Abstract
AIM
To investigate the incidence, risk factors and clinical outcomes of acute antibody-mediated rejection (ABMR) after intestinal transplantation (ITx).
METHODS
A retrospective single-center analysis was performed to identify cases of acute ABMR after ITx, based on the presence of donor-specific antibody (DSA), acute tissue damage, C4d deposition, and allograft dysfunction.
RESULTS
Acute ABMR was identified in 18 (10.3%) out of 175 intestinal allografts with an average occurrence of 10 d (range, 4-162) after ITx. All acute ABMR cases were presensitized to donor human leukocyte antigens class I and/or II antigens with a detectable DSA. A positive cross-match was seen in 14 (77.8%) cases and twelve of 18 patients (66.7%) produced newly-formed DSA following ITx. Histological characteristics of acute ABMR include endothelial C4d deposits, interstitial hemorrhage, and severe congestion with focal fibrin thrombin in the lamina propria capillaries. Multivariate analysis identified a liver-free graft and high level of panel reactive antibody as a significant independent risk factor. Despite initial improvement after therapy, eleven recipients (61.1%) lost transplant secondary to rejection. Of those, 9 (50%) underwent graft removal and 4 (22.2%) received second transplantation following acute ABMR. At an average follow-up of 32.3 mo (range, 13.3-76.4), 8 (44.4%) recipients died.
CONCLUSION
Our results indicate that acute ABMR is an important cause of intestine graft dysfunction, particularly in a liver-exclusive graft and survivors are at an increased risk of developing refractory acute rejection and chronic rejection. More effective strategies to prevent and manage acute ABMR are needed to improve outcomes.
Keywords: Intestinal transplantation, C4d deposition, Donor-specific antibody, Acute antibody-mediated rejection
Core tip: Antibody-mediated rejection (ABMR) has appeared to be an important cause of allograft failure after intestinal transplantation (ITx). This study aimed to evaluate the incidence, risk factors and clinical outcomes of acute ABMR after ITx. The incidence of acute ABMR after ITx was as high as 10.3% in our series, which was closely associated with poor graft and patient survival. Our results indicate that acute ABMR is an important cause of intestinal graft failure, especially in a liver-free allograft and survivors are at an increased risk of developing chronic rejection. Effective strategies to prevent and treat acute ABMR are needed to improve outcomes.
INTRODUCTION
Intestinal transplantation (ITx) has increasingly become a viable option for most patients with irreversible intestinal failure. Short-term patient and graft survival have improved to a great extent due to advances in surgical technology and immunosuppressive management[1,2]. However, long-term outcomes after ITx have been inferior to other solid-organ transplants, especially with less intestinal allograft survival more than 10 years[3,4]. Allograft failure secondary to acute and chronic rejection remain a significant impediment to the success of ITx[5].
Traditionally, intestine transplant rejection has been considered as a T-cell-mediated course that can be effectively controlled with T-cell targeted immunosuppressive agents. Harmful effects of antibodies to human leukocyte antigens (HLA) in intestinal allograft rejection have not been studied thoroughly although these HLA antibodies were often detectable after ITx[6-8]. To date, HLA antibodies are believed to be a major risk factor for hyperacute rejection, acute antibody-mediated rejection (ABMR) and chronic ABMR after kidney or heart transplantation[9]. Several case reports imply that HLA antibodies are also associated with lung, liver, or pancreas allograft dysfunction[10-12]. Increasing data suggest that an early diagnosis and prompt management of acute ABMR are essential for improving patient and graft outcomes[13,14].
The impact of HLA antibodies has got less attention in the assessment of acute intestinal allograft rejection. Similar to other solid-organ transplantation, many patients who require ITx become sensitized and form alloantibodies that originate either from previous exposure to blood products, pregnancies, transplants, and/or infections or de novo formation of donor-specific antibody (DSA) following transplantation[15,16]. In recent years, we and others have shown that the presence of DSA was closely associated with the incidence and severity of intestinal allograft rejection and decreased the overall graft and patient survival[17,18]. Although hyperacute rejection, caused by preformed DSA, rarely occurs in highly sensitized recipients after ITx[19], clinicopathological findings consistent with acute ABMR have increasingly been recognized as an important form of rejection[20,21]. Currently diagnostic standards for acute ABMR after ITx have not been set up yet and its incidence and clinical significance have remained unknown.
The diagnostic standards for acute ABMR in a kidney or heart transplant have been well-established. According to the guidelines, acute ABMR is defined by circulating DSA, C4d deposition, tissue pathology and clinical allograft dysfunction. In this series, we reviewed our institutional experience to identify recipients with acute ABMR that fulfill the criteria for kidney transplantation, and to evaluate the rate, risk factors and consequences after acute ABMR.
MATERIALS AND METHODS
Patient selection
Since August 2003, patients who received small bowel transplants at the University of Pittsburgh Medical Center have started to have a routine serum DSA specificities determinations, by either the purified HLA antigen-based ELISA or the Luminex single-antigen bead analysis. We performed a retrospective electronic medical records review of patients who underwent a small bowel transplant between August 2003 and May 2010. The clinical charts were reviewed as needed for additional data and the Institutional Review Board approved this study.
Donor and recipient demographics are summarized in Table 1. The transplant type consisted of a liver-exclusive transplant (isolated intestine graft and modified multivisceral graft without liver) and a liver-inclusive full multivisceral transplant. T cell complement-dependent lymphocytotoxic cross-match (CDC-XM) was performed by anti-human globulin (AHG)-enhanced method and B cell CDC-XM was performed by extended-incubation/modified Amos technique. In our practice, a positive CDC-XM was not considered as a contraindication to ITx. HLA panel reactive antibody (PRA) was determined by LAT ELISA assay. The HLA antibodies were checked by the purified HLA antigen-based ELISA prior to April 2007 and have since then been replaced by the Luminex single-antigen bead assay. A value of the mean fluorescence intensity (MFI) ≥ 1000 was considered positive. We did not routinely follow up DSA levels post-transplant and indications for DSA monitoring were usually higher PRA levels, refractory rejection, or suspicious of acute ABMR.
Table 1.
Donor and recipient demographic and clinical characteristics
| Characteristic | Transplants (n = 175) |
| Donor characteristics | |
| Age (yr) | 25.4 ± 9.9 |
| Gender (% male) | 77.7 |
| Nonwhite race (%) | 16.6 |
| Cold ischemic time (h) | 7.6 ± 1.5 |
| Recipient characteristics | |
| Age at transplantation (yr) | 43.0 ± 12.5 |
| Gender (% male) | 38.9 |
| Nonwhite race (%) | 5.9 |
| Primary diagnoses, n (%) | |
| Vascular occlusion | 59 (33.7) |
| Crohn’s disease | 34 (19.4) |
| Neoplastic disorders | 28 (16.0) |
| Motility disorders | 21 (12.0) |
| Others | 33 (18.9) |
| Donor/recipient sex mismatches (%) | 56.6 |
| Donor CMV positive/recipient negative (%) | 21.9 |
| Type of graft liver-free/liver-inclusive (%) | 61.1/38.9 |
| Two mismatches in HLA loci A/B/DR (%) | 39.1/82.1/66.9 |
| PRA at transplantation (≥ 10%) Class I (%) | 40 |
| Class II (%) | 26.3 |
| Positive T/B cell cross-match (%) | 25.7 |
| Preformed DSA (%) | 30.3 |
| Retransplantation (%) | 6.7 |
| Induction, n (%) | |
| None | 41 (23.4) |
| Zenapax | 3 (1.7) |
| Thymoglobulin | 7 (4.0) |
| Campath-1H | 124 (70.9) |
| Follow-up (mo; range) | 37.5 ± 22.7 (0.7 to 81.5) |
CMV: Cytomegalovirus; PRA: Panel reactive antibody; HLA: Human leukocyte antigens; DSA: Donor-specific antibody.
The majority of patients underwent induction therapy with alemtuzumab (Campath-1H; Genzyme, Cambridge, MA) (n = 124), administered at day 0 (30 mg each dose) and some patients received antithymocyte globulin (ATG; Genzyme, Cambridge, MA) (n = 7), the IL-2 receptor antagonist basiliximab (Simulect; Novartis, East Hanover, NJ) (n = 3) or no induction therapy (n = 41) during the early period of this study. The basic immunosuppressive regimen was tacrolimus (Prograf; Astellas, Deefield, IL) and steroids. The 12-h trough levels of tacrolimus during the initial six months were targeted at 10-15 ng/mL with Campath-1H or ATG induction therapy, and 15-25 ng/mL with Simulect induction or without any treatment. Maintenance immunosuppression was similar between a positive and negative CDC-XM. All patients with a positive preformed DSA were given a single-dose of intravenous immunoglobulin (IVIG) at 2 g/kg body weight on day of transplantation. A 5-d steroid tapering was also given followed by a 10-20 mg daily dose for at least 6 mo. Recipients with acute ABMR underwent steroid boluses and/or OKT3. No patients were given plasmapheresis or anti-B cell treatment for acute ABMR.
Diagnosis of rejection
Surveillance ileal biopsies were routinely performed twice per week for the first 2 to 3 wk after transplantation and then once a week thereafter, with increased frequency in case of clinical indications. A diagnosis of acute ABMR was based upon the criteria, including: (1) clinical evidence of graft dysfunction; (2) histological evidence of tissue damage (vascular congestion, submucosal hemorrhage, neutrophilic margination, and platelet-fibrin thrombi in the lamina propria microvasculature)[7]; (3) focal (5%-50%) or diffuse (> 50%) linear C4d deposition; and (4) circulating anti-HLA antibodies[9,22,23]. A C4d staining was done on formalin-fixed paraffin-embedded tissue when acute ABMR was clinically or histologically suspected. The histological criteria for diagnosis of acute cellular rejection (ACR) were as described previously[24]. A new rejection episode was defined by newly occurred clinical symptoms and histological evidence of acute rejection with at least 1 normal mucosal biopsy between rejection episodes. A determination of chronic rejection was based upon clinical symptoms and was further confirmed by a full-thickness specimen of partially or totally resected allografts to reveal evidence of vasculopathy and mesenteric lymphoid depletion with mesenteric sclerosis[25].
Statistical analysis
Results are shown as means and ranges, unless otherwise stated. Categorical variables were assessed with the use of the χ2 test or, when appropriate, Fisher’s exact test. Continuous variables were analyzed with the use of the Student’s t-test. Survival time was analyzed with the Kaplan-Meier method and differences were assessed by log-rank test. All data were analyzed using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium).
RESULTS
Diagnosis of acute ABMR after ITx
During the study period, 164 adults underwent 175 consecutive small bowel transplants; 11 (6.7%) patients underwent retransplantation. Donor characteristics, recipient profiles, and perioperative features are summarized in Table 1. We identified 18 cases (10.3%) that fulfilled all the criteria for acute ABMR proposed by the National Conference. Of these, 16 of 164 cases (9.8%) developed acute ABMR after primary transplantation and 2 of 11 cases (18.2%) developed acute ABMR after retransplantation (Figure 1). Recipient age at the time of transplantation was 25.4 ± 9.9 years old and thirteen cases (72.2%) were female. All patients were sensitized to HLA class I (median PRA 78.5%, range 11%-100%) and/or HLA class II antigens (median PRA 67.0%, range 1%-100%). A CDC-XM was positive in 14 (77.8%) recipients, in which anti-donor antibody titer was ≥ 1:8 in 7 cases (50.0%). Three recipients (16.7%) underwent splenectomy at the time of transplantation (2 at primary transplantation and 1 at retransplantation). Recipients developed acute ABMR at a median time of 10.0 d (range, 4-162 d). Fourteen patients presented within 30 d after transplantation (early acute ABMR) and the remaining 4 presented beyond 30 d (late acute ABMR). Fifteen patients developed a single episode of acute ABMR and three developed repeat episodes of ABMR (Table 2).
Figure 1.
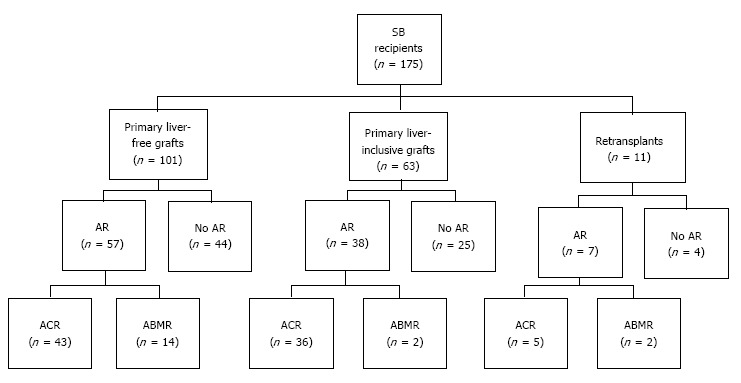
Patient distribution according to graft type and acute rejection type. SB: Small bowel; AR: Acute rejection; ACR: Acute cellular rejection; ABMR: Acute antibody-mediated rejection.
Table 2.
Characteristics of 18 patients with diagnosis of acute antibody-medicated rejection
| Case | Tx type | POD (d) |
XM |
DSA at time of Tx and/or rejection | De novo DSA | Vascular alterations | C4d | #ACR≤ 360 d | |
| T-cell | B-cell | ||||||||
| 1 | MV + K | 4 | 1:32 | 1:16 | A1, A25, B8, B18 | DR51 | ++ | Focal | 0 |
| 2 | SB | 5 | 1:256 | 1:512 | B7, B44, BW4, DQ1, DR10 | DR15, DR51 | +++ | Diffuse | 2 |
| 3 | SB | 5 | 1:2 | 1:2 | B60 | DR16 | ++ | Diffuse | 3 |
| 41 | SB | 6 | 1:8 | 1:8 | B35, B60 | A31, DQ7, DR11 | +++ | Diffuse | 0 |
| 109 | A24, B604 | DR14, DR52 | ++ | Diffuse | |||||
| 5 | SB | 7 | Neg | 1:1 | A3, B18, DR17 | None | ++ | Diffuse | 0 |
| 6 | MV | 7 | Neg | 1:8 | A26, B70, DR52 | None | ++ | Diffuse | 1 |
| 7 | MMV | 9 | 1:32 | 1:8 | A2 | None | +++ | Diffuse | 4 |
| 8 | SB | 10 | Neg | Neg | DR52 | None | ++ | Diffuse | 1 |
| 9 | MMV | 10 | 1:2 | 1:2 | B13, BW4, DR7, DR53 | DQ1 | ++ | Diffuse | 2 |
| 101 | SB + K | 11 | Neg | 1:4 | A32, B8 | A1, DR17 | ++ | Diffuse | 4 |
| 52 | A32, DQ44 | ++ | Diffuse | ||||||
| 11 | SB + P | 14 | Neg | 1:4 | A3, B64 | DQ7 | ++ | Diffuse | 0 |
| 121 | SB | 15 | Neg | Neg | A2, B50 | DQ8, DR53, DR4 | + | Diffuse | 5 |
| 112 | B7, B504 | + | Focal | ||||||
| 13 | SB | 41 | 1:256 | 1:8 | A3, BW4, B53 | DR18 | +++ | Diffuse | 2 |
| 14 | MMV | 84 | 1:4 | 1:1 | A25, B14, B18 | None | ++ | Diffuse | 1 |
| 15 | SB | 140 | Neg | Neg | A24 | A28, B78, A30, DQ7, 9 | + | Diffuse | 1 |
| 16 | SB | 162 | 1:2 | 1:2 | A24, B444 | DQ1, CW5 | ++ | Diffuse | 2 |
| 172 | SB | 4 | Neg | Neg | A28, B78, A30, DQ7, 9 | B44, B58, DR4 | +++ | Diffuse | 2 |
| 183 | MMV | 18 | 1:1 | 1:8 | A11, B7, DR12, DR17, DQ2 | None | ++ | Diffuse | 1 |
Type of transplant: SB: An isolated small bowel; MMV: A modified multivisceral graft; MV: A full multivisceral graft; P: Pancreas; K: Kidney.
Patients with repeat ABMR;
Patient with a history of ABMR after prior transplant;
Patient with prior transplant;
DSA detected at time of rejection. POD: ABMR days post-transplant; XM: Cross-match; DSA: Donor-specific antibody; ACR: Acute cellular rejection.
In all cases, we established the diagnosis of acute ABMR based on the combination of clinical evidence of graft dysfunction, histological findings, and the presence of DSA. Six (37.5%) patients with acute ABMR occurring within a week displayed evidence of graft dysfunction by severe mucosal vascular congestion and diffuse mucosal hemorrhage during endoscopic examination. The other clinical presentation includes fever, abdominal pain or distention, increased stomal output or other non-specific symptoms. The prominent pathological findings in the cases with early ABMR were vascular congestion, focal hemorrhage with focal platelet-fibrin thrombi, and capillary neutrophilic infiltration in the lamina propria and the submucosa. The mucosal biopsies obtained from acute ABMR occurring within a week showed severe vascular congestion along with diffuse mucosal hemorrhage without any evidence of crypt and epithelial injury or apoptosis. These changes gradually returned to normal by 2 to 3 wk in most cases after treatment. Four cases with late ABMR exhibited less prominent vascular congestion and hemorrhage but showed significant fibrin thrombi or neutrophilic margination (Table 2). There was no evidence of any significant vasculitis in the biopsies we evaluated. Seventeen cases showed a diffuse C4d deposition in the lamina propria and the submucosal capillaries (Figure 2). One case with a liver-inclusive transplant showed focal C4d deposition of the intestinal allograft but with significant vascular disturbance. Four of the 18 patients had pure acute ABMR without concomitant ACR within a year and the remaining 14 patients had concomitant ACR either before ABMR (n = 3), at the time of ABMR (n = 4), or after a diagnosis of ABMR (n = 9) (Table 2).
Figure 2.
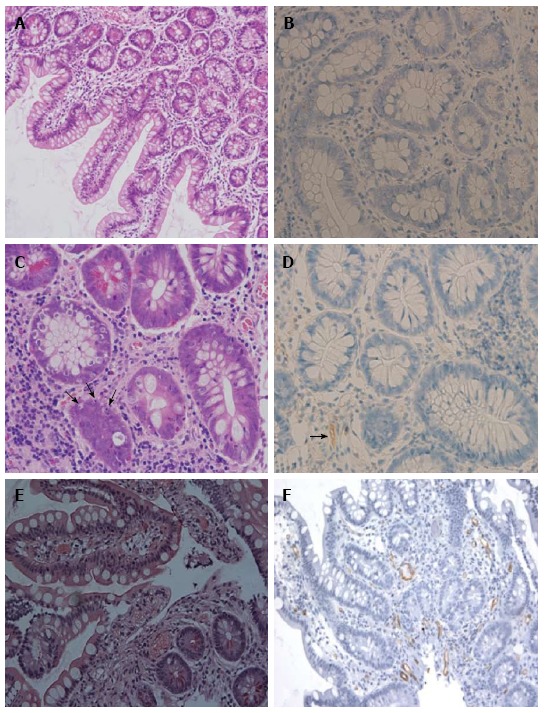
Histopatholgy of intestinal allograft. A and B: No rejection: normal mucosal architecture of small bowel biopsy after transplantation. No staining for C4d is seen in the capillaries of the lamina propria; C and D: Acute cellular rejection (ACR): There is mononuclear infiltration, crypt epithelial injury, and apoptotic bodies (arrows) in the lamina propria. Weak and focal staining for C4d (arrows) is sometimes present in a patient with ACR; E and F: Acute antibody-mediated rejection (ABMR): There is prominent hemorrhage and congestion with scattered fibrin thrombin in the lamina propria. Widespread and bright staining for C4d is present in the capillaries of the lamina propria. Magnifications: × 200 in A, E and F; × 400 in B, C and D. A, C, E: H and E; B, D, F: C4d.
All 18 patients had DSA at the time of transplant: 10 to Class I HLA, 1 to Class II HLA only, and 7 to both Class I and Class II HLA. DSA was persistent in 3 cases at the time of the second episode of acute ABMR (Table 2). These antibodies were detected in fourteen cases by the purified HLA antigen-based ELISA and in the remaining four cases by the Luminex single-antigen bead assay.
Treatment, graft loss and patient death
Our treatment approach evolved over time and the regimen was individualized based on severity of illness, clinical course and response to therapy (Table 3). All patients were initially given intravenous steroids. Thirteen patients required additional OKT3 (n = 10), ATG (n = 1), Campath-1H (n = 1), or Campath-1H followed by OKT3 (n = 1) to reverse acute ABMR.
Table 3.
Treatment and outcome of 18 patients with acute antibody-mediated rejection
| Case | Treatment | Graft status/survival (mo) | Re-Tx/graft type | Patient status/survival (mo) |
| 1 | ST/IVIG/OKT3 | CHR/30.5 | None | Dead (liver failure)/30.5 |
| 2 | ST/IVIG/OKT3/Campath | CHR/13.5 | None | Dead (ruptured pseudo-aneurysm)/18.6 |
| 3 | ST/OKT3 | Functioning/75.9 | None | Alive/75.9 |
| 41 | ST/OKT3 | CHR/5.4 | Yes/MV | Dead (pneumonia)/43.0 |
| 5 | ST/OKT3 | Functioning/17.7 | None | Alive/17.7 |
| 6 | ST/OKT3 | Functioning/56.4 | None | Alive/56.4 |
| 7 | ST/OKT3/Campath | ACR/31.7 | None | Dead (pneumonia)/31.7 |
| 8 | ST/Campath | Functioning/30.3 | None | Dead (unknown)/30.3 |
| 9 | ST | CHR/35.4 | Yes/MV | Alive/55.4 |
| 101 | ST/OKT3 | ACR/13.2 | None | Dead (sepsis)/15.5 |
| 11 | ST | Functioning/52.6 | None | Alive/52.6 |
| 121 | ST/ATG | CHR/22.6 | None | Alive/22.6 |
| 13 | ST | AHR/2.7 | Yes/MV | Alive/76.4 |
| 14 | ST | Functioning/22.5 | None | Alive/22.5 |
| 15 | ST/OKT3 | CHR/4.8 | None | Dead (sepsis)/32.8 |
| 16 | ST | CHR/12.3 | None | Alive/46.2 |
| 172 | ST/OKT3 | CHR/12.6 | Yes/SB | Dead (GI bleeding)/13.3 |
| 183 | ST/OKT3 | Functioning/37.6 | Yes/MV | Alive/37.6 |
Type of transplant: SB: An isolated small bowel; MV: A full multivisceral graft.
Patients with repeat ABMR;
Patient with a history of ABMR after prior transplant;
Patient with prior transplant. ST: Steroids; IVIG: Intravenous immunoglobulin; ACR: Acute cellular rejection; CHR: Chronic rejection.
During the study period, post-transplant HLA antibodies were checked in 158 (90.3%) cases. De novo DSA was detected in twelve of the 18 patients (66.7%): 7 to Class II HLA, 5 to both Class I and Class II HLA. The presence of de novo DSA was markedly higher in the cases with acute ABMR compared to 7.6% (5 of 66) in the cases without rejection (P < 0.0001) or 21.6% (16 of 74) in the cases with ACR (P < 0.001). Graft failure occurred in 12 (66.7%) of the 18 patients with acute ABMR. The causes of graft loss were chronic rejection in 8 cases, severe ACR in 2, persistent ABMR in 1, and unknown etiology in 1 (Table 3). Nine cases underwent enterectomy due to rejection. Of those, de novo DSA was detectable in 7 cases prior to enterectomy and was persistent after graft removal. Two cases had undetectable levels of de novo DSA by the ELISA assay before enterectomy but became detectable after enterectomy. The presence of a newly-formed DSA was closely associated with graft loss (P < 0.0001). Compared with no rejectors, intestinal graft survival was significantly lower in patients with acute ABMR (P = 0.0001) or ACR (P = 0.0009). Graft survival was lower in acute ABMR than in ACR but the differences between them did not reach statistical significance (P = 0.088) (Figure 3A). Patient survival was worse in acute ABMR or ACR than in no rejectors (P = 0.0264). There were no statistical differences in patient survival between ABMR and ACR (Figure 3B).
Figure 3.
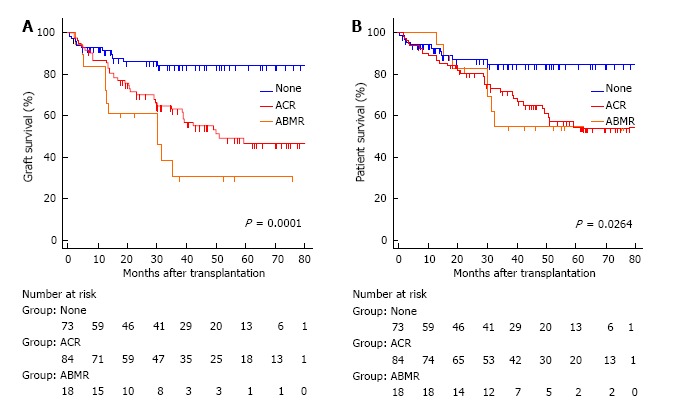
The Kaplan-Meier graft (A) and patient (B) survival for no acute rejection (none) (solid line), acute cellular rejection (dotted heavy line), and acute antibody-mediated rejection (dotted light line). The overall comparison was significantly different in graft (log-rank P = 0.0001) and patient survival (log-rank P = 0.0264). The patients with antibody-mediated rejection (ABMR) or acute cellular rejection (ACR) had significantly lower graft and patient survival than those without rejection. The graft survival was worse in ABMR than in ACR but the differences between them did not reach statistical significance (P = 0.088).
A total of five patients underwent repeat transplants, including a liver-inclusive graft in 4 and a liver-free intestinal graft in 1 patient. Of those, three patients had acute ABMR after primary transplantation and two patients had acute ABMR after retransplant. Four patients underwent repeat transplants after a diagnosis of acute ABMR and one with prior history of a liver-free graft developed acute ABMR after a liver-inclusive retransplant.
At an average follow-up of 32.3 mo (range, 13.3-76.4 mo), eight of the 18 (44.4%) patients died. The causes of patient death were sepsis in 4, massive gastrointestinal bleeding in 2, chronic liver failure in 1 and unknown etiology in 1 (Table 3). Patient 1 with a positive CDC XM developed acute ABMR in the intestinal allograft 4 d after a liver-inclusive intestine combined with kidney transplant. He responded well with a combination of steroids, IVIG and OKT3 therapies. One year after transplantation, he had progressively elevated liver enzymes with circulating de novo DSA and he subsequently died due to chronic liver failure. In three patients undergoing liver-inclusive retransplants after primary graft loss, one patient died secondary to Aspergillus pneumonia, while the other three patients were alive with a well-functioning graft at the time of last follow-up. Two patients (#17 and #18) with a prior history of primary graft loss due to rejection developed acute ABMR after retransplantation. Patient 17 had acute ABMR 4 d after a liver-free retransplant and soon developed chronic rejection within a year with persistent de novo DSA and subsequently died due to massive lower GI bleeding. Patient 18 had acute ABMR 18 d after liver-inclusive retransplant, which was successfully treated with steroids and OKT3. The higher levels of preformed DSA gradually declined in this case after transplantation and she was well with functioning graft with no evidence of de novo DSA by the Luminex assay at the time of the last follow-up.
Risk factors for acute ABMR
In the univariate analysis, younger recipients at the time of transplant, liver-free graft, and the presence of DSA were closely related to acute ABMR. The presence of the spleen in recipients tended to be associated with acute ABMR but no statistical significance was observed (P = 0.071) (Table 4). In the multivariate analysis, only the presence of DSA and a liver-free graft were significantly associated with the development of acute ABMR (Table 5). Donor age, gender, cold ischemic time, cytomegalovirus donor and recipient serology, HLA mismatches, previous transplants, and induction therapy were not significantly associated with acute ABMR, and their inclusion in the multivariate model did not exclude liver-free grafts or the presence of DSA as independent risk factors.
Table 4.
Pretransplant risk factors for acute antibody-mediated rejection (univariate analysis)
| Variables | Non-ABMR (n = 157) | ABMR (n = 18) | OR | 95%CI | P |
| Donor age (yr) | 25.6 ± 10.2 | 24.0 ± 6.5 | 0.98 | 0.93-1.04 | 0.549 |
| Female donor, n (%) | 33 (20.8) | 6 (37.5) | 0.44 | 0.15-1.29 | 0.133 |
| Cold ischemic time (h) | 7.72 ± 1.52 | 7.58 ± 1.11 | 0.94 | 0.66-1.33 | 0.711 |
| Recipient age | 43.7 ± 12.4 | 36.9 ± 12.0 | 0.96 | 0.92-0.99 | 0.028 |
| Female recipient, n (%) | 94 (59.8) | 13 (72.2) | 0.57 | 0.19-1.69 | 0.299 |
| Donor CMV positive/recipient negative, n (%) | 34 (21.4) | 4 (25.0) | 1.12 | 0.69-1.81 | 0.642 |
| Donor/recipient sex mismatches, n (%) | 88 (55.3) | 11 (68.7) | 1.78 | 0.59-5.35 | 0.308 |
| HLA mismatches ≥ 4, n (%) | 107 (67.3) | 12 (75.0) | 1.46 | 0.45-4.74 | 0.531 |
| Prior transplant, n (%) | 9 (5.7) | 2 (12.5) | 2.38 | 0.47-2.12 | 0.296 |
| Campath-1H induction, n (%) | 113 (71.1) | 12 (75.0) | 0.72 | 0.30-1.73 | 0.468 |
| Liver-free graft, n (%) | 92 (58.5) | 15 (83.3) | 3.53 | 1.08-12.7 | 0.031 |
| Presence of spleen, n (%) | 99 (63.1) | 15 (83.3) | 2.93 | 0.81-10.55 | 0.071 |
| Anti-HLA antibodies | |||||
| Positive CDC-XM, n (%) | 30 (19.1) | 14 (77.8) | 21.17 | 5.76-77.81 | < 0.0001 |
| PRA I ≥ 10%, n (%) | 53 (33.8) | 18 (100) | 33.36 | 4.32-257.52 | < 0.0001 |
| PRA II ≥ 10%, n (%) | 32 (20.4) | 14 (77.8) | 13.67 | 4.21-44.36 | < 0.0001 |
| Presence of DSA, n (%) | 37 (23.6) | 18 (100) | 55.14 | 7.09-428.38 | < 0.0001 |
ABMR: Antibody-mediated rejection; CMV: Cytomegalovirus; CDC-XM: Complement-dependent lymphocytotoxic cross-match; PRA: Panel reactive antibody; DSA: Donor-specific antibody.
Table 5.
Pretransplant risk factors for acute antibody-mediated rejection (multivariate analysis)
| Variables | OR | 95%CI | P |
| Liver-free graft | 8.791 | 2.011-38.480 | 0.004 |
| PRA class I | 16.302 | 3.092-85.801 | 0.001 |
| PRA class II | 6.023 | 1.490-24.253 | 0.012 |
PRA: Panel reactive antibody.
DISCUSSION
We believe that the established diagnostic standards for acute ABMR in kidney or heart transplants can help identify acute ABMR after ITx. We found the incidence of acute ABMR of the intestinal allograft is 10.3%, on the basis of the presence of circulating DSA, evidence of C4d deposit, acute tissue injury, and clinical graft dysfunction. To our knowledge, this is the first largest series of investigation to date to retrospectively assess the incidence of acute ABMR after ITx. The most important finding in this study was that acute ABMR is closely associated with increased graft loss and poor outcomes. Our rate of intestinal graft acute ABMR is comparable to the incidence of acute ABMR reported in the kidney transplant that ranges from 7.7% to 41%, depending on the level of pre-transplant recipient sensitization status[9,26,27]. Given the frequency and poor prognosis of acute ABMR after ITx, every effort should be made to set up or eliminate this diagnosis in the setting of graft dysfunction to more specifically direct immunosuppressive management.
C4d deposition along graft capillaries has become a critical component to the diagnosis of acute AB MR in a kidney or heart transplant. However, the clinical relevance of a positive C4d staining in an intestinal allograft remains uncertain. Post-transplant microvascular lesions in a small intestinal allograft at early time periods might be related to higher pre-transplant PRA levels or a positive CDC-XM[7]. Other studies concluded that C4d deposition had no clinical significance when assessing acute ABMR in a small bowel allograft[28,29]. Unfortunately these studies either did not correlate with the HLA antibody levels or they did not detect DSA in small-sized heterogeneous populations. Therefore, the above studies did not have sufficient evidence to include or exclude C4d as a useful marker to detect acute ABMR in intestinal allografts. Our previous publications showed that a diffuse C4d deposition was very common in CDC-XM positive recipients with the presence of DSA, while focal and trace C4d deposition was often seen in CDC-XM negative recipients in the setting of no histological evidence of ACR or evidence of ACR but in absence of DSA[18]. Our current study further demonstrates that a diffuse C4d deposition is strongly associated with vascular disturbances after ITx, indicating that it is a useful marker for a diagnosis of ABMR after ITx. Our results suggest that a diffuse C4d staining, in conjunction with the presence of DSA, clinicopathological findings and significant clinical improvement after initial treatment, strongly supports a diagnosis of acute ABMR. The clinical relevance of focal and weak C4d staining in an intestinal allograft should be further evaluated in future studies. We suggest that a C4d staining be routinely included in intestinal biopsies in sensitized recipients or in the setting o f appearance of a newly-formed DSA after ITx.
The rate of pretransplant sensitization in our current study was 30.3%, higher than 10%-15% in kidney or heart transplant recipients, indicating that intestinal recipients are an high immunological risk group. The causes of sensitization may be from previous operations, multiple blood transfusions, infections, pregnancies or retransplantation. Sensitization increases the risk of a positive CDC-XM and is associated with rejection and poor outcomes. Our results further showed that a positive CDC-XM significantly increases the risk of acute ABMR and is closely associated with graft loss, particularly in a liver-free transplant recipient. In the setting of anti-donor antibody titer ≥ 1:8, all four recipients lost grafts early on after a liver-free transplant. Similar to other solid organ transplants, our findings confirmed a close association between preformed DSA and acute ABMR, indicating that preformed DSA is a prerequisite for the occurrence of acute ABMR after ITx. In our series the majority of patients had preformed class I DSA prior to transplantation, often directed at the A, B locus, whereas the majority of de novo DSA post-transplant were against class II, which were often associated with late graft failure. The mechanisms underlying the difference between class I and class II in this clinical setting is unknown. Based on our results, we suggest special attention should be paid to recipients with high immunological risk in terms of implementing pre-transplant desensitization strategies, avoiding positive cross-match transplantation, increasing maintenance immunosuppression, and frequently monitoring DSA post-transplant.
In our series, younger recipient age was associated with acute ABMR, but this significance no longer exist when we adjusted for other factors. Our analysis identified a liver-free transplant as an independent risk factor for the occurrence of acute ABMR after ITx. Our previous paper demonstrated that the liver is relatively insensitive to antibody-mediated damage and the inclusion of a liver graft with the intestine appears to be protective in recipients of high immunological risk[30]. In contract to a liver-free graft, no or only a single mild episode of rejection occurred in three highly sensitized recipients after a liver-inclusive transplant. Our findings further confirmed that the liver as a component of multivisceral transplants might ameliorate or prevent early and late intestinal allograft loss. A liver-inclusive transplant may offer a better long-term patient and graft survival in immunological high-risk recipients. As optimized approaches for depleting HLA antibodies have not yet been set up in ITx, the use of a liver-inclusive graft may be a valuable option in highly sensitized recipients, especially in the setting of retransplantation after primary graft loss due to rejection.
Despite the initial clinical improvement in many patients, long-term outcomes were dismal because of a high incidence of chronic rejection. In this series, although a combination of steroids and T-cell targeted OKT3 achieved the initial resolution after a diagnosis of acute ABMR, the majority of grafts failed due to subsequent severe ACR or chronic rejection. Clearly, additional studies are required to identify effective strategies to control acute ABMR. The antibody-directed regimens, such as IVIG and anti-CD20, should be routinely implemented in highly sensitized recipients prior to transplantation. A new therapy, such as the proteasome inhibitor “bortezomab” and complement-targeted treatment with C1 or C5 inhibitor, has yielded encouraging preliminary results, but the long-term efficacy and safety remain to be seen.
Our study has several important limitations, including its retrospective nature, inconsistent antibody detection methods, and experience at a single institution. Although we identified 18 cases among 175 transplants over a 7-year period, the true incidence of acute ABMR may be higher after ITx. It is likely that less severe cases were unrecognized due to our evolving antibody detection methods, lack of standardized definition, and our unawareness of the importance of acute ABMR during the early study period. An additional important limitation of this study is that C4d staining of biopsies was not routinely performed in recipients with ACR to evaluate its sensitivity and specificity. Furthermore, the lack of consistent DSA monitoring post-transplantation limits our ability to assess sub-clinical acute ABMR. However, we sought to characterize a convincing series of cases to develop a preliminary definition that may serve as a foundation for future studies.
Our results indicate that acute ABMR is an important cause of intestinal graft dysfunction, particularly in a liver-exclusive transplant. After acute ABMR, patients are at an increased risk of developing refractory acute rejection and chronic rejection. Preventive and effective therapeutic approaches are needed to manage acute ABMR in intestinal transplant recipients.
ACKNOWLEDGMENTS
The authors would like to thank the surgical team (Kareem M Abu-Elmagd, Guilherme Costa, Geoffrey J Bond, Kyle Soltys, and George Mazariegos), the nursing staff at the Intestinal Rehabilitation and Transplant Center, the University of Pittsburgh Medical Center, for their excellent patient care. The authors thank Mr. Yinglun Wu for help with English grammar.
COMMENTS
Background
Antibody-mediated rejection (ABMR) is the major cause of kidney transplant failure. The incidence and clinical significance of acute ABMR after intestinal transplantation (IATx) remain unknown.
Research frontiers
ABMR has increasingly become an important area for research and clinical investigation. The aim of this study aimed to assess the incidence, risk factors and clinical outcomes of acute ABMR after ITx.
Innovations and breakthroughs
This is the first largest series of study to retrospectively examine the incidence of acute ABMR after ITx. The incidence of acute ABMR after ITx is 10.3% in the series. Both a liver-free graft and a high level of panel reactive antibody were identified as a significant independent risk factor for acute ABMR. Without appropriate management, acute ABMR was closely associated with increased graft loss and poor clinical outcomes.
Applications
The results suggest that acute ABMR must be included in the differential diagnosis of acute rejection after ITx. The prevention of acute ABMR should include desensitization, avoiding a positive cross-match donor, and considering the liver as part of an intestinal graft in highly sensitized recipients. Future studies are required to develop the optimal approaches to managing acute ABMR in ITx recipients.
Terminology
Acute ABMR is identified on the basis of circulating donor-specific antibody, C4d deposition, tissue injury and clinical allograft dysfunction after ITx.
Peer-review
This is a well written, well designed retrospective review study on acute ABMR after intestinal transplantion, put through an electronic analysis of medical records of 18 patients diagnosed with ABMR out of 175 liver-free small bowel and modified multivisceral graft transplantations, during a 7-year period.
Footnotes
Institutional review board statement: The University of Pittsburgh Medical Center Institutional Review Board approved this study.
Informed consent statement: Patients were not required to give informed consent because of observational, retrospective nature of the study.
Conflict-of-interest statement: The author declares no conflict of interests for this article.
Data sharing statement: No additional data available.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 23, 2016
First decision: September 28, 2016
Article in press: November 23, 2016
P- Reviewer: Kouraklis G, Maric I S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361:998–1008. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, Koritsky DA, Schuster B, Martin L, Cruz RJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 2009;250:567–581. doi: 10.1097/SLA.0b013e3181b67725. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Wu G, Qing A, Everly M, Cheng E, Terasaki P. Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients 2014 Data Report: Intestine. Clin Transpl. 2014:33–47. [PubMed] [Google Scholar]
- 4.Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210–219. doi: 10.1111/ajt.12979. [DOI] [PubMed] [Google Scholar]
- 5.Sudan D. The current state of intestine transplantation: indications, techniques, outcomes and challenges. Am J Transplant. 2014;14:1976–1984. doi: 10.1111/ajt.12812. [DOI] [PubMed] [Google Scholar]
- 6.Bond G, Reyes J, Mazariegos G, Wu T, Schaefer N, Demetris J, Fung JJ, Starzl TE, Abu-Elmagd K. The impact of positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival. Transplant Proc. 2000;32:1197–1198. doi: 10.1016/s0041-1345(00)01181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz P, Garcia M, Pappas P, Berney T, Esquenazi V, Kato T, Mittal N, Weppler D, Levi D, Nishida S, et al. Mucosal vascular alterations in isolated small-bowel allografts: relationship to humoral sensitization. Am J Transplant. 2003;3:43–49. doi: 10.1034/j.1600-6143.2003.30108.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Abu-Elmagd K, Bond G, Demetris AJ. A clinicopathologic study of isolated intestinal allografts with preformed IgG lymphocytotoxic antibodies. Hum Pathol. 2004;35:1332–1339. doi: 10.1016/j.humpath.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller GG, Destarac L, Zeevi A, Girnita A, McCurry K, Iacono A, Murray JJ, Crowe D, Johnson JE, Ninan M, et al. Acute humoral rejection of human lung allografts and elevation of C4d in bronchoalveolar lavage fluid. Am J Transplant. 2004;4:1323–1330. doi: 10.1111/j.1600-6143.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 11.Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA, Andreoni KA. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006;6:3022–3029. doi: 10.1111/j.1600-6143.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 12.Melcher ML, Olson JL, Baxter-Lowe LA, Stock PG, Posselt AM. Antibody-mediated rejection of a pancreas allograft. Am J Transplant. 2006;6:423–428. doi: 10.1111/j.1600-6143.2005.01185.x. [DOI] [PubMed] [Google Scholar]
- 13.Amore A. Antibody-mediated rejection. Curr Opin Organ Transplant. 2015;20:536–542. doi: 10.1097/MOT.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 14.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Lunz J, Costa G, Bond G, Cruz R, Soltys K, Mazariegos G, Sindhi R, Zeevi A, Abu-Elmagd KM. Donor specific human leukocyte antigen (HLA) antibodies and allograft survival after intestinal transplantation. Am J Transplant. 2010;10 Suppl 4:S162. [Google Scholar]
- 16.Hawksworth JS, Rosen-Bronson S, Island E, Girlanda R, Guerra JF, Valdiconza C, Kishiyama K, Christensen KD, Kozlowski S, Kaufman S, et al. Successful isolated intestinal transplantation in sensitized recipients with the use of virtual crossmatching. Am J Transplant. 2012;12 Suppl 4:S33–S42. doi: 10.1111/j.1600-6143.2012.04238.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsai HL, Island ER, Chang JW, Gonzalez-Pinto I, Tryphonopoulos P, Nishida S, Selvaggi G, Tekin A, Moon J, Levi D, et al. Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation. Transplantation. 2011;92:709–715. doi: 10.1097/TP.0b013e318229f752. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Elmagd KM, Wu G, Costa G, Lunz J, Martin L, Koritsky DA, Murase N, Irish W, Zeevi A. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 2012;12:3047–3060. doi: 10.1111/j.1600-6143.2012.04237.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz P, Carreno M, Weppler D, Gomez C, Island E, Selvaggi G, Nishida S, Moon J, Levi D, Tekin A, et al. Immediate antibody-mediated (hyperacute) rejection in small-bowel transplantation and relationship to cross-match status and donor-specific C4d-binding antibodies: case report. Transplant Proc. 2010;42:95–99. doi: 10.1016/j.transproceed.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Dick AA, Horslen S. Antibody-mediated rejection after intestinal transplantation. Curr Opin Organ Transplant. 2012;17:250–257. doi: 10.1097/MOT.0b013e3283533847. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz P. Updates on acute and chronic rejection in small bowel and multivisceral allografts. Curr Opin Organ Transplant. 2014;19:293–302. doi: 10.1097/MOT.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 22.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas M. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2014;19:315–322. doi: 10.1097/MOT.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Abu-Elmagd K, Bond G, Nalesnik MA, Randhawa P, Demetris AJ. A schema for histologic grading of small intestine allograft acute rejection. Transplantation. 2003;75:1241–1248. doi: 10.1097/01.TP.0000062840.49159.2F. [DOI] [PubMed] [Google Scholar]
- 25.Lee RG, Nakamura K, Tsamandas AC, Abu-Elmagd K, Furukawa H, Hutson WR, Reyes J, Tabasco-Minguillan JS, Todo S, Demetris AJ. Pathology of human intestinal transplantation. Gastroenterology. 1996;110:1820–1834. doi: 10.1053/gast.1996.v110.pm8964408. [DOI] [PubMed] [Google Scholar]
- 26.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 27.Mauiyyedi S, Colvin RB. Humoral rejection in kidney transplantation: new concepts in diagnosis and treatment. Curr Opin Nephrol Hypertens. 2002;11:609–618. doi: 10.1097/00041552-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Troxell ML, Higgins JP, Kambham N. Evaluation of C4d staining in liver and small intestine allografts. Arch Pathol Lab Med. 2006;130:1489–1496. doi: 10.5858/2006-130-1489-EOCSIL. [DOI] [PubMed] [Google Scholar]
- 29.de Serre NP, Canioni D, Lacaille F, Talbotec C, Dion D, Brousse N, Goulet O. Evaluation of c4d deposition and circulating antibody in small bowel transplantation. Am J Transplant. 2008;8:1290–1296. doi: 10.1111/j.1600-6143.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Cruz RJ. Liver inclusion improves outcomes of intestinal retransplantation in adults. [Corrected] Transplantation. 2015;99:1265–1272. doi: 10.1097/TP.0000000000000488. [DOI] [PubMed] [Google Scholar]


