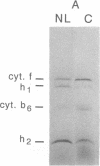Abstract
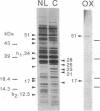
When grown under nitrogen limitation, pronounced chlororespiratory activity develops together with an altered composition of thylakoid membranes in Chlamydomonas reinhardtii. Relative to control cultures, the flash-inhibited, chlororespiration-dependent O2 consumption signal increases 10-fold. Also augmented is the light-sensitive respiratory activity responsible for the "Kok effect," reflecting competitive inhibition of chlororespiratory electron transport by photosystem I. Fluorescence measurements show that the thylakoid plastoquinone pool is extensively reduced in dark-adapted, N-limited cells. Thylakoids of N-limited cells have reduced amounts of cytochrome b6, cytochrome f, and light-harvesting complexes. However, thylakoid-bound NADH-PQ oxidoreductase, with major subunits of 51 kDa and 17 kDa, is increased 7-fold and two novel cytochromes of 34 and 12.5 kDa are highly abundant. Thus, components of photosynthetic and chlororespiratory electron transport pathways are differentially regulated by N availability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEDHEER J. C. A cooperation of two pigment systems and respiration in photosynthetic luminescence. Biochim Biophys Acta. 1963 Jan 15;66:61–71. doi: 10.1016/0006-3002(63)91167-3. [DOI] [PubMed] [Google Scholar]
- Gfeller R. P., Gibbs M. Fermentative Metabolism of Chlamydomonas reinhardtii: II. Role of Plastoquinone. Plant Physiol. 1985 Feb;77(2):509–511. doi: 10.1104/pp.77.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- La Monica R. F., Marrs B. L. The branched respiratory system of photosynthetically grown Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976 Mar 12;423(3):431–439. doi: 10.1016/0005-2728(76)90198-5. [DOI] [PubMed] [Google Scholar]
- Lampe H. H., Drews G. Die Differenzierung des Membransystems von Rhodopseudomonas capsulata hinsichtlich seiner photosynthetischen und respiratorischen Funktionen. Arch Mikrobiol. 1972;84(1):1–19. [PubMed] [Google Scholar]
- Morehouse K. M., Mason R. P. The transition metal-mediated formation of the hydroxyl free radical during the reduction of molecular oxygen by ferredoxin-ferredoxin:NADP+ oxidoreductase. J Biol Chem. 1988 Jan 25;263(3):1204–1211. [PubMed] [Google Scholar]
- Ohyama K., Kohchi T., Sano T., Yamada Y. Newly identified groups of genes in chloroplasts. Trends Biochem Sci. 1988 Jan;13(1):19–22. doi: 10.1016/0968-0004(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Peltier G., Thibault P. O(2) uptake in the light in chlamydomonas: evidence for persistent mitochondrial respiration. Plant Physiol. 1985 Sep;79(1):225–230. doi: 10.1104/pp.79.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Nitrogen-dependent regulation of photosynthetic gene expression. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2678–2682. doi: 10.1073/pnas.86.8.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saux C., Lemoine Y., Marion-Poll A., Valadier M. H., Deng M., Morot-Gaudry J. F. Consequence of Absence of Nitrate Reductase Activity on Photosynthesis in Nicotiana plumbaginifolia Plants. Plant Physiol. 1987 May;84(1):67–72. doi: 10.1104/pp.84.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Alpes I., Sadowski H., Böger P. Ferredoxin-NADP+ oxidoreductase is the respiratory NADPH dehydrogenase of the cyanobacterium Anabaena variabilis. Arch Biochem Biophys. 1988 Nov 15;267(1):228–235. doi: 10.1016/0003-9861(88)90027-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Willeford K. O., Gombos Z., Gibbs M. Evidence for Chloroplastic Succinate Dehydrogenase Participating in the Chloroplastic Respiratory and Photosynthetic Electron Transport Chains of Chlamydomonas reinhardtii. Plant Physiol. 1989 Jul;90(3):1084–1087. doi: 10.1104/pp.90.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F. A., Delepelaire P. Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol. 1984 Jan;98(1):1–7. doi: 10.1083/jcb.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]