Abstract
Objectives
The aim of the current study was to examine expression and the role, if any, of ALDH1B1 in pancreatic adenocarcinoma.
Methods
A tissue microarray of 61 pancreatic cancer patients were evaluated for protein expression of ALDH1B1 by immunohistochemistry. ALDH1B1 siRNA was used to assess the contribution of ALDH1B1 on proliferation of pancreatic cancer cells.
Results
In normal human pancreas, ALDH1B1 is abundantly expressed in glandular cells, but sparsely in the ducts (ALDH1B1 immunopositivity = 16.7 ± 1.7). In pancreatic ductal carcinoma, we found high ALDH1B1 expression in ductal cancerous tissues (ALDH1B1 immunopositivity = 197.2 ± 29.4). Analysis of ALDH1B1 expression in a human pancreatic adenocarcinoma tissue microarray showed the greatest expression in tumors that were more invasive. A variation in ALDH1B1 expression was also observed in 16 human pancreatic cancer cell lines. Knockdown of ALDH1B1 caused a 35% reduction in cell growth in the high ALDH1B1-expressing cell lines.
Conclusion
Our data show for the first time that ALDH1B1 is expressed at very high levels in human pancreatic cancer and it contributes to proliferation in these tumor cells. These data suggest a potential modulatory role for ALDH1B1 in pancreatic cancer.
Keywords: ALDH1B1, pancreatic ductal carcinoma
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer deaths in the United States with an estimated 45,220 new cases in 2013.1 This devastating disease occurs through a series of stepwise genetic alterations in which an activating mutation in the KRAS gene is the predominate factor in facilitating malignant transformation.2–4 While greater insights into the molecular events of pancreatic cancer and newer therapies have become available, the 5-year survival rate still remains extremely poor at only 4%.5 Standard of care therapy for first-line treatment of this disease consists of gemcitabine which improves the median survival by ≈ 6 months when compared to 5-fluorouracil 6 or FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) which showed a 4.3-month improvement in overall survival when compared to gemcitabine.7
Aldehyde dehydrogenases (ALDH) are a family of enzymes comprising 19 different isoforms.8 Many function in catalyzing the oxidation of aldehydes to their corresponding acids.9 In addition, several different isoforms, including ALDH1A1, ALDH1A2, ALDH1A3 and ALDH8A1, are involved in the biosynthesis of retinoic acid.9–13 Variant base pair substitutions in certain ALDHs are associated with altered protein function and distinct phenotypes in humans.14 In humans, ALDH enzymatic activity (ALDH+), measured by an Aldefluor® assay, has been identified as a marker of cancer stem cells 15–17, which are defined by the capacity to self-renew and differentiate. Recent research effort has focused on identifying which ALDH isoforms mediate the ALDH enzymatic activity and how these may modulate the development and progression of cancer. Of these, high ALDH1A1 may play an important role given its high expression is associated with more aggressive tumors and worse outcomes in bladder, ovarian, lung, prostate and pancreatic cancers.18–21
In pancreatic cancer, ALDH+ cells have been shown to have stem cell features, evident by enhanced clonogenicity in vitro and tumor growth in mice.22 Interestingly, ALDH+ cells express many genes common to the mesenchymal phenotype, have an increased capacity to migrate and invade and have a higher incidence in metastatic lesions.21 In pancreatic cancer patients, expression of ALDH1A1 in tumors is associated with a poorer survival rate.21,23 These studies suggest that cells with ALDH enzymatic activity may be important in disease progression thereby contribute to the negative outcomes in patients with pancreatic cancer. In colorectal cancer (CRC), we observed a significant elevation in ALDH1B1 (relative to ALDH1A1) indicating that ALDH1B1 may play an important role in this disease. In this study, we explore the potential role of ALDH1B1 in pancreatic cancer by determining its expression in tumor tissue and investigating its influence on pancreatic cancer cell proliferation.
MATERIALS AND METHODS
Human tissues
Tumor specimens were obtained from consenting patients at Johns Hopkins University in accordance with protocols approved by the Institutional Review Board. Fresh pancreas adenocarcinoma patient tumor specimens were collected from 80 patients. These specimens were assembled onto tissue microarrays (TMA) with duplicate samples and both intra- and inter-slide controls to control for edge effects and variation in slide staining.
Immunohistochemical staining (IHC)
IHC was performed according to previously descried procedures. Briefly, epitope retrieval was done using Retrieval Solution (Leica Microsystems, Bannockburn, IL) at 90°C for 10 min after deparffinzing the TMAs (Chen et al, BBRC). The TMAs were then rehydrated and incubated with Protein Blocker (Open Biosystems, Huntsville, AL). Polyclonal anti-human ALDH1B124 (1:750 dilution in Protein Blocker) was applied for 60 min at room temperature. Thereafter, sections were incubated for 10–20 min at room temperature with corresponding HRP-conjugated secondary antibodies (BioCare Medical, Concord, CA) (1:500 dilution). Slides underwent a final incubation with DAB (Open Biosystems, Huntsville, AL) for 10 min, and counterstained with hematoxylin.
IHC staining analysis
Sections of pancreas adenocarcinoma samples were evaluated for ALDH1B1 staining as described previously.16 Briefly, the IHC signal was rated semi-quantitatively in positively-stained sections for the ALDH1B1 antibody in each tissue for intensity (I) on a scale of 1–3 (1 = week staining, 2 = moderate staining, and 3 = intense staining) and for extensiveness (E, % of tumor cells in TMA with positive IHC signal). Samples were evaluated and scored by two independent blinded observers (SS and DJO) using the criteria established by international experts on precursor lesions for pancreatic ductal adenocarcinoma.25 Briefly, PanIN-1 is characterized by tall columnar cells containing abundant mucin; PanIN-2 have cells with nuclear abnormality along with pseudostratified structure and PanIN-3 have features similar to PanIN-2 with “budding off” of small groups of epithelial cells into the lumen. Those samples lacking enough tumor tissue for evaluation were excluded from the final analysis. The IHC expression score (S) for the antibody in each sample was calculated as S = I × E. Data are reported as mean ± SEM.
Cell lines and culture and analysis
Pancreas cancer cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1% non-essential amino acids, and 1% penicillin/streptomycin, and were maintained at 37 °C under an atmosphere containing 5% CO2. Cells from the Panc1, A32.1 or L3.6 cell lines were plated in a 96 well plate (1,000 cells/well) and incubated overnight prior to exposure to ALDH1B1 siRNA or scramble RNA (control) (Life technologies, Grand Island, NY) for 72 hr. For the evaluation of proliferation, a sulforhodamine B (SRB) assay was used. This entailed the media being removed and cells being fixed with cold 10% trichloroacetic acid for 30 min at 4°C. Cells were then rinsed with distilled water, incubated with 0.4% SRB for 30 min at room temperature, washed with 1% acetic acid, followed by stain solubilization with 10mM Tris at room temperature. Absorbance (λ = 565nm) of each well was then measured using a plate reader (Biotek Synergy2, Winooski, VT, USA). ALDH1B1 quantitative real-time PCR was performed to determine gene transcription repression efficiency of siRNA integration.
RNA Isolation
Baseline gene expression levels of ALDH1B1 in pancreas cancer cell lines and evaluation of ALDH1B1 mRNA knockdown in the Panc1, A32.1, and L3.6 cell lines were examined by RT-PCR. Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) according to manufacturer’s protocol with an additional DNA digestion step. The total RNA concentration and integrity were measured using a NanoDrop (Thermo Fisher Scientific, Waltham, MA) and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), respectively. cDNA was synthesized using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. Validated and pre-designed primer/probes for ALDH1B1 (assay ID: Hs00265114_s1) and GAPDH (assay ID: Hs02758991_g1) were purchased from Applied Biosystems. Samples were amplified using the ABI Step One Plus RT-PCR system (Applied Biosystems, Foster City, CA),. Relative expression of the mRNA analyzed was estimated using the formula: 2−ΔCT, where ΔCT = CT (ALDH1B1) − CT (GAPDH).
ALDH1B1 shRNA knockdown
ALDH1B1 shRNA in pRFP-C-RS plasmid vector and pRS vector-negative control (scramble) were purchased from OriGene (Rockville, MD, USA, # TF314844). Stable clones were generated by transfecting Panc1 cells in a 6-well plate with 1 μg of each of the shRNA plasmids using Clontech transfection reagent (Clontech, Mountain View, CA, USA) according to the manufacturer’s recommendations. Successful knockdown of ALDH1B1 was confirmed by Western blot analysis.
Xenograft in athymic mice
All protocols used were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver. Scramble control and ALDH1B1 shRNA knockdown Panc1 cells (see above) were injected into the left and right flanks of 4 to 6 wk-old female athymic (nu+/nu+) mice (Harlan Laboratories, Indianapolis, IN). Mice were monitored daily for signs of toxicity. The size of the tumor was evaluated twice each week by caliper measurement using the following formula: tumor volume = (length × width2) × 0.52.
Pancreas Explant model
Patient-derived pancreas adenocarcinoma specimens were obtained (Panc 122 and 129) from consenting patients at the University of Colorado Hospital (Aurora, Colorado) in accordance with protocols approved by the Colorado Multiple Institutional Review Board (COMIRB # 07-0570). Tumor material not required for clinical histopathological analysis was collected and placed in RPMI medium supplemented with 10 μM HEPES, 4.5 g/L glucose, 1 μM pyruvate sodium, 200 U/mL penicillin, and 200 μg/mL streptomycin. After being rinsed several times in fresh medium, tumor tissue was cut into 2–3 mm3 pieces in antibiotic-containing medium and coated in matrigel. Four - six wk old female athymic (nu+/nu+) mice were obtained from Harlan laboratories (Washington, DC) under an approved research protocol by the Institutional Animal Care and Use Committee. The tumor pieces were then implanted on the flank of mice and expansion of the F1–F3 generations were carried out as previously described.26 Tumors were expanded in the left and right flanks of mice.
Flow cytometric analysis of ALDH enzymatic activity in pancreas explants
Explant tumor tissues were excised from mice, finely minced and placed in DMEM medium containing 10% FBS, 20 U/ml collagenase, and 1 μg/ml DNase. Following incubation for 60 min at 37°C, any remaining intact tissue was disrupted by passage through 100 μm and 70 μm filters. Red blood cells were lysed using an ACK lysis buffer (Invitrogen, Carlsbad, CA). Dispersed cells were collected by centrifugation and cell counts/viability were determined on the countess. Cell viability was >80% for all samples. The cell pellets were resuspended in Aldefluor® buffer (Stem cell technologies, Vancouver, CA)) at a concentration of 1 × 106 cells/ml. Activated Aldefluor® (5 μl/1 × 106 cells/ml) was added and immediately thereafter,,500 μl was transferred to another tube containing DEAB buffer (negative control). All samples were incubated at 37°C for 45 min and then centrifuged and washed with 500 μl of Aldefluor® buffer. Cells possessing ALDH enzymatic activity fluorescence after treatment with Aldefluor®, i.e., ALDH+ cells. Anti-mouse alexa-647 H-2Kb/H-2Db, CD45, CD31 antibodies were added to each sample at a concentration of 1:20. The samples were rotated at room temperature for 20 min and then washed two times with Aldefluor® assay buffer. All samples were analyzed by the University of Colorado Cancer Center flow cytometry core facility. To determine the tumorigenicity of ALDH+ cells, ALDH+ and ALDH negative (ALDH−; i.e., those not fluorescing in response to Aldefluor®) cells were sorted by flow cytometry and placed in culture containing stem cell media. For the analysis of RT-PCR in ALDH+ and ALDH− populations, cells were sorted directly into RLT lysis buffer and RT-PCR was performed as described above.
RESULTS
Expression of ALDH1B1 in normal pancreas and pancreatic adenocarcinoma
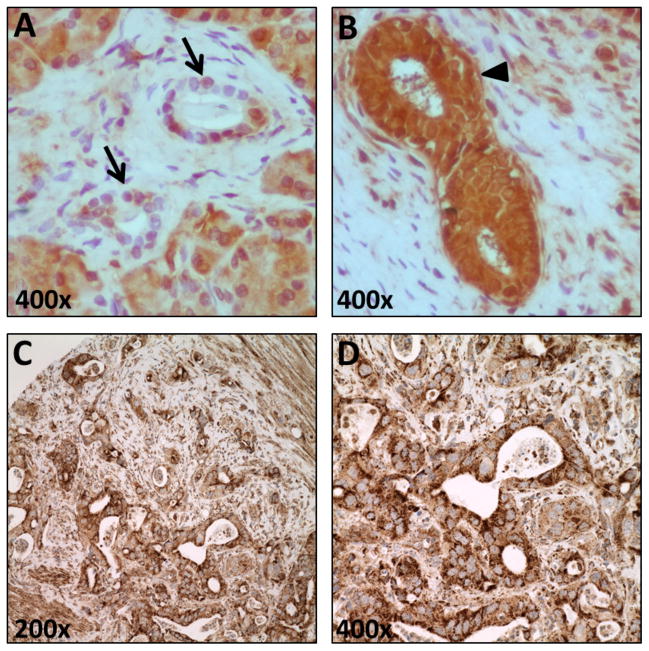
The expression of ALDH1B1 protein in normal pancreas (n=3) was compared with pancreatic ductal adenocarcinomas tissues (n=9) from our patient-derived explants by immunohistochemical (IHC) staining. When compared to normal pancreatic ducts (IHC score = 16.7 ± 1.7) (figure 1A), ALDH1B1 immunopositivity was elevated (P < 0.05) in pancreatic ductal tumors (IHC score = 197.2 ± 29.4) (figure 1B). ALDH1B1 expression was then assessed in 61 pancreatic ductal adenocarcinomas using a tissue microarray (TMA). The IHC score for ALDH1B1 in TMA (Table 1), regardless of tumor differentiation levels, was comparable to that of the tumor explants. Interestingly, there appeared to be an increase in ALDH1B1 expression in invasive ductal pancreas adenocarcinoma (PanIN2–3). Representative images are presented in figures 1C and D.
Figure 1.
Immunohistochemical (IHC) staining of ALDH1B1 in normal and cancerous pancreatic tissues. Representative tissue sections of normal pancreas (A) and pancreatic ductal adenocarcinoma (B) are presented. Normal pancreatic duct cells (A, arrows) show lower immunopositivity than pancreatic ductal adenocarcinoma (B, arrow head) in pancreas explant. (C–D) Representative micrographs of ALDH1B1 staining in the pancreas adenocarcinoma TMA.
Table 1.
ALDH1B1 immunopositivity in pancreatic cancer tissue microarrays (TMA) obtained from patients with pancreas adenocarcinoma. TMAs were scored by tumor differentiation type or by Pancreatic Intraepithelial Neoplasia (PanIN) duct lesions.
| Tumor features | n/N a | Immunopositivity score b |
|---|---|---|
| Differentiation | ||
| Well | 6/8 | 198 ± 32 |
| Moderate | 18/27 | 148 ± 19 |
| Poor | 16/26 | 144 ± 21 |
| Duct lesions c | ||
| PanIN1 | 1/1 | 56 |
| PanIN2 | 10/14 | 192 ± 29 |
| PanIN3 | 29/46 | 144 ± 14 |
n = number of cancer samples that were positive for ALDH1B1 and N = total number of samples examined.
Immunopositivity score was calculated as the product of staining intensity and the percentage of ALDH1B1+ cells. Results are expressed as mean ± s.e.m.
Duct lesions in the pancreas were graded according to the PanIN diagnostic criteria 23.
- PanIN-1: Tall columnar cells containing abundant mucin.
- PanIN-2: Cells have nuclear abnormality along with pesudostratified structure.
- PanIN-3: Features similar to PanIN-2 with “budding off” of small groups of epithelial cells into the lumen.
Variability in ALDH1B1 gene expression in pancreas cell lines and in ALDH+ population in pancreas explants
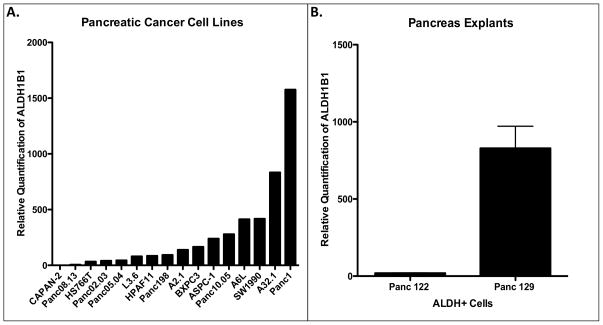
Given that ALDH1B1 is elevated in pancreatic cancer tissue, gene expression levels of ALDH1B1 were assessed in 16 pancreatic cell lines by RT-PCR. The expression of ALDH1B1 mRNA varied widely among these lines, ranging from high levels in PANC1 cells to very low levels in CAPAN-2 cells (Fig. 2A). ALDH1B1 mRNA expression was then evaluated in the ALDH+ cell population isolated from the explant tumor tissues derived from Panc122 and Panc129 cells. ALDH1B1 mRNA was 50-fold higher in ALDH+ cells from Panc129 cells than in those derived from Panc122 cells (Fig. 2B). These results demonstrate that there can be great variability in the expression of ALDH1B1 in the ALDH+ population of different pancreas explants.
Figure 2.
Variable ALDH1B1 gene expression levels in pancreatic cell lines and in the ALDH+ population of pancreas explants. (A) Gene expression levels of ALDH1B1 in sixteen pancreas cancer cell lines. B) ALDH+ cells were FACS isolated from two distinct pancreas explants (Panc122, Panc129) and evaluated for ALDH1B1 gene expression by RT-PCR.
Proliferation depends on ALDH1B1 expression in pancreas cancer cell lines with high ALDH1B1 expression
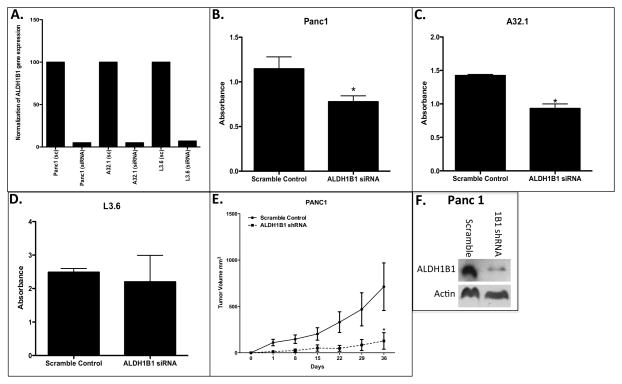
To investigate the importance of ALDH1B1 on proliferation, the effect of siRNA-mediated knockdown of the ALDH1B1 expression was examined in two high- and one low-expressing pancreatic cancer cell lines. In all of the cell lines tested, the siRNA treatment suppressed ALDH1B1 expression by over 90%, irrespective of initial expression levels (Fig 3A). Suppression of ALDH1B1 in the high ALDH1B1-expressing Panc1 and A32.1 cell lines resulted in a 37% and 33% reduction in proliferation, respectively (Fig. 3 B and C); no effect was observed in the L3.6 cell line (Fig D). To confirm these in vitro results, the effects of ALDH1B1 knockdown (shRNA) on tumor growth of the Panc1 cell line was evaluated using an in vivo xenograft model. A decrease in tumor growth was observed in the Panc1 cell line in which ALDH1B1 expression had been suppressed (Fig E). The efficacy of shRNA transfection in reducing ALDH1B1 protein in the Panc1 tumors was confirmed by Western blot analysis at the end of study (Fig 3F). This suggests that ALDH1B1 may contribute to proliferation of those pancreatic cancer cells expressing higher levels ALDH1B1.
Figure 3.
Suppression of ALDH1B1 expression reduces proliferation in ALDH1B1 high-expressing pancreas cell lines. (A) Gene expression of ALDH1B1 in scramble (sc) expressed as a percentage of that in ALDH1B1 siRNA-treated (siRNA) cells. siRNA knockdown of ALDH1B1 reduces growth of the (B) Panc1, (C) A32.1 and (D) L3.6 pancreas cell lines that were treated with scramble RNA or siRNA (to suppress ALDH1B1 expression). Data represent mean + SEM from 3–4 experiments. * P < 0.05, Student’s unpaired t-test, compared to scramble control.
Cell population density was estimated by the absorbance associated with the sulforhodamine B assay. (E) Growth of tumors induced by injection of Panc1 cells transfected with ALDH1B1 shRNA (dashed) or scramble shRNA (solid) into the left and right flanks of female athymic (nu+/nu+) mice. Tumor volume was calculated as (length × width2 × 0.52). Data are presented as mean ± SEM (n = 10 tumors per group). * P < 0.05, Student’s unpaired t-test, compared with tumors in scramble shRNA group at same time point. (F) Evaluation of ALDH1B1 protein expression by Western blot analysis in the ALDH1B1 shRNA (1B1 shRNA) and scramble Panc1 (Scramble) tumors at the end of study.
DISCUSSION
ALDH enzymatic activity is a well-established marker of cancer stem cells in many solid malignancies.16,17,27–29 This population of cells is postulated to possess the ability to facilitate tumor formation and chemotherapeutic resistance. In pancreatic cancer, ALDH enzymatic activity is elevated in tumors with a more aggressive phenotype. In addition, ALDH1A1 protein expression is associated with reduced survival 21,23 and ALDH1A3 gene expression has been identified to be elevated in pancreatic cancer.30 Although ALDH1A1 and ALDH1A3 have been proposed to be major contributors to ALDH enzymatic activity and cancer development, we have recently determined that the ALDH1B1 isozyme was upregulated in colorectal cancer.24 In the present study, we demonstrated that ALDH1B1 protein expression was upregulated in pancreatic cancer. Normal pancreas tissue exhibited very weak staining of ALDH1B1 while pancreatic cancer tissues had a marked elevation in ALDH1B1 expression. Using a pancreatic adenocarcinoma tissue microarray, ALDH1B1 protein expression appeared to be greatest in tumors that were more invasive, in particular PanIN 2–3. These results indicate that ALDH1B1 expression is upregulated in pancreatic cancer and may be an important modulator of tumor progression.
We further explored the role of ALDH1B1 in pancreatic cancer by evaluating gene expression of ALDH1B1 in a series of pancreatic cell lines and in the ALDH+ population of pancreas explants. A range of gene expression was observed in the various cell lines. In addition, within the ALDH+ population of pancreatic explant-derived cells, we documented a fifty-fold difference in ALDH1B1 expression, which was dependent on the source of the cancer tissue from which the explant grew. Thus, it is plausible that some pancreas tumors (e.g., ALDH1B1 high ALDH+ cells) may be dependent on ALDH1B1 for growth and disease progression while others (e.g., ALDH1B1 low ALDH+ cells) may rely on another ALDH isozyme, such as ALDH1A1, which has been proposed as an important factor facilitating tumorigenesis and progression in pancreatic cancer.21,22
Given the observed elevations in ALDH1B1 expression in pancreatic adenocarcinoma, we were interested in determining whether ALDH1B1 is capable of modulating proliferation of tumor cells. Small interfering RNA (siRNA)-induced suppression of ALDH1B1 expression resulted in 35% reduction in proliferation of cells that constitutively expressed high ALDH1B1 levels. The same treatment failed to affect the growth rate of cells that expressed low levels of ALDH1B1. These results suggest that ALDH1B1 may contribute to the growth of tumors in cells that possess high levels of ALDH1B1. A recent study has demonstrated that ALDH1B1 appears to be a marker of normal stem cell/progenitor cell populations and may be important for the regeneration of pancreatic cells following injury.31 If ALDH1B1 is indeed a characteristic of normal adult stem cells, dysregulation in favor of increased ALDH1B1 expression may contribute to the development of the self-renewal phenotype of the cancer stem cell in the pancreas. The precise mechanism by which increased ALDH influences the ability of a cell to proliferate and/or differentiate remains to be established. The capacity to promote retinoic acid formation from retinol is one mechanism proposed for ALDH1A132, and could apply to ALDH1B1. In addition, as a mitochondrial enzyme33, ALDH1B1 could change cellular metabolism in such a way that it provides a survival advantage to the cancer cells. Nonetheless, additional studies are needed in order to delineate the interaction between ALDH1B1 and oncogenic/tumor suppressor pathways involved in modulating tumor growth and survival.
The results of the present study implicate a potential role for ALDH1B1 as a modulator of pancreatic adenocarcinoma cell. The importance of ALDH1B1 in pancreatic cancer relative to other ALDH isozymes, such as ALDH1A1, remains to be established. The observations that (i) ALDH1B1 was most highly expressed in the most invasive PanIN 2–3 tumors, and (ii) decreased ALDH1B1 expression repressed the proliferation of some populations of pancreatic cancer cells provide a rational, albeit preliminary, basis for targeting this isozyme as a novel treatment of pancreatic cancer. Certainly, a clearer understanding of the mechanism by which ALDH1B1 modulates pancreatic cancer cell function would facilitate the development of such treatments.
Acknowledgments
Financial support: National Institutes of Health Grants AA022057 and AA021724. Supported in part by NIH grant EY11490 and a Skaggs Foundation Grant.
We thank Johns Hopkins University for the pancreas cancer tissue microarray.
Footnotes
All authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. doi: 10.1158/0008-5472.CAN-04-0807. [DOI] [PubMed] [Google Scholar]
- 4.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;365:768–769. doi: 10.1056/NEJMc1107627. [DOI] [PubMed] [Google Scholar]
- 8.Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 10.Black W, Vasiliou V. The aldehyde dehydrogenase gene superfamily resource center. Hum Genomics. 2009;4:136–142. doi: 10.1186/1479-7364-4-2-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penzes P, Wang X, Napoli JL. Enzymatic characteristics of retinal dehydrogenase type I expressed in Escherichia coli. Biochim Biophys Acta. 1997;1342:175–181. doi: 10.1016/s0167-4838(97)00102-7. [DOI] [PubMed] [Google Scholar]
- 12.Rexer BN, Zheng WL, Ong DE. Retinoic acid biosynthesis by normal human breast epithelium is via aldehyde dehydrogenase 6, absent in MCF-7 cells. Cancer Res. 2001;61:7065–7070. [PubMed] [Google Scholar]
- 13.Zhao D, McCaffery P, Ivins KJ, et al. Molecular identification of a major retinoic-acid-synthesizing enzyme, a retinaldehyde-specific dehydrogenase. Eur J Biochem. 1996;240:15–22. doi: 10.1111/j.1432-1033.1996.0015h.x. [DOI] [PubMed] [Google Scholar]
- 14.Marchitti SA, Brocker C, Stagos D, et al. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti S, Locatelli F, Papadimitriou D, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 16.Ma S, Chan KW, Lee TK, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 17.Arcaroli JJ, Powell RW, Varella-Garcia M, et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a gamma-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol. 2012;6:370–381. doi: 10.1016/j.molonc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng S, Yang X, Lassus H, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Su Y, Mei Y, et al. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest. 2010;90:234–244. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MP, Fleming JB, Wang H, et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahlert C, Bergmann F, Beck J, et al. Low expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer. BMC Cancer. 2011;11:275. doi: 10.1186/1471-2407-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Orlicky DJ, Matsumoto A, et al. Aldehyde dehydrogenase 1B1 (ALDH1B1) is a potential biomarker for human colon cancer. Biochem Biophys Res Commun. 2011;405:173–179. doi: 10.1016/j.bbrc.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 26.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 27.Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu P, Clanton DJ, Snipas TS, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int J Cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- 29.Clay MR, Tabor M, Owen JH, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia J, Parikh H, Xiao W, et al. An integrated transcriptome and epigenome analysis identifies a novel candidate gene for pancreatic cancer. BMC Med Genomics. 2013;6:33. doi: 10.1186/1755-8794-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannou M, Serafimidis I, Arnes L, et al. ALDH1B1 is a potential stem/progenitor marker for multiple pancreas progenitor pools. Dev Biol. 2013;374:153–163. doi: 10.1016/j.ydbio.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcato P, Dean CA, Giacomantonio CA, et al. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 33.Stagos D, Chen Y, Brocker C, et al. Aldehyde dehydrogenase 1B1: molecular cloning and characterization of a novel mitochondrial acetaldehyde-metabolizing enzyme. Drug Metab Dispos. 2010;38:1679–1687. doi: 10.1124/dmd.110.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]