-mediated telomere fusions during both vegetative growth and nitrogen starvation. Furthermore, by directly measuring repair, we show that levels of the two modes of repair vary through the cell cycle by a factor of 10, with NHEJ being higher in G1 and HR being higher in G2.
Results and Discussion
Occurrence of pre-START G1 during logarithmic growth induces taz1- telomere fusions
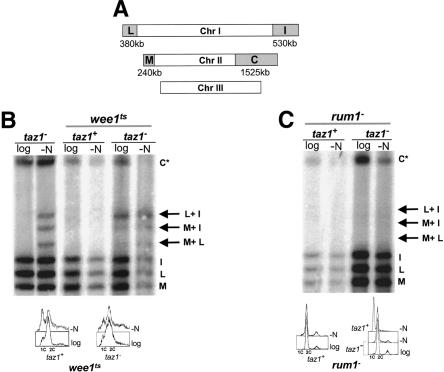
Logarithmically growing fission yeast increase in size during the G2 phase of the cell cycle, and upon completion of mitosis, cells generally possess sufficient mass to proceed directly to S phase. Thus, the vegetative cell cycle lacks a pre-START G1 phase. However, nitrogen-starved cells arrest in G1. As fission yeast prefers haploidy, these G1 cells contain only one copy of each chromosome. taz1- cells accumulate telomere fusions when subjected to nitrogen starvation, yet these same cells lack telomere fusions, and indeed show virtually wild-type levels of viability, when grown in rich medium (Ferreira and Cooper 2001). To determine whether the taz1- telomere fusions are a consequence of the G1 arrest induced by nitrogen starvation, we investigated cells in which a pre-START G1 phase occurs during vegetative growth. wee1-50 cells enter mitosis prematurely, forming smaller cells than wild type. These cells cannot initiate replication until sufficient cell mass is attained, and remain in G1 for extended periods with low levels of Cdc2 kinase activity (Russell and Nurse 1987). wee1-50 taz1- cultures were grown at the semipermissive temperature of 32°C and divided into halves. One half was starved for nitrogen for 24 h at 32°C, whereas the other was grown in nitrogen-rich medium; aliquots of both were taken for FACS and PFGE analyses. Southern blotting with telomere probes revealed that telomere fusions were present not only in the nitrogen-starved culture, but also in vegetatively growing wee1-50 taz1- cells (Fig. 1B). Thus, by recreating pre-START G1 during the vegetative cell cycle, we induce telomere fusions in taz1- cells growing in nitrogen-rich medium.
Figure 1.
The stage of the cell cycle determines whether taz1- cells sustain telomere fusions. (A) Diagram of telomeric NotI restriction fragments on chromosome I and II (C, I, L, and M); chromosome III lacks NotI restriction sites. (B,C) Southern blot analysis of genomic DNA digested with NotI, separated by PFGE, and probed with a telomeric oligonucleotide. Telomeric restriction fragments are indicated. C* comprises the C restriction fragment as well as the C + L, C + M, and C + I fusions that cannot be resolved under these conditions. (B) Inactivation of wee1+ extends early G1 and induces fusions of taz1- telomeres during logarithmic growth. The most prominent fusion band (top arrow) corresponds to circularized chromosome I. Prolonged growth of wee1ts cells at the semipermissive temperature typically distorts the FACS profile due to delayed cytokinesis in small cells. (C) rum1+ deletion prevents G1 arrest during nitrogen starvation, and prevents starvation from inducing fusion between taz1- telomeres.
The telomere fusions seen in vegetatively growing wee1-50 taz1- cells are primarily intramolecular (i.e., resulting in self-circularization), whereas both inter- and intramolecular fusions are seen in nitrogen starved taz1- cells (Fig. 1B). Intrachromosomal fusions also predominate in logarithmically growing taz1- cells impaired in HR (Ferreira and Cooper 2001). Schizzosaccaromyces pombe cells possessing circular chromosomes are viable (Naito et al. 1998; Nakamura et al. 1998), whereas inter-chromosomal fusions generate lethal dicentric chromosomes and are only maintained in nondividing (i.e., G1-arrested) cultures. Therefore, the predominance of intrachromosomal fusions in wee1-50 taz1- cells supports the idea that the fusions occur while the cells are actively dividing in rich medium.
Absence of G1 arrest during nitrogen starvation prevents telomere fusions
If prolonging the G1 period during vegetative growth can induce taz1- telomere fusions, then the absence of G1 arrest during nitrogen starvation might prevent telomere fusions. To test this, we generated taz1- strains lacking the CDK inhibitor Rum1. Upon nitrogen starvation, rum1- cells are unable to restrain S phase and arrest with replicated chromosomes and high levels of Cdc2 kinase activity. Nonetheless, rum1- mutants do activate the Ste11 transcription factor and its targets in response to nitrogen starvation (Stern and Nurse 1998). As expected, neither rum1- nor rum1- taz1- strains were able to arrest in G1, even after prolonged nitrogen starvation and maintained 2C DNA content (Fig. 1C). Remarkably, rum1- taz1- cells exhibit no telomere fusions during nitrogen starvation, (Fig. 1C) indicating that the Rum1-dependent G1 arrest, and not some event downstream of Ste11 activation, activates the pathway that results in taz1- telomere fusions. Thus, by indirectly manipulating the activity of the Cdc2 kinase, we can manipulate the susceptibility of taz1- telomeres to undergo NHEJ-induced fusions in a manner independent of nutritional status, with low Cdc2 activity inducing telomere fusions and high Cdc2 activity preventing them.
Telomeric 3′ overhangs persist in G1 cells
taz1- telomeres possess extensive 3′ G-strand overhangs during logarithmic growth (Tomita et al. 2003). In principle, such overhangs should promote the strand invasions that initiate HR, whereas NHEJ would require overhang removal. Indeed, upon inhibition of TRF2 function in human cells, NHEJ-dependent telomere fusions are accompanied by a reduction in 3′-overhang signal (van Steensel et al. 1998; Smogorzewska et al. 2002). To investigate whether elevated NHEJ stems from a global loss of 3′ overhangs in G1 taz1- cells, we used native in-gel hybridization analysis. Telomeric restriction fragments from asynchronous and nitrogen-starved cultures were electrophoresed under nondenaturing conditions and hybridized to a G-strand telomere probe. Both cycling and G1-blocked taz1- cells show intense hybridization (Fig. 2A), whereas no signal is detectable using a complementary C-rich strand probe (data not shown). The overhang signal also persists in taz1- cells lacking telomere fusions (via pku70- and lig4- deletion). A caveat of the native hybridization method is that it can only address global changes in the abundance of overhanging DNA and does not address individual telomeres. Hence, some telomeres may lose overhangs, whereas others gain them, generating no net change in signal strength. Nevertheless, we speculate that the NHEJ machinery can engage taz1- overhang-containing telomeres as substrates, and that overhang removal is concomitant with the end-joining reaction. Furthermore, our observation that NHEJ-dependent telomere fusions do occur in logarithmically growing taz1- cells that lack Rad22 (Ferreira and Cooper 2001) argues against the idea that G2 taz1- telomeres are incompetent for NHEJ.
Figure 2.
taz1- telomeres exhibit extensive ssDNA overhangs in G1. (A) Telomere 3′ overhangs are detected in nitrogen-starved taz1-, pku70- taz1, lig4- taz1- cells. In-gel hybridization of EcoRI-digested genomic DNA to a G-strand telomere oligonucleotide in nondenaturing and denaturing conditions. (B) Quantitation of telomeric 3′ overhangs. Hybridization in each lane was quantified, and the ratio of nondenatured/denatured signal for each sample was normalized against the ratio derived from logarithmically growing taz1- cells. Error bars represent standard deviation for a minimum of three independent experiments.
DSB repair pathways are cell cycle regulated
The appearance of telomere fusions in log-phase taz1-rad22- cells suggested that HR precludes NHEJ at dysfunctional telomeres during vegetative growth (Ferreira and Cooper 2000). Thus, unprotected telomeres may be recipients of whatever DNA repair pathway predominates generally in the cell at a given cell cycle stage. This idea implies that cell cycle regulation of DNA repair would be seen not only at telomeres, but also throughout the genome. To test this, we directly measured the two main modes of DSB repair in different stages of the cell cycle. We used nitrogen-starved cells as representative of the G1 phase and logarithmically growing cells as representative of the S, G2, and M phases. FACS analysis indicated that upon nitrogen starvation, 60%-70% of cells arrest in G1, whereas ∼100% of the cells in logarithmically growing cultures exhibit only a 2C DNA peak.
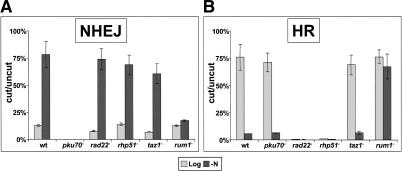
Plasmid-based assays have been used in both fission and budding yeasts to investigate the genetic requirements for NHEJ and HR (Orr-Weaver et al. 1981; Keeney and Boeke 1994; Boulton and Jackson 1996; Muris et al. 1997; Baumann and Cech 2000; Manolis et al. 2001). To assess NHEJ, a plasmid containing a replication origin is linearized within sequences that lack homology to the yeast genome, and then transformed into yeast cells. The uncut plasmid is transformed in parallel to normalize for differences in transformation efficiencies between strains. Logarithmically growing S. pombe cells display a low plasmid end-joining efficiency (Fig. 3A) and, as observed previously, this end-joining is almost entirely dependent on pku70+ (Baumann and Cech 2000; Manolis et al. 2001). Strikingly, end-joining levels were seven- to 10-fold higher in nitrogen-starved G1 cells (Fig. 3A). However, this elevation of NHEJ was absent in nitrogen-starved rum1- cells that are unable to undergo G1 arrest (Fig. 3A). Thus, Ku-dependent DSB repair is up-regulated in G1-arrested cells, consistent with our hypothesis that taz1- telomeres fuse during G1 arrest, because NHEJ is generally elevated under these conditions. Moreover, the genetic requirement of rum1+ for elevated NHEJ suggests that Rum1-dependent down-regulation of Cdc2 kinase is a key event in the switch between the two modes of repair.
Figure 3.
NHEJ and HR are reciprocally regulated through the cell cycle. (A) NHEJ is up-regulated in G1 (-N) arrested cells. Levels of plasmid end joining are represented as the number of transformants obtained with linear vs. circular plasmid. Cells lacking Rum1 fail to arrest in G1 and NHEJ levels remain low. (B) HR is down-regulated in G1 nitrogen-starved cells. Integration of a linear leu1+ pJK148 plasmid into the genome is expressed as the ratio of tranformants for the linearized vector (which lacks an origin of replication) vs. supercoiled pJK148-ars1. Rum1 is required for down-regulation of HR during nitrogen starvation.
A straightforward model of direct competition between NHEJ and HR pathways would predict that, in the absence of HR, NHEJ levels would rise during logarithmic growth. However, we did not detect an increase in plasmid end-joining efficiency in logarithmically growing HR-deficient rad22- or rhp51- mutants (Fig. 3A). This suggests that the two pathways are regulated independently, such that in the absence of one, the other mode of repair is still subject to cell cycle control. Alternatively, DNA ends that attempt HR in the absence of rad22+ or rhp51+ may be reversed and channeled to NHEJ.
To measure HR during different stages of the cell cycle, we used a plasmid-integration assay in which leu1- strains are transformed with a plasmid that lacks a replication origin and is linearized within the leu1+ auxotrophic marker. These strains must integrate the plasmid to survive in medium lacking leucine. A circular plasmid carrying an origin of replication and leu1+ was transformed in parallel to normalize for transformation efficiencies. Levels of plasmid integration were high during logarithmic growth and dependent on rhp51+ (Fig. 3B), as previously reported (Muris et al. 1997). Deletion of rad22+, the fission yeast RAD52 homolog, also led to a severe reduction in HR, in agreement with van den Bosch et al. (2001) and in contrast to the modest effect on HR reported for a truncation of rad22+ (Ostermann et al. 1993; Muris et al. 1997). Conversely to NHEJ, plasmid integration levels were reduced 10- to 15-fold in G1-arrested cells (Fig. 3B). Southern blotting demonstrated that ∼90% of the integration events occurred at the chromosomal leu1 locus (data not shown), confirming that HR had been the pathway for integration. rum1- cells failed to exhibit reduced HR during nitrogen starvation, suggesting that down-regulation of HR depends upon G1 arrest. In agreement with a gene-targeting transfection assay in mammalian cells (Pierce et al. 2001a), deletion of pku70+ did not lead to an elevation of HR (Fig. 3B).
Sister chromatid cohesion is a candidate regulator of HR through the cell cycle (Jessberger 2002), as cohesion is established during S phase and is required for efficient DSB repair. Coupled with the observed preference for sister chromatids over homologs as substrates for HR (Kadyk and Hartwell 1992; Moynahan and Jasin 1997), these observations suggest that HR dominates in G2 through the availability of a cohesed sister chromatid. Hence, the prevalence of HR at taz1- telomeres during G2 could simply reflect this availability. However, we were unable to promote telomere fusions during logarithmic growth by disrupting the cohesion complex in a rad21ts taz1- double mutant (data not shown). Furthermore, the plasmid recircularization and integration events that we used as measures of NHEJ and HR occur in the absence of cohesion between damaged and undamaged substrates.
Thus, the two major modes of DSB repair are regulated reciprocally, such that during logarithmic growth, when cells harbor two copies of each chromosome, HR dominates over NHEJ. Conversely, in G1-arrested cells that contain only one copy of each chromosome, the levels of these two modes of repair are inverted, with lower HR and higher NHEJ. These patterns of general repair levels mirror the activities that we observe at taz1- telomeres, and support the idea that cell cycle regulation of repair directs the consequences of telomere dysfunction. The telomere fusions that follow expression of dominant-negative TRF2 in human cells can arise in both G1 and G2 (Smogorzewska et al. 2002), although the latter may reflect a requirement for passage through S phase to allow displacement of wild-type TRF2 by the dominant-negative form. Nonetheless, a relative paucity of fusions between dysfunctional telomeres during G2 in fission yeast versus human cells is consistent with the idea that mammalian cells have generally higher levels of NHEJ than the yeast systems.
NHEJ is required for radiation resistance during G1
Previous studies in yeasts showed that survival following DSB induction depended entirely on HR, whereas NHEJ mutants were insensitive to DSBs (Siede et al. 1996; Manolis et al. 2001). Our observation that NHEJ levels increase during G1 prompted us to test whether NHEJ becomes important for survival of DSBs during G1 arrest. Using wild-type cells and mutants in the HR (rad22- and rhp51-) and NHEJ (pku70- and lig4-) pathways, we analyzed the viability of logarithmic and nitrogen-starved cultures exposed to varying doses of γ-irradiation (Fig. 4A,B). Whereas logarithmically growing HR mutants were extremely sensitive to γ-irradiation, NHEJ mutants exhibited viabilities comparable to that of wild-type cells (Fig. 4A). During nitrogen starvation, however, NHEJ mutants were 10-fold more sensitive to γ-irradiation than wild-type cells, denoting a role for NHEJ in survival of radiation during G1 (Fig. 4A,B). Interestingly, wild-type cells lose viability more severely during G1 than during logarithmic growth (Fig. 4B), perhaps indicating that elevated use of potentially inaccurate NHEJ during G1 confers some lethality. HR genes are also important for survival of γ-irradiation during nitrogen starvation (Fig. 4A,B), although dependence on HR is less severe in nitrogen-starved than logarithmic cultures (Fig. 4B). This could reflect HR that occurs following release from nitrogen starvation, the ∼30% of cells that do not arrest in G1 upon nitrogen starvation, or HR-related processes that occur during G1 arrest.
Figure 4.
NHEJ is required for survival and repair of damage induced by γ-radiation during G1. (A,B) Logarithmic (Log) and nitrogen starved (-N) cultures were treated with varying doses of γ-radiation. NHEJ mutants are only sensitive during the G1 arrest induced by nitrogen starvation. (A) Serial dilution assay. (B) Quantitative survival analysis. (C) Cells were exposed to 100 Gy of γ radiation and allowed to recover for varying amounts of time. G1-arrested wild-type (wt) cells undergo DSB repair faster than NHEJ mutants, whereas NHEJ is dispensable for repair during log growth. (D) Quantitation of the data in C. Ethidium bromide signals corresponding to broken DNA were normalized to the signal for intact chromosome II for each lane. The value obtained at time 0 was designated as 1.00.
NHEJ is required for efficient DSB repair in G1
As assessment of survival can only address the success of DSB repair and not when it was undertaken, we directly monitored the recovery of whole chromosomes following breakage by ionizing radiation. G1-arrested cells were exposed to 100 Gy of ionizing radiation to induce DSBs, then kept in G1 and analyzed by PFGE to monitor DSB repair as a function of time. Whereas whole chromosomes from untreated cells are visible as three intact bands (Fig. 4C), the irradiated cells accumulate degraded DNA appearing as a smear of higher-mobility DNA fragments. Remarkably, G1-arrested wild-type cells not only sustain less DNA damage, but also recover chromosome integrity faster than pku70- and lig4- mutants (Fig. 4C,D). This result demonstrates that repair occurs while the cells are blocked in G1, and that efficient repair requires the NHEJ pathway. pku70- and lig4- mutants only started to regain intact chromosomes upon prolonged recovery periods, consistent with the moderate decline of viability of NHEJ mutants. In IR-treated logarithmic cultures, recovery rates were independent of pku70- and lig4- (Fig. 4D), reinforcing the idea that NHEJ is a prominent mode of DSB repair during G1 exclusively.
Perspectives
As manipulating Cdc2 kinase activity can alter the relative levels of NHEJ and HR, we infer that Cdc2 is the ultimate determinant of the choice of repair pathway, with low levels of Cdc2 activity dictating elevated NHEJ and high levels directing HR. Indeed, replication studies have established that levels of Cdc2 kinase activity distinguish cells that have or have not accomplished genome duplication. This same kinase may direct DSBs to error-free repair pathways when sister chromatids are present. These ideas complement a recent report (Caspari et al. 2002) describing a mutation in the B-type cyclin Cdc13 that reduces the formation of Rhp51 foci and impairs a later step in HR in response to DSBs during G2. It will be of great interest to identify the targets of Cdc2 kinase that control the regulation of DNA repair.
Materials and methods
Strains and medium
Strains are listed in Table 1. Cultures were grown at 32°C in standard YES or EMM medium with or without NH4Cl and any required supplements.
Table 1.
S. pombe strains used in this work
| Strain | Genotype | Source |
|---|---|---|
| Wild type | h−ade6-M210 leu1-32 ura4-D18 | |
| taz1− | h−taz1::ura4+ade6-M210 leu1-32 ura4-D18 | J.P. Cooper |
| wee1ts | wee1-50 ura4-D18 | P. Nurse |
| wee1tstaz1− | wee-1-50 taz1::ura4+ura4-D18 | This study |
| rum1− | rum1::ura4+ura4-D18 | S. Moreno |
| rum1−taz1− | rum1::ura4+ura4-D18 taz1::kanr | This study |
| rum1−leu1− | h−rum1::ura4+ade6-M216 leu1-32 ura4-D18 | P. Nurse |
| pku70−leu1− | pku70::kanrhis3-D1 leu1-32 ura4-D18 ade6− | P. Baumann |
| pku70−kans | h−pku70::ura4+leu1-32 ura4-D18 ade6-M210 | This study |
| rad22−ura4− | h+rad22::LEU2 his3-D1 leu1-32 ura4-D18 | T. Nakamura |
| rad22−leu1− | h−rad22::ura4+ade6-M210 leu1-32 ura4-D18 | This study |
| rhp51− | h+rhp51::ura4+ade6-704 leu1-32 ura4-D18 | A.M. Carr |
Gene disruption strains
Gene disruption was performed using the method of Bahler et al. (1998), and verified by PCR using primers for the integrating constructs and flanking genomic sequences.
Nitrogen starvation and FACS analysis
Cultures were grown to log phase (0.5-1 × 107 cells/mL), washed extensively with EMM-N, resuspended in EMM-N at a density of 1-5 × 106 cells/mL, and starved for 36-72 h. FACS was performed on ethanol-fixed cells on a Becton Dickson FACScan.
Pulse-field gel electrophoresis, in-gel hybridization, and Southern blotting
Pulsed-field gel electrophoresis was performed as previously described (Ferreira and Cooper 2001). Telomere overhangs were analyzed as in Tomita et al. (2003). Overhang signals were quantified using Molecular Dynamics ImageQuant software.
Plasmid assays
The NHEJ plasmid assay was performed essentially as described (Boulton and Jackson 1996). The plasmid pKan1 (Haering et al. 2000) was linearized with KpnI and cells transformed in triplicate with 1 μg of linear or circular pKan1. Cells were spread on YES containing 100 μg/mL G418 and colonies counted after 5 d at 32°C.TheHRplasmid assay was similar to that previously described (Keeney and Boeke 1994). Strains auxotrophic for leu1+ were transformed in triplicate with 1 μg NdeI-linearized pJK148 as above. To assess the uptake of DNA, an aliquot of competent cells of each strain was transformed with 1 μg of pJK148-ars1 (same as pJK148, but with the EcoRI fragment of ars1 filled in and cloned into the SmaI site of pJK148). Transformants were selected on EMM lacking leucine, and integration frequencies calculated by dividing the number of pJK148 leu1+ colonies by the number of pJK148-ars1 leu1+ colonies. For each such ratio, the average and standard deviation of at least three replicates of each transformation is presented.
Radiation sensitivity and DSB repair assay
Cultures were either diluted in triplicate to yield 200 colonies per plate or serially diluted 1:5 in 96-well plates and spotted on YES medium. The cells were immediately exposed to γ-rays using an IBL 637 Irradiator (137Cs source at a rate of 3.45 Gy/min). Plates were then incubated for 3-5 d at 32°C. The results are presented as the percentage of survivors of a given dose relative to the viabilities of the same strains unirradiated. Each experiment was repeated three times.
DSB repair assay
Logarithmic and nitrogen-starved cultures were exposed to 100 Gy γ-radiation in liquid medium. Samples were collected at various times and processed for PFGE as described above. Whole chromosomes were separated on a CHEF-DRIII apparatus (Bio-Rad) according to the manufacturer's specifications. Gels were stained with ethidium bromide and digitized using a UVP VisiDoc-IT system without oversaturation. Broken DNA and intact chromosome signals were quantified using NIH Image version 1.63 software.
Acknowledgments
We thank the members of the Cooper lab for discussion and J. Hayles and F. Uhlmann for critically reading the manuscript. We thank the laboratories of T. Carr, T. Cech, S. Moreno, A. Pastink, and P. Nurse for sharing strains and plasmids. This work was supported by the NIH, the Human Frontiers Science Program, the Pew Scholars Program in the Biomedical Sciences, and Cancer Research UK. M.G.F. is a recipient of a Cancer Research UK postdoctoral fellowship.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.315804.
References
- Bahler J., Wu, J.Q., Longtine, M.S., Shah, N.G., McKenzie III, A., Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943-951. [DOI] [PubMed] [Google Scholar]
- Baumann P. and Cech, T.R. 2000. Protection of telomeres by the Ku protein in fission yeast. Mol. Biol. Cell. 11: 3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S.J. and Jackson, S.P. 1996. Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. EMBO J. 15: 5093-5103. [PMC free article] [PubMed] [Google Scholar]
- Caspari T., Murray, J.M., and Carr, A.M. 2002. Cdc2-cyclin B kinase activity links Crb2 and Rqh1-topoisomerase III. Genes & Dev. 16: 1195-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.P., Nimmo, E.R., Allshire, R.C., and Cech, T.R. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast [see comments]. Nature 385: 744-747. [DOI] [PubMed] [Google Scholar]
- Cooper J.P., Watanabe, Y., and Nurse, P. 1998. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination [see comments]. Nature 392: 828-831. [DOI] [PubMed] [Google Scholar]
- Ferreira M.G. and Cooper, J.P. 2001. The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol. Cell 7: 55-63. [DOI] [PubMed] [Google Scholar]
- Ferreira M.G., Miller, K.M., and Cooper, J.P. 2004. Indecent exposure: When telomeres become uncapped. Mol. Cell 13: 7-18. [DOI] [PubMed] [Google Scholar]
- Haering C.H., Nakamura, T.M., Baumann, P., and Cech, T.R. 2000. Analysis of telomerase catalytic subunit mutants in vivo and in vitro in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. 97: 6367-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R. 2002. The many functions of SMC proteins in chromosome dynamics. Nat. Rev. Mol. Cell. Biol. 3: 767-778. [DOI] [PubMed] [Google Scholar]
- Kadyk L.C. and Hartwell, L.H. 1992. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics 132: 387-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis E. and Wilson, T.E. 2002. Enhancement of Saccharomyces cerevisiae end-joining efficiency by cell growth stage but not by impairment of recombination. Genetics 161: 1015-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J., Broccoli, D., Dai, Y., Hardy, S., and de Lange, T. 1999. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283: 1321-1325. [DOI] [PubMed] [Google Scholar]
- Keeney J.B. and Boeke, J.D. 1994. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 136: 849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Oestreich, S., and de Lange, T. 2000. Identification of human Rap1: Implications for telomere evolution. Cell 101: 471-483. [DOI] [PubMed] [Google Scholar]
- Lisby M., Rothstein, R., and Mortensen, U.H. 2001. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. 98: 8276-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolis K.G., Nimmo, E.R., Hartsuiker, E., Carr, A.M., Jeggo, P.A., and Allshire, R.C. 2001. Novel functional requirements for non-homologous DNA end joining in Schizosaccharomyces pombe. EMBO J. 20: 210-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan M.E. and Jasin, M. 1997. Loss of heterozygosity induced by a chromosomal double-strand break. Proc. Natl. Acad. Sci. 94: 8988-8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris D.F.R., Vreeken, K., Schmidt, H., Ostermann, K., Clever, B., Lohman, P.H.M., and Pastink, A. 1997. Homologous recombination in the fission yeast Schizosaccharomyces pombe: Different requirements for the rhp51+, rhp54+ and rad22+ genes. Curr. Genet. 31: 248-254. [DOI] [PubMed] [Google Scholar]
- Naito T., Matsuura, A., and Ishikawa, F. 1998. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat. Genet. 20: 203-206. [DOI] [PubMed] [Google Scholar]
- Nakamura T.M., Cooper, J.P., and Cech, T.R. 1998. Two modes of survival of fission yeast without telomerase. Science 282: 493-496. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T.L., Szostak, J.W., and Rothstein, R.J. 1981. Yeast transformation: A model system for the study of recombination. Proc. Natl. Acad. Sci. 78: 6354-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann K., Lorentz, A., and Schmidt, H. 1993. The fission yeast rad22 gene, having a function in mating-type switching and repair of DNA damages, encodes a protein homolog to Rad52 of Saccharomyces cerevisiae. Nucleic Acids Res. 21: 5940-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A.J., Hu, P., Han, M., Ellis, N., and Jasin, M. 2001a. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes & Dev. 15: 3237-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A.J., Stark, J.M., Araujo, F.D., Moynahan, M.E., Berwick, M., and Jasin, M. 2001b. Double-strand breaks and tumorigenesis. Trends Cell. Biol. 11: S52-S59. [DOI] [PubMed] [Google Scholar]
- Russell P. and Nurse, P. 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49: 559-567. [DOI] [PubMed] [Google Scholar]
- Sandell L.L. and Zakian, V.A. 1993. Loss of a yeast telomere: Arrest, recovery, and chromosome loss. Cell 75: 729-739. [DOI] [PubMed] [Google Scholar]
- Siede W., Friedl, A.A., Dianova, I., Eckardt-Schupp, F., and Friedberg, E.C. 1996. The Saccharomyces cerevisiae Ku autoantigen homologue affects radiosensitivity only in the absence of homologous recombination. Genetics 142: 91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A., Karlseder, J., Holtgreve-Grez, H., Jauch, A., and de Lange, T. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12: 1635-1644. [DOI] [PubMed] [Google Scholar]
- Stern B. and Nurse, P. 1998. Cyclin B proteolysis and the cyclin-dependent kinase inhibitor rum1p are required for pheromone-induced G1 arrest in fission yeast. Mol. Biol. Cell 9: 1309-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sasaki, M.S., Sonoda, E., Morrison, C., Hashimoto, M., Utsumi, H., Yamaguchi-Iwai, Y., Shinohara, A., and Takeda, S. 1998. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 17: 5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., Matsuura, A., Caspari, T., Carr, A.M., Akamatsu, Y., Iwasaki, H., Mizuno, K., Ohta, K., Uritani, M., Ushimaru, T., et al. 2003. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol. Cell. Biol. 23: 5186-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch M., Vreeken, K., Zonneveld, J.B., Brandsma, J.A., Lombaerts, M., Murray, J.M., Lohman, P.H., and Pastink, A. 2001. Characterization of RAD52 homologs in the fission yeast Schizosaccharomyces pombe. Mutat. Res. 461: 311-323. [DOI] [PubMed] [Google Scholar]
- van Gent D.C., Hoeijmakers, J.H., and Kanaar, R. 2001. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2: 196-206. [DOI] [PubMed] [Google Scholar]
- van Steensel B., Smogorzewska, A., and de Lange, T. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401-413. [DOI] [PubMed] [Google Scholar]