Abstract
Prostate cancer rates vary substantially by race, ethnicity, and geography. These disparities can be explained by variation in access to screening and treatment, variation in exposure to prostate cancer risk factors, and variation in the underlying biology of prostate carcinogenesis (including genomic propensity of some groups to develop biologically aggressive disease). It is clear that access to screening and treatment are critical influencers of prostate cancer rates, yet even among geographically diverse populations with similar access to care (e.g., low and medium income countries), African descent men have higher prostate cancer rates and poorer prognosis. To date, the proportion of prostate cancer that can be explained by environmental exposures is small, and the impact of these factors across different racial, ethnic, or geographical populations is poorly understood. In contrast, prostate cancer has one of the highest heritabilities of all major cancers. Numerous genetic susceptibility markers have been identified from family-based studies, candidate gene association studies, and genome-wide association studies. Some prostate cancer loci, including the risk loci found at chromosome 8q24, have consistent effects in all groups studied to date. However, replication of many susceptibility loci across race, ethnicity, and geography remains limited, and additional studies in certain populations (particularly in men of African descent) are needed to better understand the underlying genetic basis of prostate cancer.
Variation in Prostate Cancer Rates by Race, Ethnicity, and Geography
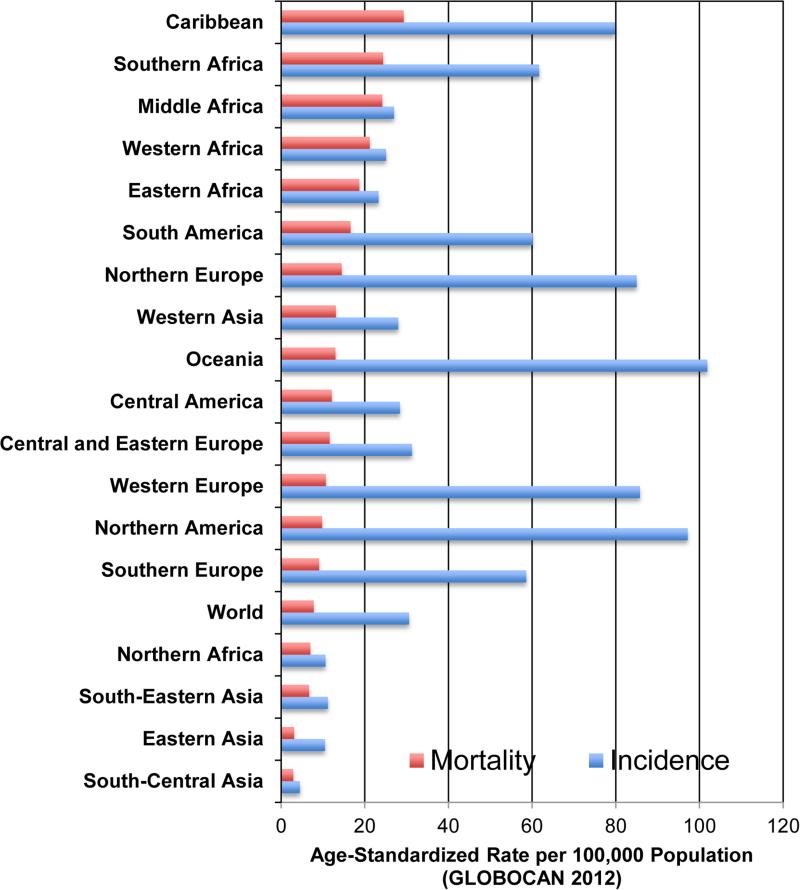
Prostate cancer is the leading non-cutaneous cancer in men in many parts of the world, although incidence and mortality rates vary substantially by population (Figure 1). Prostate cancer incidence rates tend to be highest in more developed parts of the world (e.g., North America, Western and Northern Europe, and Australia), in part reflecting access to medical care, including screening and early detection. In contrast, prostate cancer mortality is highest in men of African descent. Afro-Caribbean (AC) and Sub-sSaharan African (SSA) men suffer from the highest prostate cancer mortality in the world with rates ranging from 18.7-29.3 deaths per 100,000 populations based on 2012 GLOBOCAN data 1. African American (AA) men have a similarly high mortality rate of 43 per 100,000 in the period 2008-20112, 3. These rates are substantially higher than other US men, including Whites (19.8 per 100,000), Hispanics (17.8 per 100,000) and Asian/Pacific Islanders (9.4 per 100,000). In contrast, East, Southeast, and South Central Asian men have the lowest rates of prostate cancer death (2.9, 31, and 6.7 per 100,000, respectively; Figure 1). Of note, North African men have rates near those of Asians and lower than the global average (7 per 100,000).
Figure 1.
Global Prostate Cancer Incidence and Mortality by World Region
Additional epidemiological data support the high rates and poor outcomes of prostate cancer among African descent men. A population survey of unselected Ghanaian men demonstrated prostate cancer prevalence higher than that reported in AA men 4, suggesting that prostate cancer in SSA men is more common than reported from tumor registries. Autopsy studies confirm that the highest rates of latent prostate cancer is found in African descent men, with the lowest rates in Asian men 5. A survey of biopsy-detected prostate cancer in SSA men revealed a high proportion of Gleason 8+ tumors 6. Despite these consistent observations, variation in access to medical care and extent of cancer registration also vary by geography and confound the ability to make strong inferences about the relationship between race, ethnicity, and geography and prostate cancer aggressiveness based on epidemiological evidence alone.
Based on both epidemiological and biological data, there is growing evidence that prostate cancer risk, aggressiveness, and prognosis vary substantially by race, ethnicity, and geography. The evidence for a role of germline genomics in explaining this variation is explored in the subsequent sections.
Hereditary Prostate Cancer
Prostate cancer exhibits the highest reported heritability of any major cancer 7-10, yet unlike other cancers, the ability to define hereditary prostate cancer syndromes and identify hereditary cancer genes has been limited. Family-based linkage studies of hereditary prostate cancer focused largely on European descent populations to identify a series of genes responsible for hereditary prostate cancer. These include HPC1 (1q24-25) 11-13, PCAP (1q42-43) 13-15, HPCX (Xq27-28) 16, CAPB (1q36) 13, 15, HPC20 (20q13) 17, HOXB13 18, 19 and others. Among these, a series of loci were identified specifically or confirmed in Non-European descent families by family studies, including 12q24 20, 1q24-25, 2p16, and 2p21 20, 21, and 1p36 in Japanese 22 and AA21. Additional linkage signals have been detected in AA pedigrees at 2p21, 11q22, 17p11, 22q12, and Xq21 23 among others. Many of these loci were validated across ethnic and geographic populations, suggesting common origins for some hereditary prostate cancer susceptibility.
Despite the success of these discovery efforts, genetic testing for hereditary prostate cancer and recommendations for reduction of that risk based on genetic information have not evolved into clinical practice as has been the case for many other cancer sites. Recently, potentially clinically meaningful associations have been identified, including associations of inherited mutations in BRCA2 and aggressive prostate cancer, with implications for treatment 24, 25.
Some loci identified by family studies have also been implicated in GWAS studies, and have been validated in multiple ethnic and racial groups. Among these are 12q24, 1q24-25 and 8q24 26-28. While the loci that would be detected by family studies vs. association (GWAS) studies are not expected to be the same, the fact that some loci are found commonly across populations and by using multiple methods provides interesting evidence for the role of these genes across the spectrum of prostate cancer etiology.
Candidate Gene Studies
Candidate gene association studies in prostate cancer and other diseases were quite common before the advent of the genome-wide association study. These studies began with the identification of pathways and genes that played a biologically plausible role in the etiology of a disease. From this information, associations of individual SNPs or haplotypes were studied. Many such studies have been published, but this approach came under scrutiny because they often used inappropriate sampling designs and were underpowered to detect relevant effects. Many associations could not be replicated, although many were replicated and may still represent valid candidate susceptibility genes. Similarly, few candidate associations have also been detected in GWAS studies, which tend to use a more stringent methodology. Variation (and lack of replication) in results of associations across race, ethnicity, and geography were generally not accounted for in making determinations about the validity of associations. Highly variable allele and haplotype frequencies, different patterns of linkage disequilibrium, and population stratification across groups were generally not taken into consideration, and could easily have led to lack of replication across studies and populations. Similar issues continue to affect GWAS studies (See below).
Genes involved in DNA damage and repair, carcinogen metabolism, inflammation, steroid hormone metabolism, and many others have been reported in candidate gene association studies and have involved populations worldwide (reviewed by Naylor 29). Of the many candidates that have been considered few have also been reported in linkage or GWAS studies. Examples of candidate genes that have been identified using large gene panels or GWAS include the androgen receptor (AR; 26), kallekrein genes (e.g., KLK3, that encodes prostate specific antigen; 30-32), telomere-related genes (TERT, TET; 26, 33), and loci containing carcinogen metabolism (UGT1A8, CYP21A2; 34), microRNAs (34) or matrix metalloprotein genes (34). Many smaller studies have been undertaken in diverse populations around the world. However, the majority of these reports have not been validated in independent samples, and most of these candidate loci have not been reported in GWAS studies.
Genome-Wide Association Studies
Numerous prostate cancer susceptibility loci with a p-value of 10−8 have been reported to date using genome-wide association study (GWAS) approaches according to the NHGRI-EBI Catalog of Published Genome-Wide Association Studies (July 8, 2016; https://www.ebi.ac.uk/gwas/). CaP susceptibility loci are found on all chromosomes except 15, 16, 21, and 23. A summary of currently reported GWAS loci is found in Table 1.
Table 1.
Reported Prostate Cancer GWAS (July 8, 2016). Includes associations reported at p<10−8 and validated in at least one independent sample.
| Locus | Mapped Gene(s) | Most Significantly Associated SNP(s) - Risk Allele |
|---|---|---|
| 1p36.22 | PEX14 | rs636291-A |
| 1q21.3 | KCNN3 | rs1218582-G, rs17599629-G, rs17599629-G |
| 1q32.1 | LOC105371702 - SLC41A1, MDM4 | rs1775148-C, rs4245739-A |
| 2p11.2 | GGCX - VAMP8 | rs10187424-A |
| 2p15 | EHBP1 | rs721048-A, rs2430386-T |
| 2p21 | THADA | rs1465618-? |
| 2p24.1 | C2orf43 | rs13385191-G |
| 2p25.1 | LOC105373426 - NOL10, GRHL1 | rs9287719-C, rs11902236-A, rs9287719-C |
| 2q31.1 | ITGA6 | rs12621278-? |
| 2q37.3 | MLPH | rs2292884-G |
| 2q37.3 | FARP2 | rs3771570-A |
| 2q37.3 | LOC105373955 - LOC105373957 | rs7584330-C |
| 3p11.2 | LINC00506 | rs17181170-?, rs9284813-? |
| 3p12.1 | LOC285232 - LINC00506 | rs17023900-G, rs2660753-T, rs17023900-G |
| 3q13.2 | SIDT1 | rs7611694-A |
| 3q21.3 | EEFSEC | rs10934853-A |
| 3q23 | ZBTB38 | rs6763931-T |
| 3q26.2 | PRKCI | rs71277158-T |
| 3q26.2 | LOC105374210 | rs10936632-A |
| 4q13.3 | LOC105377273, AFM | rs10009409-T, rs10009409-T, rs1894292-G |
| 4q22.3 | PDLIM5 | rs12500426-?, rs17021918-? |
| 4q24 | LOC643675 - TET2 | rs7679673-C, rs7679673-? |
| 5p12 | FGF10 | rs2121875-G |
| 5p15.33 | TERT | rs7725218-G, rs12653946-T, rs2242652-G |
| 5q35.2 | LOC105377732 | rs6869841-A |
| 6p21.1 | FOXP4 | rs1983891-T |
| 6p21.32 | NOTCH4 - LOC101929163 | rs3096702-A |
| 6p21.32 | BTNL2 - HLA-DRA | rs115306967-G |
| 6p21.33 | CCHCR1 | rs130067-G |
| 6p22.1 | TRIM31, TRIM31-AS1 | rs115457135-A |
| 6p24.2 | NEDD9 | rs4713266-C, rs4713266-C |
| 6q14.1 | MYO6 | rs9443189-G |
| 6q21 | ARMC2 | rs2273669-G |
| 6q22.1 | RFX6 | rs339331-T |
| 6q25.2 | RGS17 | rs1933488-A |
| 6q25.3 | SLC22A1 - SLC22A2 | rs651164-?, rs9364554-T, rs651164-G, rs7758229-T |
| 7p12.3 | TNS3 | rs56232506-A, rs56232506-A |
| 7p15.3 | LINC01162 | rs12155172-A |
| 7q21.3 | LMTK2 | rs6465657-?, rs6465657-C |
| 8p21.2 | SLC25A37 - NKX3-1 | rs10503733-T |
| 8p21.2 | EBF2 | rs11135910-A, rs1512268-?, rs1512268-? |
| 8q24.21 | PRNCR1 - LOC105375752, CCAT2, CASC8, CASC21, PCAT2 | rs12682344-G, rs6983267-G, rs10505477-A, rs16901979-A, rs445114-T, rs6983267-G, rs1016343-T, rs13254738-C, rs6983267-G, rs16901979-A, rs1456315-?, rs6983267-G, rs6983267-G, rs6983561-C, rs16901979-A, rs1447295-A, rs10505483-T, rs16902094-G, rs6983267-G, rs4242384-C, rs4242384-C, rs1447295-A, rs1447295-A, rs4242382-A, rs1447295-A, rs4242384-?, rs7837688-?, rs1456315-?, rs188140481-A, rs4242382-A |
| 9p21.3 | CDKN2B-AS1 | rs17694493-G |
| 9q31.2 | RAD23B - LINC01509 | rs817826-C |
| 10q11.22 | MSMB - LOC105378288 | rs10993994-?, rs76934034-T, rs76934034-T, rs10993994-T, rs10993994-T, rs3123078-?, rs10993994-T, s10993994-T |
| 10q24.32 | LOC105378460, TRIM8 | rs3850699-A |
| 10q26.12 | LINC01153 - LOC105378523 | rs11199874-? |
| 11p15.5 | MIR4686 - ASCL2 | rs7126629-C, rs7127900-? |
| 11q13.3 | LOC105369366 - LOC105369367 | rs7130881-G, rs10896449-G, rs11228565-A, rs7931342-G, rs7130881-?, rs7929962-T |
| 11q22.2 | MMP7 - MMP20 | rs11568818-A |
| 11q23.2 | HTR3B | rs11214775-G, rs11214775-G |
| 12q13.11 | LOC105369750, TUBA1C - LOC101927267, KRT78 - RPL7P41 | rs80130819-A, rs80130819-A, rs10875943-C, rs902774-A |
| 12q24.21 | LOC105369996 - TBX5 | rs10774740-G, rs1270884-A |
| 13q22.1 | RNU6-66P - LINC00393 | rs9600079-T |
| 14q22.1 | FERMT2, LOC105370500 | rs8008270-G |
| 14q23.1 | SIX1 - SIX4 | rs7153648-C |
| 14q24.1 | RAD51B, LOC100996664, LOC105370544 | rs7141529-G |
| 14q24.2 | LOC101928075 | rs8014671-G |
| 15q21.1 | LOC105370802 - LOC105370803 | rs4775302-? |
| 16q22.2 | PHLPP2 | rs12051443-A |
| 17p13.3 | VPS53 - FAM57A | rs684232-G |
| 17q12 | HNF1B | rs4430796-A, rs4430796-A, rs7501939-C, rs7501939-?, rs7501939-?, rs8064454-C |
| 17q21.32 | FLJ40194 - MIR6129 | rs11650494-A |
| 17q21.33 | ZNF652 | rs7210100-? |
| 17q24.3 | CASC17 | rs1859962-G, rs1859962-G, rs4793529-T, rs1859962-?, rs17765344-A |
| 18q23 | SALL3 - ATP9B | rs7241993-G |
| 19q13.2 | PCAT19, DPF1 - PPP1R14A | rs11672691-G, rs8102476-C, rs11672691 -G |
| 19q13.33 | KLK3 | rs2735839-G, rs17632542-T |
| 19q13.42 | MIR4752 - LILRA5 | rs103294-C |
| 20q13.13 | ADNP | rs12480328-T |
| 20q13.33 | LOC105372710, ZGPAT | rs2427345-G, rs6062509-A |
| 21q22.3 | TMPRSS2 - LOC105372809 | rs1041449-G |
| 22q11.21 | TBX1 | rs2238776-G |
| 22q13.2 | RPS25P10 - BIK | rs5759167-G, rs5759167-? |
| Xp11.22 | NUDT11 - LINC01496, XAGE3, CXorf67 | rs5945619-C, rs2807031-C, rs1327301-?, rs2807031-C, rs5945572-A |
| Xp22.2 | SHROOM2 | rs2405942-A |
| Xq12 | BMI1P1 - OPHN1 | rs5919432-A |
| Xq13.1 | NLGN3 - GJB1, TEX11 - SLC7A3 | rs4844289-G, rs4844289-G, rs6625711-A |
As of July 2016, nearly 200 independent GWAS loci have been associated with CaP (NHGRI-EBI Catalog of Published Genome-Wide Association Studies, July 8, 2016; https://www.ebi.ac.uk/gwas/; Table 1). This includes numerous loci with multiple independent associations in the same region. In addition, associations have been reported with early onset CaP, aggressive CaP, or gene × gene interactions. Prostate cancer susceptibility loci are found on all chromosomes (Table 1). The majority of prostate cancer GWAS loci have been discovered in European descent populations. Of the 191 independent and replicated associations that reach genome-wide significance (i.e., p<10−8), 123 (64%) were reported in European and European descent populations, 21 (11%) in Asian or Asian descent populations, and 45 (24%) in sample sets using multiple populations (e.g., European, Asian, AA, Hispanic, etc.). Only one locus (17q21) has reached genome-wide level significance in African descent populations to date 35. This locus has since been validated as a prostate cancer risk locus in European descent populations 34. A novel locus at Chromosome 10p14 was reported in a GWAS undertaken in a Ghanaian population 36, although this association did not reach genome-wide significance.
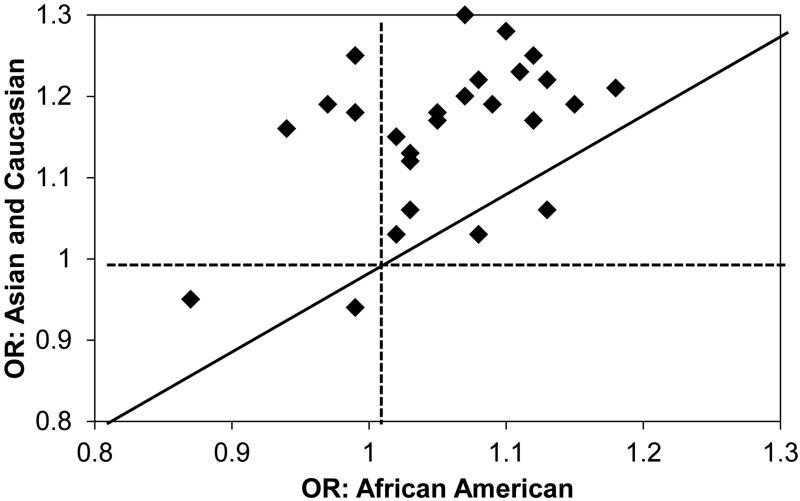
Many loci have been consistently shown to be associated with prostate cancer risk across populations, and suggest that these are strongly associated with risk in most settings. Han et al. demonstrated limited heterogeneity in the directionality of associated prostate cancer variants across populations 37-43. However, many loci detected in European or Asian descent populations have not been replicated in African descent populations, or the magnitude of effect was less (or directionally opposite) by race 38-40. A number of studies have attempted to validate reported associations in AA. Xu et al. 38 studied 868 cases and 878 controls and validated the loci at 8q24 (p=0.034 to p=2×10−5) and 3p12 (p=0.029). Waters et al. 39 studied 860 cases and 575 controls, and validated KLK2/3 (19q13.33) and NUDT10/11 (Xp11.22). Finally, Hooker et al. 41 validated 8q24 (p=1×10−4), 11q13.2 (p=0.009), HNF1B/TCF2 (17q12; p=0.008), KLK2/3 (19q13.33; p=0.04), and NUDT11 (Xp11.22; p=0.05) in 454 cases and 301 controls. The validated loci were not consistent across these studies, perhaps due to relatively small sample sizes in each study. Chang et al.42 studied a sample of nearly 8,000 men of African descent in the US and UK. This report only involved those loci that had been previously reported in non-African descent populations. They reported that the majority of the loci identified as prostate cancer susceptibility loci in White or Asian populations were not replicated in AA men. Only JAZF1, MSMB, NUDT10/11 and a locus on 11q13 were validated as having effects similar to those in non-African descent populations. The remainder of the associations in AA men exhibited smaller effects than those reported in non-AA populations. Some of the AA associations were even in the opposite direction of the non-AA reports, and in many cases the 95% confidence intervals for AA men did not overlap the non-AA estimates. Using a GWAS approach, Haiman et al. also did not replicate most of the previously reported loci identified in European or Asian descent populations 35, 44. Using over 9,500 AA prostate cancer cases and controls, Han et al. 37 reported that of established 82 GWAS hits, only 68 (83%) were directionally consistent with the original report, and 37% were significant at p<0.05. Similarly, the effect size of many loci is also smaller for the same locus in African descent populations compared to the original report of these loci in European or Asian descent populations (Figure 2).
Figure 2.
Odds Ratio (OR) Effects Estimated in Caucasian, Asian, and AAGWAS Studies.
In addition to a lack of replication for many loci, it has also been observed that AA exhibited smaller effects than those reported in non-AA populations. Some of the AA associations were even in the opposite direction to those of the non-AA reports, and in many cases the 95% confidence intervals for AA men did not overlap the non-AA estimates. Chang et al. 42 also reported that the magnitude of the odds ratio effect was smaller in AA men compared with the original reports in European or Asian descent populations (Figure 2).
A number of hypotheses have been proposed to explain the difficulty in replication and systematic differences in magnitudes of genetic effects by race and ethnicity. First, the underlying genomic susceptibility, and thus biology, for prostate cancer may differ fundamentally across racial or ethnic groups. This explanation is unlikely to be the case, as it implies that the biological basis for prostate cancer differs by race or ethnicity. However, this hypothesis cannot be ruled out based on available data.
A more likely explanation is that the risk alleles in European/Asian descent individuals are not the same as in African descent individuals. It is likely that the risk alleles and the underlying population structure of prostate cancer susceptibility loci differ by ethnicity, race, or geography, and that these differences are likely to influence the ability to detect genetic associations 45. This hypothesis is supported by well-described differences in the genomic architecture of the genome by race and ethnicity. Linkage disequilibrium and haplotype diversity differ substantially by race and ethnicity 45, 46, as do allele frequencies at many loci across the genome. The capture of genomic variability is incomplete, and it is likely that many African-specific alleles have yet to be detected that may provide a better capture of the African genome. Thus, it is not hard to imagine that prostate cancer risk alleles and the frequencies of these alleles vary substantially across populations to affect the ability to detect associations in a GWAS setting. To address this hypothesis, additional studies using non-Caucasian populations will be required that employ genotyping panels that adequately capture African genomic variability. While generally unreported in the GWAS literature, similar arguments can be made regarding genomics of other populations that are genomically divergent from Caucasians, including Native Americans, middle eastern groups, and aboriginal populations in the Arctic, Oceania, and elsewhere.
Furthermore, it is likely that the underlying etiology of prostate cancer is not only influenced by genes but also by exposures and gene by environment interactions. The different magnitudes of effect observed across race, ethnicity and geography (e.g., Figure 2) could be explained by the influence of contextual factors that influence prostate cancer susceptibility through gene-environment interactions that may vary by population. The number of confirmed environmental factors or exposures that influence prostate cancer risk and outcome are limited 47, but it is still possible that underlying genetic susceptibility may influence the effect of exposures that are not detectable on their own. To the degree that the frequencies of both the exposures and the susceptibility genotypes vary by race or ethnicity (which they are likely to do), it is possible that differences in etiology or severity of prostate cancer could be explained by complex interactions of these factors. There have been published examples of gene-environment interactions in prostate cancer, including novel and biological plausible interactions (e.g., 48, 49). However, most of these have not been validated in independent sets or across populations, and the large post-GWAS evaluations of GWAS loci have not shown convincing interaction results 50. No major or replicated interaction studies have been undertaken across racially or ethnically diverse groups to be able to test the hypothesis that differences in gene-environment interactions could explain differences in prostate cancer etiology or severity.
Unlike many prostate cancer susceptibility loci for which variation and lack of replication has been observed, genetic variation on chromosome 8q24 has been widely and consistently associated with prostate and numerous other cancers across many populations 51. Multiple independent regions conferring prostate cancer susceptibility have been identified at this locus 27, 28, 52, although no gene has been designated to be responsible for this cancer risk. Instead, regulation of the downstream gene MYC or regulation by lncRNAs has been reported 53-55. Disease-associated variation at 8q24 has been confirmed in multiple populations. Indeed, the 8q24 locus was associated with prostate cancer in early admixture mapping studies 56 that identify susceptibility loci by exploiting different disease risks and genotype distributions by race. Ethnic specific mutations and haplotypes have been reported in African and European descent populations 57, 58. The associations at 8q24 have been confirmed not only in African American, but also in two African populations 58. Thus, the genetic contribution to prostate cancer risk at this locus can be considered a master regulator of cancer at multiple sites as well as across populations.
Tumor Biomarkers
In addition to the strong evidence for inherited genomic factors in CaP etiology, somatic alterations in prostate tumors may also play a role in CaP etiology. The biomarkers identified to date may improve screening for CaP (e.g., as an alternative or supplement to PSA testing) and might inform treatment choices and prognosis. A number of prostate tumor biomarkers have been identified that may define heterogeneity of CaP etiology 59, 60, have clinical implications for surveillance and treatment (reviewed in 61), or correlate with aggressive phenotypes62-69. Among these are the TMPRSS2:ERG gene fusion/translocation70, Ki-67 expression64, biomarkers involved in androgen metabolism65, 71 and genomic classifiers that use a whole-transcriptome microarray assays to analyze gene activity in prostate cancer specimens72. In general, the majority of the studies of these biomarkers has been in European- or Asian-descent populations, are there are limited data that evaluate whether these markers have similar distributions or confer similar effects on outcomes in African descent populations. A few papers have begun to report differences in biomarkers between AA and Caucasian men, including AMCAR, ERG, SPINK1, NXK3.1, GOLM1, AR, Ki67, and SRD5A2 73, 74.
TMPRSS2:ERG translocations have been reported as having a different frequency by race. Magi-Galluzzi et al. 75 reported that the frequency of TMPRSS2:ERG translocations was highest in Japanese (71%) and Caucasians (62%) and much lower in AA (20%). Yamoah et al. 73 also reported significant differences in ERG expression between Caucasian and AA CaP cases. While the prognostic value of TMPRSS2:ERG translocations and ERG expression is not clear, it does not seem to correlate with clinical outcome in most studies76. However, the underlying marker distribution may identify tumor heterogeneity that informs CaP etiology and disparities. For example, it is becoming clear that the relationship of potential CaP risk factors differs by TMPRSS2:ERG translocation status 59, 60, 77. While not formally evaluating a racially diverse group, Pettersson et al. 78 reported that the relationship of obesity and lethal CaP varied by TMPRSS2:ERG translocation status. Since obesity has been associated with poorer CaP outcomes in some studies, and AA and Hispanic men tend to have greater rates of obesity than other racial/ethnic groups, this biomarker may prove valuable in understanding poor outcomes in some men.
Challenges and Needs
The observation of highly variable prostate cancer rates by race, ethnicity and geography has interesting biological, clinical, and public health implications. However, there are relatively little data that explain these disparities. There have been few consistent associations of exposures or environmental factors with prostate cancer etiology across populations. Prostate cancer has the highest heritability of any major cancer7, 79, and many genetic susceptibility loci have been identified, primarily in men of European and Asian descent. However, most GWAS-identified loci have not been replicated in African descent populations35, 42, 44, 80. There is a pressing need to identify African-specific alleles and thereby elucidate the etiology of prostate cancer in AA and Afro-Caribbean men, who have the highest prostate cancer mortality rates in the world.
A number of benefits will derive from an improved elucidation of prostate cancer genetics across populations. Improved understanding of population genetics by multi-ethnic studies of prostate cancer and other diseases can inform our understanding of genetics in general. Population genomic features strongly influence the ability to detect and interpret genetic associations, and may provide benefits and challenges to disease gene detection81. Diversity in population genomic structure and differences in allele frequency and linkage disequilibrium mean that a single approach to all association studies may not be successful 45, 81. The lack of replication across race, ethnicity, and geography is not only a feature of prostate cancer43, but of many GWAS studies of disease and non-disease traits82, and can be explained by a variety of factors including limited capture of ethnic-specific alleles, limited consideration of population-specific linkage disequilibrium, and inadequate knowledge of population substructure. These explanations are consistent with the observation that most GWAS studies to date have been undertaken using SNP panels based on the European or Asian genome, with limited representation of the African or other minority population genomes83. A more complete representation of the human genome may improve our ability to identify susceptibility loci, particularly in Africa where haplotype diversity in SSA is large and levels of linkage disequilibrium are relatively low. Localization and fine mapping of susceptibility alleles and improved evaluation of population structure in African descent populations to avoid biases due to population stratification will be facilitated by studies of representative populations from around the world 45.
Second, by understanding evolutionary and population genetics relationships across races, ethnicities, and geographies, we may be better able to understand why risk allele frequencies (and thus population risk differences) vary across populations. Basic knowledge about population genetics in ancestral populations may also improve the understanding of admixed populations, including African Americans and Hispanics/Latinos.
ACKNOWLEDGEMENTS
This work was supported by NIH grants P60-MD006900 and U01-CA184734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; Lyon: 2013. [Google Scholar]
- 2.SEER . Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute; [Google Scholar]
- 3.Howlader N,NA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2008. In SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; Bethesda, MD: 2011. [Google Scholar]
- 4.Hsing AW, Yeboah E, Biritwum R, Tettey Y, De Marzo AM, Adjei A, Netto GJ, Yu K, Li Y, Chokkalingam AP, Chu LW, Chia D, Partin A, Thompson IM, Quraishi SM, Niwa S, Tarone R, Hoover RN. High prevalence of screen detected prostate cancer in West Africans: implications for racial disparity of prostate cancer. J Urol. 2014;192(3):730–5. doi: 10.1016/j.juro.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebbeck TR, Haas GP. Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol. 2014;21(5):7496–506. [PMC free article] [PubMed] [Google Scholar]
- 6.Jalloh M, Friebel TM, Thiam FS, Niang L, Sy C, Siby T, Fernandez P, Mapulanga V, Maina S, Mante SD, Yeboah E, Kyei M, Ankomah R, Amegbor J, Adusei B, Yegbe P, Watya S, Kaggwa S, Haiman C, Henderson BE, Narashimhamurthy M, Abuidris D, Eljili E, Mohamadani AA, Mohamed M, Mansoor MO, Mohamed E, Elballal A, Zeigler-Johnson CM, Heyns CF, Gueye SM, TR R. Evaluation of Routine Prostate Biopsies Performed in Six African Countries. 2013 In Press. [Google Scholar]
- 7.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 8.Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, Pukkala E, Christensen K, Adami HO, Holm NV, Nuttall E, Hansen S, Hartman M, Czene K, Harris JR, Kaprio J, Mucci LA. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303–10. doi: 10.1158/1055-9965.EPI-13-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhage BA, Aben KK, Witjes JA, Straatman H, Schalken JA, Kiemeney LA. Site-specific familial aggregation of prostate cancer. Int J Cancer. 2004;109(4):611–7. doi: 10.1002/ijc.20015. [DOI] [PubMed] [Google Scholar]
- 10.Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH, McIntosh C, Nuttall E, Brandt I, Penney KL, Hartman M, Kraft P, Parmigiani G, Christensen K, Koskenvuo M, Holm NV, Heikkilä K, Pukkala E, Skytthe A, Adami HO, Kaprio J, Collaboration, N. T. S. o. C. N. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA. 2016;315(1):68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooney KA, McCarthy JD, Lange E, Huang L, Miesfeldt S, Montie JE, Oesterling JE, Sandler HM, Lange K. Prostate cancer susceptibility locus on chromosome 1q: a confirmatory study. J Natl Cancer Inst. 1997;89(13):955–9. doi: 10.1093/jnci/89.13.955. [DOI] [PubMed] [Google Scholar]
- 12.Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A. 1992;89(8):3367–71. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry R, Schaid DJ, Smith JR, French AJ, Schroeder JJ, McDonnell SK, Peterson BJ, Wang ZY, Carpten JD, Roberts SG, Tester DJ, Blute ML, Trent JM, Thibodeau SN. Linkage analyses at the chromosome 1 loci 1q24-25 (HPC1), 1q42.2-43 (PCAP), and 1p36 (CAPB) in families with hereditary prostate cancer. Am J Hum Genet. 2000;66(2):539–46. doi: 10.1086/302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuhausen SL, Farnham JM, Kort E, Tavtigian SV, Skolnick MH, Cannon-Albright LA. Prostate cancer susceptibility locus HPC1 in Utah high-risk pedigrees. Hum Mol Genet. 1999;8(13):2437–42. doi: 10.1093/hmg/8.13.2437. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Zheng SL, Chang B, Smith JR, Carpten JD, Stine OC, Isaacs SD, Wiley KE, Henning L, Ewing C, Bujnovszky P, Bleeker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB. Linkage of prostate cancer susceptibility loci to chromosome 1. Hum Genet. 2001;108(4):335–45. doi: 10.1007/s004390100488. [DOI] [PubMed] [Google Scholar]
- 16.Schleutker J, Matikainen M, Smith J, Koivisto P, Baffoe-Bonnie A, Kainu T, Gillanders E, Sankila R, Pukkala E, Carpten J, Stephan D, Tammela T, Brownstein M, Bailey-Wilson J, Trent J, Kallioniemi OP. A genetic epidemiological study of hereditary prostate cancer (HPC) in Finland: frequent HPCX linkage in families with late-onset disease. Clin Cancer Res. 2000;6(12):4810–5. [PubMed] [Google Scholar]
- 17.Berry R, Schroeder JJ, French AJ, McDonnell SK, Peterson BJ, Cunningham JM, Thibodeau SN, Schaid DJ. Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am J Hum Genet. 2000;67(1):82–91. doi: 10.1086/302994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breyer JP, Avritt TG, McReynolds KM, Dupont WD, Smith JR. Confirmation of the HOXB13 G84E germline mutation in familial prostate cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1348–53. doi: 10.1158/1055-9965.EPI-12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Lange EM, Lu L, Zheng SL, Wang Z, Thibodeau SN, Cannon-Albright LA, Teerlink CC, Camp NJ, Johnson AM, Zuhlke KA, Stanford JL, Ostrander EA, Wiley KE, Isaacs SD, Walsh PC, Maier C, Luedeke M, Vogel W, Schleutker J, Wahlfors T, Tammela T, Schaid D, McDonnell SK, DeRycke MS, Cancel-Tassin G, Cussenot O, Wiklund F, Grönberg H, Eeles R, Easton D, Kote-Jarai Z, Whittemore AS, Hsieh CL, Giles GG, Hopper JL, Severi G, Catalona WJ, Mandal D, Ledet E, Foulkes WD, Hamel N, Mahle L, Moller P, Powell I, Bailey-Wilson JE, Carpten JD, Seminara D, Cooney KA, Isaacs WB, Genetics, I. C. f. P. C. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum Genet. 2013;132(1):5–14. doi: 10.1007/s00439-012-1229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledet EM, Sartor O, Rayford W, Bailey-Wilson JE, Mandal DM. Suggestive evidence of linkage identified at chromosomes 12q24 and 2p16 in African American prostate cancer families from Louisiana. Prostate. 2012;72(9):938–47. doi: 10.1002/pros.21496. [DOI] [PubMed] [Google Scholar]
- 21.Brown WM, Lange EM, Chen H, Zheng SL, Chang B, Wiley KE, Isaacs SD, Walsh PC, Isaacs WB, Xu J, Cooney KA. Hereditary prostate cancer in African American families: linkage analysis using markers that map to five candidate susceptibility loci. Br J Cancer. 2004;90(2):510–4. doi: 10.1038/sj.bjc.6601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui H, Suzuki K, Ohtake N, Nakata S, Takeuchi T, Yamanaka H, Inoue I. Genomewide linkage analysis of familial prostate cancer in the Japanese population. J Hum Genet. 2004;49(1):9–15. doi: 10.1007/s10038-003-0099-y. [DOI] [PubMed] [Google Scholar]
- 23.Baffoe-Bonnie AB, Kittles RA, Gillanders E, Ou L, George A, Robbins C, Ahaghotu C, Bennett J, Boykin W, Hoke G, Mason T, Pettaway C, Vijayakumar S, Weinrich S, Jones MP, Gildea D, Riedesel E, Albertus J, Moses T, Lockwood E, Klaric M, Faruque M, Royal C, Trent JM, Berg K, Collins FS, Furbert-Harris PM, Bailey-Wilson JE, Dunston GM, Powell I, Carpten JD. Genome-wide linkage of 77 families from the African American Hereditary Prostate Cancer study (AAHPC). Prostate. 2007;67(1):22–31. doi: 10.1002/pros.20456. [DOI] [PubMed] [Google Scholar]
- 24.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami K, Guy M, Sawyer E, Wilkinson R, Ardern-Jones A, Ellis S, Frost D, Peock S, Evans DG, Tischkowitz M, Cole T, Davidson R, Eccles D, Brewer C, Douglas F, Porteous ME, Donaldson A, Dorkins H, Izatt L, Cook J, Hodgson S, Kennedy MJ, Side LE, Eason J, Murray A, Antoniou AC, Easton DF, Kote-Jarai Z, Eeles R. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, Campa D, Riboli E, Key T, Gronberg H, Hunter DJ, Kraft P, Thun MJ, Ingles S, Chanock S, Albanes D, Hayes RB, Neal DE, Hamdy FC, Donovan JL, Pharoah P, Schumacher F, Henderson BE, Stanford JL, Ostrander EA, Sorensen KD, Dork T, Andriole G, Dickinson JL, Cybulski C, Lubinski J, Spurdle A, Clements JA, Chambers S, Aitken J, Gardiner RA, Thibodeau SN, Schaid D, John EM, Maier C, Vogel W, Cooney KA, Park JY, Cannon-Albright L, Brenner H, Habuchi T, Zhang HW, Lu YJ, Kaneva R, Muir K, Benlloch S, Leongamornlert DA, Saunders EJ, Tymrakiewicz M, Mahmud N, Guy M, O'Brien LT, Wilkinson RA, Hall AL, Sawyer EJ, Dadaev T, Morrison J, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Lophatonanon A, Southey MC, Hopper JL, English DR, Wahlfors T, Tammela TL, Klarskov P, Nordestgaard BG, Roder MA, Tybjaerg-Hansen A, Bojesen SE, Travis R, Canzian F, Kaaks R, Wiklund F, Aly M, Lindstrom S, Diver WR, Gapstur S, Stern MC, Corral R, Virtamo J, Cox A, Haiman CA, Le Marchand L, Fitzgerald L, Kolb S, Kwon EM, Karyadi DM, Orntoft TF, Borre M, Meyer A, Serth J, Yeager M, Berndt SI, Marthick JR, Patterson B, Wokolorczyk D, Batra J, Lose F, McDonnell SK, Joshi AD, Shahabi A, Rinckleb AE, Ray A, Sellers TA, Lin HY, Stephenson RA, Farnham J, Muller H, Rothenbacher D, Tsuchiya N, Narita S, Cao GW, Slavov C, Mitev V, Easton DF, Eeles RA. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. 2011;43(8):785–91. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, Albers-Akkers MT, Godino-Ivan Marcos J, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39(5):631–7. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 28.Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF, Jr., Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39(5):645–9. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 29.Naylor SL. SNPs associated with prostate cancer risk and prognosis. Front Biosci. 2007;12:4111–31. doi: 10.2741/2375. [DOI] [PubMed] [Google Scholar]
- 30.Lose F, Batra J, O'Mara T, Fahey P, Marquart L, Eeles RA, Easton DF, Al Olama AA, Kote-Jarai Z, Guy M, Muir K, Lophatananon A, Rahman AA, Neal DE, Hamdy FC, Donovan JL, Chambers S, Gardiner RA, Aitken JF, Yaxley J, Alexander K, Clements JA, Spurdle AB, Kedda MA, BioResource APC. Common variation in Kallikrein genes KLK5, KLK6, KLK12, and KLK13 and risk of prostate cancer and tumor aggressiveness. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Lange EM, Johnson AM, Wang Y, Zuhlke KA, Lu Y, Ribado JV, Keele GR, Li J, Duan Q, Li G, Gao Z, Li Y, Xu J, Isaacs WB, Zheng S, Cooney KA. Genome-wide association scan for variants associated with early-onset prostate cancer. PLoS One. 2014;9(4):e93436. doi: 10.1371/journal.pone.0093436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipe DW, Evans DM, Kemp JP, Eeles R, Easton DF, Kote-Jarai Z, Al Olama AA, Benlloch S, Donovan JL, Hamdy FC, Neal DE, Davey Smith G, Lathrop M, Martin RM. Genetic variation in prostate-specific antigen-detected prostate cancer and the effect of control selection on genetic association studies. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1356–65. doi: 10.1158/1055-9965.EPI-13-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berndt SI, Wang Z, Yeager M, Alavanja MC, Albanes D, Amundadottir L, Andriole G, Beane Freeman L, Campa D, Cancel-Tassin G, Canzian F, Cornu JN, Cussenot O, Diver WR, Gapstur SM, Grönberg H, Haiman CA, Henderson B, Hutchinson A, Hunter DJ, Key TJ, Kolb S, Koutros S, Kraft P, Le Marchand L, Lindström S, Machiela MJ, Ostrander EA, Riboli E, Schumacher F, Siddiq A, Stanford JL, Stevens VL, Travis RC, Tsilidis KK, Virtamo J, Weinstein S, Wilkund F, Xu J, Lilly Zheng S, Yu K, Wheeler W, Zhang H, Sampson J, Black A, Jacobs K, Hoover RN, Tucker M, Chanock SJ, Consortium, A. A. P. C. G. Two susceptibility loci identified for prostate cancer aggressiveness. Nat Commun. 2015;6:6889. doi: 10.1038/ncomms7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Røder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC, Kote-Jarai Z, Easton DF, Initiative, C. C. R. U. G. E. p. o. G.-O.; Bioresource, A. P. C.; Oncology, U. G. P. C. S. C. B. A. o. U. S. S. o.; Collaborators, U. P. P. t. f. c. a. T. S.; Consortium, P. P. C. A. G. t. I. C.-A. A. i. t. G. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–91. 391e1–2. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, Diver WR, Witte JS, Hsing AW, Nemesure B, Rebbeck TR, Cooney KA, Xu J, Kibel AS, Hu JJ, John EM, Gueye SM, Watya S, Signorello LB, Hayes RB, Wang Z, Yeboah E, Tettey Y, Cai Q, Kolb S, Ostrander EA, Zeigler-Johnson C, Yamamura Y, Neslund-Dudas C, Haslag-Minoff J, Wu W, Thomas V, Allen GO, Murphy A, Chang BL, Zheng SL, Leske MC, Wu SY, Ray AM, Hennis AJ, Thun MJ, Carpten J, Casey G, Carter EN, Duarte ER, Xia LY, Sheng X, Wan P, Pooler LC, Cheng I, Monroe KR, Schumacher F, Le Marchand L, Kolonel LN, Chanock SJ, Berg DV, Stram DO, Henderson BE. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43(6):570–3. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook MB, Wang Z, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chung CC, Chokkalingam AP, Chu LW, Yeager M, Hutchinson A, Yu K, Rand KA, Haiman CA, Hoover RN, Hsing AW, Chanock SJ, Consortium AAPCG. A genome-wide association study of prostate cancer in West African men. Hum Genet. 2014;133(5):509–21. doi: 10.1007/s00439-013-1387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Y, Signorello LB, Strom SS, Kittles RA, Rybicki BA, Stanford JL, Goodman PJ, Berndt SI, Carpten J, Casey G, Chu L, Conti DV, Rand KA, Diver WR, Hennis AJ, John EM, Kibel AS, Klein EA, Kolb S, Le Marchand L, Leske MC, Murphy AB, Neslund-Dudas C, Park JY, Pettaway C, Rebbeck TR, Gapstur SM, Zheng SL, Wu SY, Witte JS, Xu J, Isaacs W, Ingles SA, Hsing A, Easton DF, Eeles RA, Schumacher FR, Chanock S, Nemesure B, Blot WJ, Stram DO, Henderson BE, Haiman CA, Consortium P, Consortium EG-O. Generalizability of established prostate cancer risk variants in men of African ancestry. Int J Cancer. 2015;136(5):1210–7. doi: 10.1002/ijc.29066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Kibel AS, Hu JJ, Turner AR, Pruett K, Zheng SL, Sun J, Isaacs SD, Wiley KE, Kim ST, Hsu FC, Wu W, Torti FM, Walsh PC, Chang BL, Isaacs WB. Prostate cancer risk associated loci in African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2145–9. doi: 10.1158/1055-9965.EPI-09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters KM, Le Marchand L, Kolonel LN, Monroe KR, Stram DO, Henderson BE, Haiman CA. Generalizability of associations from prostate cancer genome-wide association studies in multiple populations. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1285–9. doi: 10.1158/1055-9965.EPI-08-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooker S, Hernandez W, Chen H, Robbins C, Torres JB, Ahaghotu C, Carpten J, Kittles RA. Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate. 70(3):270–5. doi: 10.1002/pros.21061. [DOI] [PubMed] [Google Scholar]
- 41.Hooker S, Hernandez W, Chen H, Robbins C, Torres JB, Ahaghotu C, Carpten J, Kittles RA. Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate. 2010;70(3):270–5. doi: 10.1002/pros.21061. [DOI] [PubMed] [Google Scholar]
- 42.Chang BL, Spangler E, Gallagher S, Haiman CA, Henderson B, Isaacs W, Benford ML, Kidd LR, Cooney K, Strom S, Ingles SA, Stern MC, Corral R, Joshi AD, Xu J, Giri VN, Rybicki B, Neslund-Dudas C, Kibel AS, Thompson IM, Leach RJ, Ostrander EA, Stanford JL, Witte J, Casey G, Eeles R, Hsing AW, Chanock S, Hu JJ, John EM, Park J, Stefflova K, Zeigler-Johnson C, Rebbeck TR. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol Biomarkers Prev. 2011;20(1):23–32. doi: 10.1158/1055-9965.EPI-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishak MB, Giri VN. A systematic review of replication studies of prostate cancer susceptibility genetic variants in high-risk men originally identified from genome-wide association studies. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1599–610. doi: 10.1158/1055-9965.EPI-11-0312. [DOI] [PubMed] [Google Scholar]
- 44.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, Diver WR, Witte JS, Chanock SJ, Kolb S, Signorello LB, Yamamura Y, Neslund-Dudas C, Thun MJ, Murphy A, Casey G, Sheng X, Wan P, Pooler LC, Monroe KR, Waters KM, Le Marchand L, Kolonel LN, Stram DO, Henderson BE. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7(5):e1001387. doi: 10.1371/journal.pgen.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teo YY, Small KS, Kwiatkowski DP. Methodological challenges of genome-wide association analysis in Africa. Nat Rev Genet. 2010;11(2):149–60. doi: 10.1038/nrg2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reich DE, Cargill M, Bolk S, Ireland J, Sabeti PC, Richter DJ, Lavery T, Kouyoumjian R, Farhadian SF, Ward R, Lander ES. Linkage disequilibrium in the human genome. Nature. 2001;411(6834):199–204. doi: 10.1038/35075590. [DOI] [PubMed] [Google Scholar]
- 47.Boffetta P, Tubiana M, Hill C, Boniol M, Aurengo A, Masse R, Valleron AJ, Monier R, de Thé G, Boyle P, Autier P. The causes of cancer in France. Ann Oncol. 2009;20(3):550–5. doi: 10.1093/annonc/mdn597. [DOI] [PubMed] [Google Scholar]
- 48.Neslund-Dudas C, Levin AM, Beebe-Dimmer JL, Bock CH, Nock NL, Rundle A, Jankowski M, Krajenta R, Dou QP, Mitra B, Tang D, Rebbeck TR, Rybicki BA. Gene-environment interactions between JAZF1 and occupational and household lead exposure in prostate cancer among African American men. Cancer Causes Control. 2014;25(7):869–79. doi: 10.1007/s10552-014-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koutros S, Berndt SI, Hughes Barry K, Andreotti G, Hoppin JA, Sandler DP, Yeager M, Burdett LA, Yuenger J, Alavanja MC, Beane Freeman LE. Genetic susceptibility loci, pesticide exposure and prostate cancer risk. PLoS One. 2013;8(4):e58195. doi: 10.1371/journal.pone.0058195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrdahl M, Canzian F, Joshi AD, Travis RC, Chang-Claude J, Auer PL, Gapstur SM, Gaudet M, Diver WR, Henderson BE, Haiman CA, Schumacher FR, Le Marchand L, Berg CD, Chanock SJ, Hoover RN, Rudolph A, Ziegler RG, Giles GG, Baglietto L, Severi G, Hankinson SE, Lindström S, Willet W, Hunter DJ, Buring JE, Lee IM, Zhang S, Dossus L, Cox DG, Khaw KT, Lund E, Naccarati A, Peeters PH, Quirós JR, Riboli E, Sund M, Trichopoulos D, Prentice RL, Kraft P, Kaaks R, Campa D. Post-GWAS gene-environment interplay in breast cancer: results from the Breast and Prostate Cancer Cohort Consortium and a meta-analysis on 79,000 women. Hum Mol Genet. 2014;23(19):5260–70. doi: 10.1093/hmg/ddu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le Roux L, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Balter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38(6):652–8. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 52.Haiman CA, Patterson N, Freedman ML, Myers SR, Pike MC, Waliszewska A, Neubauer J, Tandon A, Schirmer C, McDonald GJ, Greenway SC, Stram DO, Le Marchand L, Kolonel LN, Frasco M, Wong D, Pooler LC, Ardlie K, Oakley-Girvan I, Whittemore AS, Cooney KA, John EM, Ingles SA, Altshuler D, Henderson BE, Reich D. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39(5):638–44. doi: 10.1038/ng2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, He HH, Brown M, Liu XS, Davis M, Caswell JL, Beckwith CA, Hills A, Macconaill L, Coetzee GA, Regan MM, Freedman ML. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci U S A. 2010;107(21):9742–6. doi: 10.1073/pnas.0910668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung S, Nakagawa H, Uemura M, Piao L, Ashikawa K, Hosono N, Takata R, Akamatsu S, Kawaguchi T, Morizono T, Tsunoda T, Daigo Y, Matsuda K, Kamatani N, Nakamura Y, Kubo M. Association of a novel long non-coding RNA in 8q24 with prostate cancer susceptibility. Cancer Sci. 2011;102(1):245–52. doi: 10.1111/j.1349-7006.2010.01737.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim T, Cui R, Jeon YJ, Lee JH, Sim H, Park JK, Fadda P, Tili E, Nakanishi H, Huh MI, Kim SH, Cho JH, Sung BH, Peng Y, Lee TJ, Luo Z, Sun HL, Wei H, Alder H, Oh JS, Shim KS, Ko SB, Croce CM. Long-range interaction and correlation between MYC enhancer and oncogenic long noncoding RNA CARLo-5. Proc Natl Acad Sci U S A. 2014;111(11):4173–8. doi: 10.1073/pnas.1400350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM, Oakley-Girvan I, Whittemore AS, Cooney KA, Ingles SA, Altshuler D, Henderson BE, Reich D. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103(38):14068–73. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung CC, Hsing AW, Yeboah Edward, Biritwum R, Tettey Y, Adjei A, Cook MB, De Marzo A, Netto G, Tay E, Boland JF, Yeager M, Chanock SJ. A comprehensive resequence-analysis of 250 kb region of 8q24.21 in men of African ancestry. Prostate. 2014;74(6):579–89. doi: 10.1002/pros.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y, Rand KA, Hazelett DJ, Ingles SA, Kittles RA, Strom SS, Rybicki BA, Nemesure B, Isaacs WB, Stanford JL, Zheng W, Schumacher FR, Berndt SI, Wang Z, Xu J, Rohland N, Reich D, Tandon A, Pasaniuc B, Allen A, Quinque D, Mallick S, Notani D, Rosenfeld MG, Jayani RS, Kolb S, Gapstur SM, Stevens VL, Pettaway CA, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chokkalingam AP, John EM, Murphy AB, Signorello LB, Carpten J, Leske MC, Wu SY, Hennis AJ, Neslund-Dudas C, Hsing AW, Chu L, Goodman PJ, Klein EA, Zheng SL, Witte JS, Casey G, Lubwama A, Pooler LC, Sheng X, Coetzee GA, Cook MB, Chanock SJ, Stram DO, Watya S, Blot WJ, Conti DV, Henderson BE, Haiman CA. Prostate Cancer Susceptibility in Men of African Ancestry at 8q24. J Natl Cancer Inst. 2016;108(7) doi: 10.1093/jnci/djv431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, Hicks JL, Wilson KM, Rider JR, Sesso HD, Fiorentino M, Flavin R, Finn S, Giovannucci EL, Loda M, Stampfer MJ, De Marzo AM, Mucci LA, Lotan TL. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graff RE, Meisner A, Ahearn TU, Fiorentino M, Loda M, Giovannucci EL, Mucci LA, Pettersson A. Pre-diagnostic circulating sex hormone levels and risk of prostate cancer by ERG tumour protein expression. Br J Cancer. 2016;114(8):939–44. doi: 10.1038/bjc.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Netto GJ. Clinical applications of recent molecular advances in urologic malignancies: no longer chasing a “mirage”? Adv Anat Pathol. 2013;20(3):175–203. doi: 10.1097/PAP.0b013e3182863f80. [DOI] [PubMed] [Google Scholar]
- 62.Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P, Hautmann RE, Gschwend JE, Kuefer R, Rubin MA. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, De Marzo AM, Rubin MA. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31(6):882–8. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 64.Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS, Reuter V, Eastham J, Moller H, Kattan MW, Gerald W, Cooper C, Scardino P, Cuzick J. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100(6):888–93. doi: 10.1038/sj.bjc.6604951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28(7):928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27(3):253–63. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fine SW, Gopalan A, Leversha MA, Al-Ahmadie HA, Tickoo SK, Zhou Q, Satagopan JM, Scardino PT, Gerald WL, Reuter VE. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol. 2010;23(10):1325–33. doi: 10.1038/modpathol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bjartell AS, Al-Ahmadie H, Serio AM, Eastham JA, Eggener SE, Fine SW, Udby L, Gerald WL, Vickers AJ, Lilja H, Reuter VE, Scardino PT. Association of cysteine-rich secretory protein 3 and beta-microseminoprotein with outcome after radical prostatectomy. Clin Cancer Res. 2007;13(14):4130–8. doi: 10.1158/1078-0432.CCR-06-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG. Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci U S A. 1994;91(24):11733–7. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–9. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 71.Wandel C, Witte JS, Hall JM, Stein CM, Wood AJ, Wilkinson GR. CYP3A activity in African American and European American men: population differences and functional effect of the CYP3A4*1B5'-promoter region polymorphism. Clin Pharmacol Ther. 2000;68(1):82–91. doi: 10.1067/mcp.2000.108506. [DOI] [PubMed] [Google Scholar]
- 72.Erho N, Buerki C, Triche TJ, Davicioni E, Vergara IA. Transcriptome-wide detection of differentially expressed coding and non-coding transcripts and their clinical significance in prostate cancer. Journal of oncology. 2012;2012:541353. doi: 10.1155/2012/541353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamoah K, Johnson MH, Choeurng V, Faisal FA, Yousefi K, Haddad Z, Ross AE, Alshalafa M, Den R, Lal P, Feldman M, Dicker AP, Klein EA, Davicioni E, Rebbeck TR, Schaeffer EM. Novel Biomarker Signature That May Predict Aggressive Disease in African American Men With Prostate Cancer. J Clin Oncol. 2015;33(25):2789–96. doi: 10.1200/JCO.2014.59.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khani F, Mosquera JM, Park K, Blattner M, O'Reilly C, MacDonald TY, Chen Z, Srivastava A, Tewari AK, Barbieri CE, Rubin MA, Robinson BD. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20(18):4925–34. doi: 10.1158/1078-0432.CCR-13-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, Esgueva R, Klein E, Rubin MA, Zhou M. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71(5):489–97. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 76.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, Martin NE, Kunz L, Penney KL, Ligon AH, Suppan C, Flavin R, Sesso HD, Rider JR, Sweeney C, Stampfer MJ, Fiorentino M, Kantoff PW, Sanda MG, Giovannucci EL, Ding EL, Loda M, Mucci LA. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1497–509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, Rider JR, Fiorentino M, Finn S, Kenfield SA, Loda M, Giovannucci EL, Rosner B, Mucci LA. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103(3):851–60. doi: 10.3945/ajcn.115.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, Finn S, Graff RE, Penney KL, Rider JR, Nuttall EJ, Martin NE, Sesso HD, Pollak M, Stampfer MJ, Kantoff PW, Giovannucci EL, Loda M, Mucci LA. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst. 2013;105(24):1881–90. doi: 10.1093/jnci/djt332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemminki K, Sundquist J, Bermejo JL. How common is familial cancer? Ann Oncol. 2008;19(1):163–7. doi: 10.1093/annonc/mdm414. [DOI] [PubMed] [Google Scholar]
- 80.Cook M, Wang Z, Yeboah E, Adjei A, Tettey Y, Biritwum R, Tay E, Truelove A, Niwa S, Chu S, Yeager M, Hutchinson A, Yu K, Haiman C, Consortium AAPCG, Hoover R, Hsing A, Chanock S. A genome-wide association study of prostate cancer in West African men. AACR. 2013:2552. doi: 10.1007/s00439-013-1387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seldin MF, Pasaniuc B, Price AL. New approaches to disease mapping in admixed populations. Nat Rev Genet. 2011;12(8):523–8. doi: 10.1038/nrg3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kraja AT, Hunt SC, Rao DC, Dávila-Román VG, Arnett DK, Province MA. Genetics of hypertension and cardiovascular disease and their interconnected pathways: lessons from large studies. Curr Hypertens Rep. 2011;13(1):46–54. doi: 10.1007/s11906-010-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–33. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]