Abstract
The culturability of Escherichia coli, Ralstonia eutropha and Bacillus subtilis after incubation in phosphate-buffered saline at either 5°C or 30°C was determined. The culturability of B. subtilis showed little dependence on temperature. The culturability of E. coli rapidly decreased at 30°C but remained almost constant at 5°C. In contrast, the culturability of R. eutropha decreased by three orders of magnitude at 5°C within 24 h but only moderately decreased (one order of magnitude) at 30°C. Remarkably, prolonged incubation of R. eutropha at 30°C resulted in a full recovery of colony forming units in contrast to only a partial recovery at 5°C. Ralstonia eutropha cells at 30°C remained culturable for 3 weeks while culturability at 5°C constantly decreased. The effect of temperature was significantly stronger in a polyhydroxybutyrate-negative mutant. Our data show that accumulated polyhydroxybutyrate has a cold-protective function and can prevent R. eutropha entering the viable but not culturable state.
Keywords: survival, viable but not culturable, VBNC, polyhydroxybutyrate
VBNC state of Ralstonia eutropha and its relationship to accumulated PHB.
INTRODUCTION
Laboratory protocols often suggest that bacterial cultures should be refrigerated during harvest and washing procedures, if different culture media are used in two subsequent growth experiments. The background of this advice is that biochemical reactions slow down with decreasing temperature and that the energy required for maintenance metabolism is higher at a temperature of 30/37°C compared to a temperature near 0°C. Cooled cells should enter a state of ‘enforced hibernation’ and should therefore survive for a long period even in the absence of appropriate nutrients. However, a reduction of the temperature has strong effects on biomolecules: proteins might denature, the permeability and fluidity of biological membranes is affected and the efficiency of repair mechanisms is also reduced. These negative effects can compensate the beneficial effects of low temperatures. However, only little is known about the sum of negative and positive effects on the survival of microbial species at reduced temperatures. We therefore compared the culturability of bacteria by incubation in carbon-source-free buffered medium at a temperature of 5°C to survival near the optimal growth temperature (30°C). We chose R. eutropha, a Gram-negative β-proteobacterium (alternative designation Cuprividus necator), that is a biotechnologically important species due to its ability to accumulate polyhydroxybutyrate (PHB) (Schlegel, Von Bartha and Gottschalk 1961; Pohlmann et al.2006; for reviews see Pötter and Steinbüchel 2006; Rehm 2010; Jendrossek and Pfeiffer 2014). Ralstonia eutropha H16 has also become a model organism for the biochemistry/biophysics of hydrogenases (Burgdorf et al.2005; Radu et al.2016), nitrogen metabolism (Pohlmann et al.2000; Luette et al.2012), bacterial fixation of carbon dioxide (Bowien and Kusian 2002; Dangel and Tabita 2015), for a production platform of products from renewable resources (Brigham et al.2012; Karstens et al.2014; Riedel et al.2014; Volodina, Raberg and Steinbüchel 2015) and other topics (Tumlirsch, Sznajder and Jendrossek 2015). A PHB-negative mutant (R. eutropha ΔphaC) was also investigated to determine a potential influence of PHB on survival at different temperatures. For comparison, we included Escherichia coli and Bacillus subtilis as widely used model organisms.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Ralstonia eutropha H16 (DSM428), R. eutropha ΔphaC (Pfeiffer and Jendrossek 2012), Eschericha coli K12 (ATCC10798) and Bacillus subtilis subsp. spezizenii (DSM15029) were used. Eschericha coli was grown in lysogeny broth (LB); all other species were grown in nutrient broth (NB) medium. Temperature was 30°C for all liquid cultures. A quantity of 0.2 volumes of an overnight seed culture was used to inoculate a 20 ml main culture. Cells were in the late exponential growth phase after 5–6 h and were harvested by centrifugation (10 min, 5000 rpm, room temperature). Alternatively, Schlegel's mineral salts medium (MSM) with 1% (wt/vol.) of sodium gluconate was used in some experiments (Schlegel, Von Bartha and Gottschalk 1961). The cell pellets were suspended in phosphate-buffered saline (PBS; 1 g l−1 NaCl in 100 mm potassium phosphate buffer, pH 7) or in MSM medium, without a carbon source in the case of MSM-grown cells, and centrifuged again. The pellets were suspended in 6 ml PBS (or in 6 ml MSM in case of a MSM-grown culture) and stored (with shaking) at either 5°C (3 ml) or at 30°C (3 ml) in Falcon tubes.
Determination of culturability
Samples were diluted with PBS. Portions of 100 μl of appropriate dilutions were plated each on LB (E. coli) or NB agar (all other species) and the colony counts were determined after growth at 30 or 37°C (E. coli). Viable cell counts (vcc) are given in colony forming units per millilitre (cfu ml−1). Dilutions were generally performed in triplicate and the average values were calculated. Two biological replicates (for R. eutropha four biological replicates) were performed. Each figure shows the results of one biological replicate.
Other techniques
The total number of cells was determined by counting the cells of an appropriate dilution (in PBS) in a Neuberg's counting chamber. The content of PHB was determined by gas chromatography after acid methanolysis of lyophilised cells as described elsewhere (Brandl et al.1988; Sznajder and Jendrossek 2014). PHB content was also assessed by fluorescence microscopy after staining the cells with Nile red (Sznajder, Pfeiffer and Jendrossek 2015).
RESULTS AND DISCUSSION
Resting cells of E. coli survive better at 5°C compared to 30°C
The viable cell counts (vcc) of E. coli K12 only marginally decreased within the first 24 h regardless of the incubation temperature. However, incubation at 30°C for longer periods resulted in a constant decrease of culturability by about three orders of magnitude within 3 weeks (Fig. 1). Only a moderate decrease was determined at 5°C. Apparently, the reduction of metabolism by the decreased temperature prolonged survival whereas culturability of E. coli in the absence of nutrients at 30°C is low. This may be explained by an adaptation to the natural habitat: as long as E. coli is in the intestine the temperature is constantly high and the supply of nutrients is assured by the host. Therefore, E. coli is not used to the absence of nutrients at high temperature and rapidly dies at non-physiological conditions (no nutrients, high temperature). In contrast, leaving the intestine results in a sudden reduction of the supply of nutrients and in a simultaneous decrease of the temperature. Eschericha coli seems to be adapted to this change of environmental conditions and can survive for periods of weeks without nutrients at reduced temperature until it is taken up by a new host.
Figure 1.
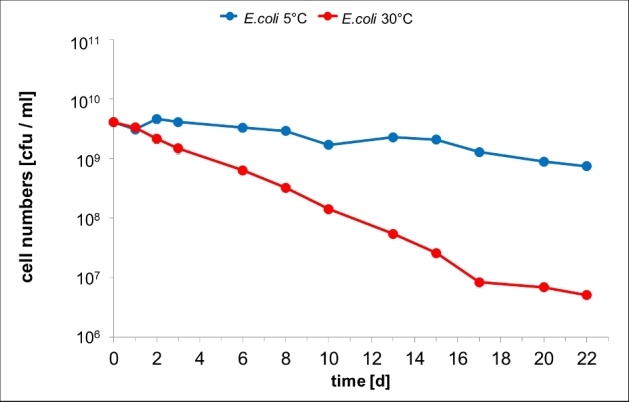
Viable cell counts of E. coli during incubation in PBS. Cells were grown in LB medium for 5.5 h, harvested by centrifugation and then suspended in PBS buffer and incubated at 30°C (red line) or at 5°C (blue line). At indicated points of time viable cell counts [colony-forming units (cfu) ml−1] were determined as described in ‘Materials and methods’. Error bars indicate standard deviation but are hardly visible due to log scale.
Ralstonia eutropha is sensitive to cold temperatures
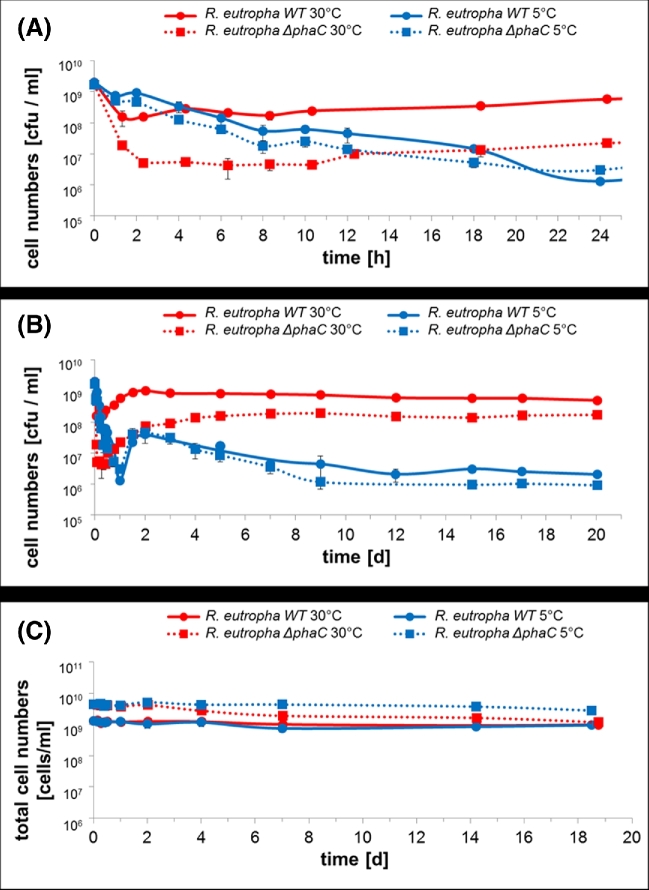
NB-grown cells had a level of 20–30% of accumulated PHB at the time of harvest as revealed by Nile red staining and by gas chromatograph analysis. The culturability of PBS buffer-incubated R. eutropha decreased constantly by three orders of magnitude within the first 24 h of incubation at 5°C (Fig. 2A). On the second day of incubation the vcc reproducibly recovered by one order of magnitude before the vcc constantly and slowly decreased in the next 3 weeks (Fig. 2B). The culturability of the parallel culture of R. eutropha at 30°C (Fig. 2) decreased by only one order of magnitude after 1–2 h after which the vcc increased to the original value within 48 h and then remained constantly high. The difference in vcc between 30°C and 5°C amounted to at least two orders of magnitude between the end of week 1 and end of week 3. The total number of cells (viable and non-viable) remained constantly high regardless of the incubation temperature (between 1 × 109 and 5 × 109 cells ml−1) over the whole time period (Fig. 2C).
Figure 2.
Viable cell counts and total cell numbers of R. eutropha H16 during incubation in PBS. Cells were grown in NB medium for 5.5 h, harvested by centrifugation and then suspended in PBS and incubated at 30°C (red line) or at 5°C (blue line). At indicated points of time viable cell counts [cfu ml−1] were determined as described in ‘Materials and methods’. Error bars indicate standard deviation. In (A), the time scale of the first 24 h is enlarged relative to the time scale of 20 days in (B). Solid lines refer to the wild type and dotted lines represent the PHB-negative ΔphaC mutant. In (C), the total cell numbers [cells ml−1] are given. The same log scale of 5 decades as for (A) and (B) is given in (C) for better comparability.
The rapid increase of vcc after the initial decrease (Fig. 2B) at 5°C cannot be explained by active growth. An increase of vcc by one order of magnitude or more in a buffer without nutrients at 5°C is not possible and such an increase was not recorded for the total number of cells. We assume that the conditions during harvest and incubation at 5°C led to a metabolic shock of the R. eutropha that transferred them into a viable but not culturable state (VBNC). A decrease of temperature is a well-known factor to provoke prokaryotic species to enter the VBNC state (Xu et al.1982; Kell et al.1998; Oliver, 2005, 2010; Li et al.2014). Remarkably, R. eutropha enters the VBNC state already after incubation at low temperature for only a few hours while other species need two or more days before a substantial fraction of the cells enters the VBNC state (Oliver 2005). Although the VBNC state has been mostly described for pathogenic bacteria (Li et al.2014), non-pathogenic bacteria can also enter this state (Su et al.2016). The VBNC state has also been provoked by dehydration (Pedersen and Jacobsen 1993). However, provocation of the VBNC state by temperature has not been described for R. eutropha strains before.
Accumulated PHB enhances culturability of R. eutropha at 30°C
Accumulated PHB helps R. eutropha to survive in the absence of an exogenous carbon source (Handrick, Reinhardt and Jendrossek 2000). However, a possible effect of PHB on the VBNC state of bacteria has not yet been investigated (Oliver 2005). To investigate the effect of accumulated PHB on culturability we performed the same experiment with a PHB-negative mutant that cannot synthesise PHB because of the absence of the key enzyme of PHB synthesis (ΔphaC, PHB synthase gene) (York et al.2001; Pfeiffer and Jendrossek 2012). The time course of the vcc of the refrigerated culture of the PHB-negative mutant was similar to the wild type (WT) strain at 5°C (Fig. 2A and B, dotted graphs). However, a remarkable result was obtained when we compared the WT with the mutant at 30°C: a sharp decrease of vcc by ∼2.5 orders of magnitude was determined for the mutant within the first 2 h while in the WT the vcc decreased by only one order of magnitude in the first 2 h. The vcc of WT cells was generally 1.5–2 orders of magnitude higher in the first 24 h of incubation (Fig. 2A). This result indicated a protective effect of accumulated PHB. After this initial decrease a slow but constant recovery of the vcc by 1.5 orders of magnitude was determined for the next 5 days for the mutant after which the vcc remained constant for 3 weeks (Fig. 2B). As for the wild type, no substantial change in the total number of cells was determined for the ΔphaC mutant over the 3 week period of the experiment.
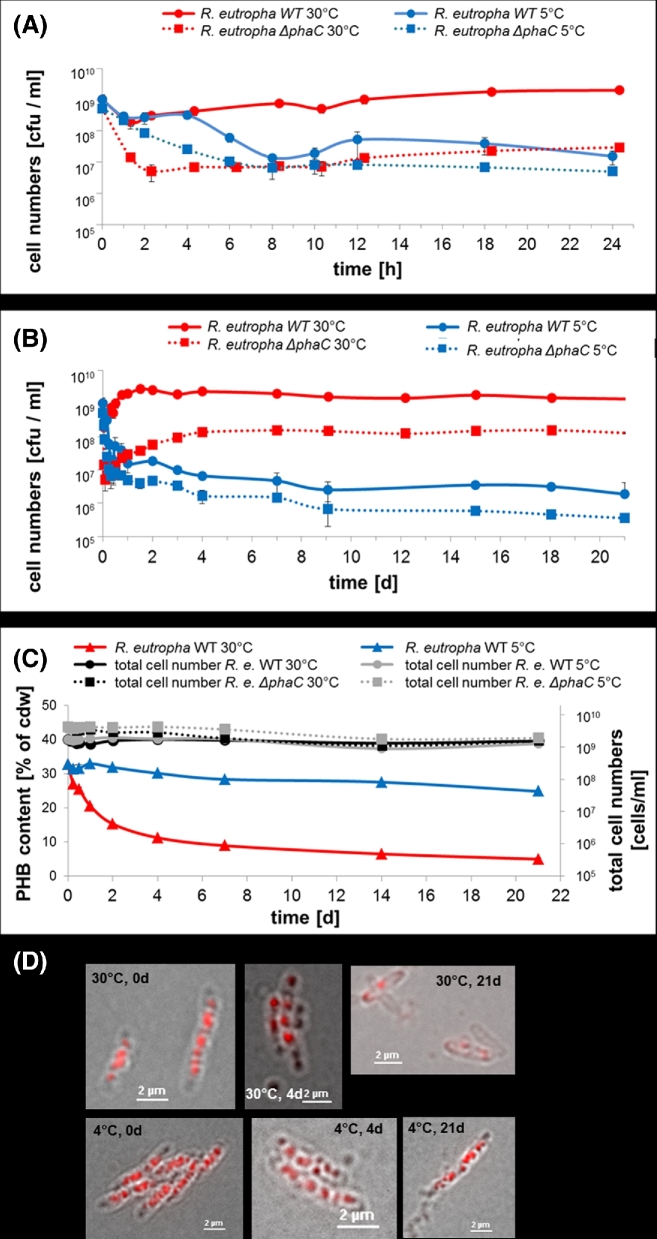
To investigate the effect of PHB on culturability we repeated the experiment with an NB culture to which 0.2% of gluconate was added and additionally determined the time course of the PHB contents. The increase of the C/N ratio resulted in a PHB content of ∼30–35% at the time of harvest. Figure 3A and B shows the time course of vcc for the WT and the mutant in PBS at 5 and 30°C. A low decrease in the vcc by approximately one order of magnitude was determined for WT cells at 30°C in the first 2 h which was compensated by an increase in vcc in the first 2 days of incubation after which the vcc remained almost constant. The PHB content (% of cellular dry weight) decreased from about 31% to one-third (∼10%) after 1 week and then slowly decreased further to ∼7% after 3 weeks (Fig. 3C and D). In contrast, a decrease in the vcc of two orders of magnitude was determined within 8 h at 5°C before the cells partially recovered at ∼12–18 h. Afterwards, a slow but constant decrease in the vcc was determined at 5°C. The PHB content at 5°C decreased much slower than at 30°C and after 3 weeks only a minor reduction from 31% to 25% was determined reflecting the low activity of the metabolism in general and, in particular, of the intracellullar PHB depolymerase at 5°C. The vcc of the PHB-negative mutant decreased dramatically by two to three orders of magnitude within 2 or 8 h at 30 or 5°C, respectively. At 30°C a substantial recovery of the vcc of approximately one order of magnitude was determined while recovery at 5°C was poor. At all points of time between t = 3 days and 3 weeks, the vcc of the cells kept at 30°C was two orders of magnitude higher than that of the refrigerated cells. The vcc of the mutant decreased independently of the temperature and confirmed the beneficial effect of PHB on culturability. However, mutant cells recovered at 30°C suggesting that recovery is mainly temperature dependent and is only marginally dependent on previously accumulated PHB. Accordingly, the mutant hardly recovered at 5°C and the vcc slowly but constantly decreased. The total number of cells changed only marginally over the 3 week period (Fig. 3C).
Figure 3.
Viable cell counts, total cell numbers and PHB contents of R. eutropha H16 and R. eutropha ΔphaC cells during incubation in PBS. Cells were grown in NB medium supplemented with 0.2% of sodium gluconate for 5.5 h, harvested by centrifugation and then suspended in PBS and incubated at 30°C (red line) or at 5°C (blue line). Wild type cells (solid lines) had ∼32% accumulated PHB and ΔphaC cells (dotted lines) were free of any storage PHB. At indicated points of time, viable cell counts [cfu ml−1] (A and B), and total number of cells [cells ml−1] and PHB content [% of cellular dry weight (cdw), mean of two determinations] (C) were determined. In (A), the time scale of the first 24 h is enlarged relative to the time scale of 3 weeks in (B). The same log scale of 5 decades is given in all graphs for better comparability. Examples of microscopical images of Nile red-stained wild type cells (overlay of bright field image and fluorescence image) are shown in (D). Note, the decrease in the number of red-stained PHB granules after 21 days at 30°C but not at 5°C. Error bars indicate standard deviation. PHB contents of the phaC mutant were not determined because the inability to synthesise PHB in the absence of PHB synthase has been frequently reported.
Composition of the growth medium affects the culturability of R. eutropha cells
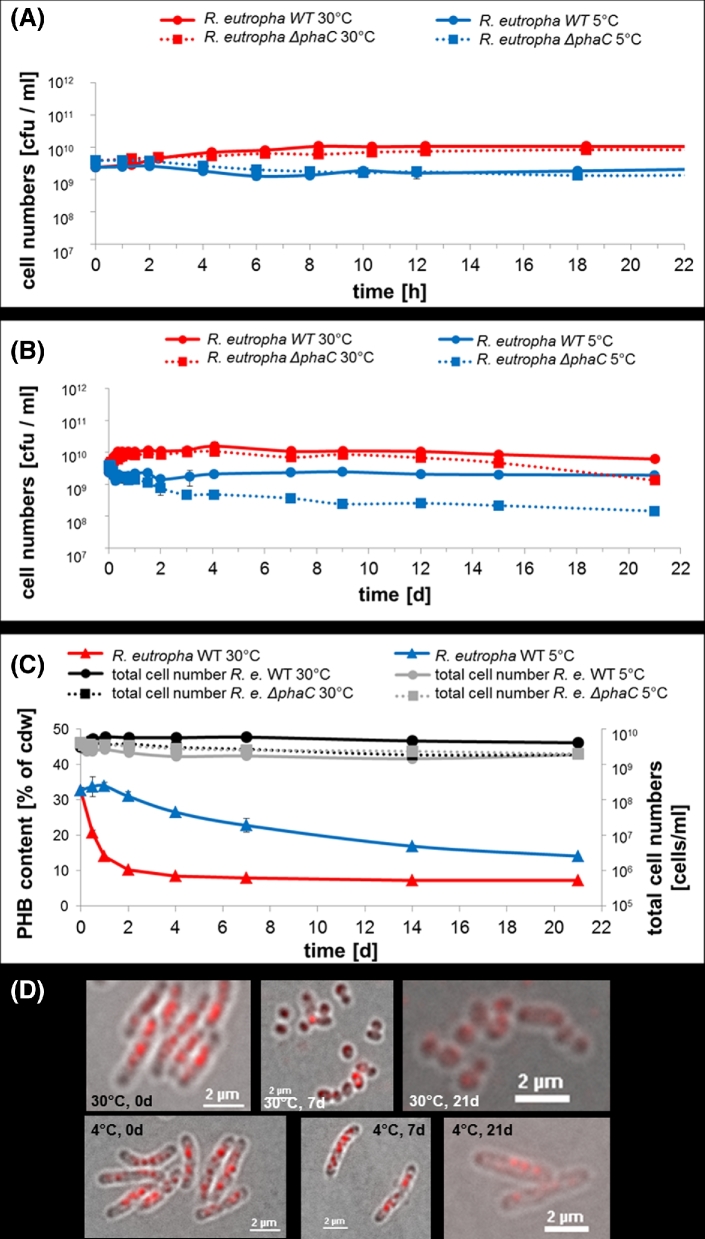
The medium composition has an impact on metabolism and may affect the sensitivity to stress factors. We therefore determined the course of vcc after growth of R. eutropha in MSM (Fig. 4A and B). MSM-grown R. eutropha cells showed a lesser response to incubation in cold buffer than cells that had been grown in NB medium (maximal difference in vcc only 1.5 orders of magnitude compared to up to 3 orders of magnitude in NB medium): the vcc of WT cells even increased during the first 8 h of incubation in PBS at 30°C by about half an order of magnitude and remained constantly high for the next 2 weeks before a slight decrease was determined in the third week of the experiment. The vcc of the WT at 5°C showed a slight reduction with subsequent partial recovery and then remained almost constant during the total incubation period. On average, the vcc of the WT at 30°C was five to eight times higher than at 5°C. The PHB content decreased from 33 to 7% at the end of the experiment at 30°C (Fig. 4C). At 5°C the PHB content decreased to only 16–18% in the 3 week period. The differences in the PHB contents were also evident by fluorescence microscopical analysis (Fig. 4D). Interestingly, the utilisation of PHB at 30°C was accompanied by a substantial shortening of most of the cells.
Figure 4.
Viable cell counts, total cell numbers and PHB contents of mineral salts medium-grown R. eutropha H16 and R. eutropha ΔphaC cells during incubation in PBS. Cells were grown in mineral salts medium supplemented with 1% of sodium gluconate for 10 h as described in ‘Materials and methods’, harvested by centrifugation and then suspended in MSM without a carbon source and incubated at 30°C (red line) or at 5°C (blue line). Wild type cells (solid lines) had ∼33% accumulated PHB. At indicated points of time, viable cell counts [cfu ml−1] (A and B), total number of cells [cells ml−1] and PHB content [% of cellular dry weight (cdw), mean of two determinations] (C) were determined. In (A), the time scale of the first 24 h is enlarged relative to the time scale of 3 weeks in (B). The same log scale of 5 decades is given in all graphs for better comparability. Examples of microscopical images of Nile red-stained wild type cells (overlay of bright field image and fluorescence image) are shown in (D). Note, the shortening of the cells and decrease in the number of red-stained PHB granules after 21 days at 30°C but not at 5°C. Error bars indicate standard deviation. PHB contents of the phaC mutant were not determined because the inability to synthesise PHB in the absence of PHB synthase has been frequently reported.
The vcc of the PHB-free mutant (ΔphaC) at 30°C proceeded similarly to the WT. However, the initial increase was less pronounced and the slight decrease started earlier than was determined for the WT. The total number of WT cells (30°C) slightly increased within the first 48 h similar to the increase of the vcc of the wild type at 30°C (Fig. 4C). Our data indicated that some cells of a mineral salts medium-grown culture with accumulated PHB can perform one cell division or can at least finish an already initiated cell division in a carbon-source-free buffer resulting in the observed slight increase of the vcc and the total number of cells. After 48 h, the total number of cells did not change any more. The total number of cells of WT at 5°C or of the ΔphaC mutant in both conditions changed only to a minor extent and slowly decreased by a factor of two within the 3 week period. These results show that PHB helps to keep the percentage of vcc high but the absence of PHB has little effect at 30°C. At 5°C, the vcc of the PHB-free mutant constantly and slowly decreased, and at the end of the 3 weeks the vcc had decreased by about one order of magnitude compared to the WT at 5°C. In comparison to NB-grown cells, cells of a MSM-culture showed less sensitivity to stress by the storage condition. As a result, only a few cells have entered the VBNC state in the first hours of incubation. At 5°C, the vcc of the WT did not decrease during the 3 weeks of the experiment. However, the vcc of the PHB-negative mutant constantly decreased at reduced temperature and this indicated that accumulated PHB has a positive effect on culturability at a reduced temperature.
Dependence of culturability of Bacillus strains from temperature
To find out whether the unexpected good survival of R. eutropha cells at 30°C could also be determined for other species we performed a similar experiment with B. subtilis as a representative of Gram-positive species. As shown in Fig. 5, the vcc of the 30°C and the 5°C cultures strongly decreased during the first 3 days. After this period, the decrease in the vcc slowed down at both incubation temperatures. Notably, the vcc decreased more strongly at 5°C than at 30°C resulting in a 10-fold higher vcc at 30°C compared to 5°C between day 10 and day 20. Microscopical examination of the cells at all points of time indicated that endospores were never formed under conditions of storage in PBS. These data indicate that also in B. subtilis long term survival in carbon-source-free buffer is slightly better at 30°C compared to 5°C. However, evidence for a VBNC state was not observed for B. subtilis.
Figure 5.
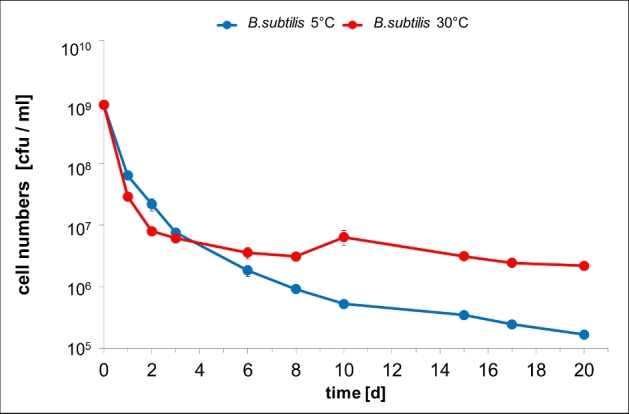
Viable cell counts of Bacillus subtilis during incubation in PBS. Cells were grown in NB medium for 5.5 h, harvested by centrifugation and then suspended in PBS buffer and incubated at 30°C (red line) or at 5°C (blue line). At indicated points of time viable cell counts [cfu ml−1] were determined. Error bars indicate standard deviation.
CONCLUSIONS
Our data show that exponentially grown R. eutropha cells are highly sensitive to sudden changes in the environmental conditions such as incubation in a carbon-source-free buffer at reduced temperature and rapidly enter the VBNC state. The presence of previously accumulated PHB and incubation near the optimal growth temperature helps R. eutropha not to enter the VBNC state or – if already entered – to recover from VBNC. Researchers working with R. eutropha should therefore avoid storage of the cells on ice or at reduced temperature if culturability of the cells is important for the success of the performed experiment, especially if experiments involve cells of cultures on complex media. Another outcome of this study is the finding that accumulated PHB has a protective function on the culturability of R. eutropha in addition to the function as a C reservoir supporting the observation of others that accumulated PHB enhances the resistance of cells against stresses such as reactive oxygen species (Koskimäki et al.2016).
Acknowledgments
We thank Anna Schweter and Simone Reinhardt for their help in some experiments.
Conflict of interest. None declared.
REFERENCES
- Bowien B, Kusian B. Genetics and control of CO2 assimilation in the chemoautotroph Ralstonia eutropha. Arch Microbiol 2002;178:85–93. [DOI] [PubMed] [Google Scholar]
- Brandl H, Gross RA, Lenz RW et al. . Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 1988;54:1977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham CJ, Zhila N, Shishatskaya E et al. . Manipulation of Ralstonia eutropha carbon storage pathways to produce useful bio-based products Wang X, Chen J, Quinn P. Reprogramming Microbial Metabolic Pathways. Vol. 64, Dordrecht Netherlands:Springer, 2012, 343–66. [DOI] [PubMed] [Google Scholar]
- Burgdorf T, Lenz O, Buhrke T et al. . [NiFe]-hydrogenases of Ralstonia eutropha H16: modular enzymes for oxygen-tolerant biological hydrogen oxidation. J Mol Microbiol Biotechnol 2005;10:181–96. [DOI] [PubMed] [Google Scholar]
- Dangel AW, Tabita FR. Amino acid substitutions in the transcriptional regulator CbbR lead to constitutively active CbbR proteins that elevate expression of the cbb CO2 fixation operons in Ralstonia eutropha (Cupriavidus necator) and identify regions of CbbR necessary for gene activation. Microbiology 2015;161:1816–29. [DOI] [PubMed] [Google Scholar]
- Handrick R, Reinhardt S, Jendrossek D. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J Bacteriol 2000;182:5916–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrossek D, Pfeiffer D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 2014;16:2357–73. [DOI] [PubMed] [Google Scholar]
- Karstens K, Zschiedrich CP, Bowien B et al. . Phosphotransferase protein EIIANtr interacts with SpoT, a key enzyme of the stringent response, in Ralstonia eutropha H16. Microbiology 2014;160:711–22. [DOI] [PubMed] [Google Scholar]
- Kell DB, Kaprelyants AS, Weichart DH et al. . Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek 1998;73:169–87. [DOI] [PubMed] [Google Scholar]
- Koskimäki JJ, Kajula M, Hokkanen J et al. . Methyl-esterified 3-hydroxybutyrate oligomers protect bacteria from hydroxyl radicals. Nat Chem Biol 2016;12:332–8. [DOI] [PubMed] [Google Scholar]
- Li L, Mendis N, Trigui H et al. . The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol 2014;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luette S, Pohlmann A, Zaychikov E et al. . Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16. Appl Environ Microbiol 2012;78:7884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD. The viable but nonculturable state in bacteria. J Microbiol 2005;43:93–100. [PubMed] [Google Scholar]
- Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 2010;34:415–25. [DOI] [PubMed] [Google Scholar]
- Pedersen JC, Jacobsen CS. Fate of Enterobacter cloacae Jp120 and Alcaligenes eutrophus Ae0106(Pr0101) in soil during water-stress: effects on culturability and viability. Appl Environ Microbiol 1993;59:1560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D, Jendrossek D. Localization of poly(3-hydroxybutyrate) (PHB) granule-associated proteins during PHB granule formation and identification of two new phasins, PhaP6 and PhaP7, in Ralstonia eutropha H16. J Bacteriol 2012;194:5909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann A, Cramm R, Schmelz K et al. . A novel NO-responding regulator controls the reduction of nitric oxide in Ralstonia eutropha. Mol Microbiol 2000;38:626–38. [DOI] [PubMed] [Google Scholar]
- Pohlmann A, Fricke WF, Reinecke F et al. . Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 2006;24:1257–62. [DOI] [PubMed] [Google Scholar]
- Pötter M, Steinbüchel A. Biogenesis and structure of polyhydroxyalkanoate granules. Microbiol Monogr 2006;1:1–28. [Google Scholar]
- Radu V, Frielingsdorf S, Lenz O et al. . Reactivation from the Ni-B state in [NiFe] hydrogenase of Ralstonia eutropha is controlled by reduction of the superoxidised proximal cluster. Chem Commun (Camb) 2016;52:2632–5. [DOI] [PubMed] [Google Scholar]
- Rehm BHA. Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 2010;8:578–92. [DOI] [PubMed] [Google Scholar]
- Riedel SL, Lu J, Stahl U et al. . Lipid and fatty acid metabolism in Ralstonia eutropha: relevance for the biotechnological production of value-added products. Appl Microbiol Biotechnol 2014;98:1469–83. [DOI] [PubMed] [Google Scholar]
- Schlegel HG, Von Bartha R, Gottschalk G. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature 1961;191:463–5. [DOI] [PubMed] [Google Scholar]
- Su X, Guo L, Ding L et al. . Induction of viable but nonculturable state in Rhodococcus and transcriptome analysis using RNA-seq. PLoS One 2016;11:e0147593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznajder A, Jendrossek D. To be or not to be a poly(3-hydroxybutyrate) (PHB) depolymerase: PhaZd1 (PhaZ6) and PhaZd2 (PhaZ7) of Ralstonia eutropha, highly active PHB depolymerases with no detectable role in mobilization of accumulated PHB. Appl Environ Microbiol 2014;80:4936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznajder A, Pfeiffer D, Jendrossek D. Comparative proteome analysis reveals four novel polyhydroxybutyrate (PHB) granule-associated proteins in Ralstonia eutropha H16. Appl Environ Microbiol 2015;81:1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlirsch T, Sznajder A, Jendrossek D. Formation of polyphosphate by polyphosphate kinases and its relationship to poly(3-hydroxybutyrate) accumulation in Ralstonia eutropha strain H16. Appl Environ Microbiol 2015;81:8277–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volodina E, Raberg M, Steinbüchel A. Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit Rev Biotechnol 2015;36:1–14. [DOI] [PubMed] [Google Scholar]
- Xu HS, Roberts N, Singleton FL et al. . Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol 1982;8:313–23. [DOI] [PubMed] [Google Scholar]
- York GM, Junker BH, Stubbe J et al. . Accumulation of the PhaP phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J Bacteriol 2001;183:4217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]