Abstract
AIM
To identify the outcome measures that have been used in randomized controlled trials (RCTs) of exercise training in solid organ transplant (SOT) recipients and to link these outcomes to the International Classification of Functioning, Disability and Health (ICF) framework.
METHODS
Electronic literature searches of MEDLINE, EMBASE, CINAHL, Cochrane, Scopus, and Web of Science were performed. We sought RCTs that investigated the effect of exercise training in SOT recipients. Reference lists of all eligible publications were searched for other appropriate studies not identified by the electronic search. A complete list of outcome measures used in the RCTs was generated and each of these was linked to an ICF category.
RESULTS
Four hundred and thirteen articles were retrieved, of which 35 met our inclusion criteria. The studies included were designed to compare the effects of exercise training programs to usual care or to another exercise training program and reported on recipients of heart (n = 21), kidney (n = 9), lung (n = 3) or liver (n = 2) transplant. Of the 126 outcome measures identified, 62 were used as primary outcome measures. The most commonly occurring primary outcomes were aerobic capacity using the peak VO2 (n = 13), quality of life using the short-form-36 (n = 8), and muscle strength (n = 7). These outcome measures were linked to 113 ICF categories and the majority of outcomes fall into the body function domain (n = 93).
CONCLUSION
There is little standardization in outcome measures used in RCTs of exercise interventions in SOT recipients. The ICF framework can be used to select a core set of outcomes that cross all domains of ICF and that would be appropriate to all SOT recipients.
Keywords: Solid organ transplantation; Systematic review; Rehabilitation; Exercise; Outcome measures; International Classification of Functioning, Disability and Health
Core tip: Over 30 randomized controlled trials (RCTs) have been conducted to examine the effectiveness of exercise training on outcomes in solid organ transplant recipients. However, the synthesis of findings across studies has been limited by the lack of similar outcomes. We identified 126 unique outcomes used in RCTs of exercise training and categorized them according to the International Classification of Functioning, Disability and Health framework. Most commonly, outcomes fell into the domains of body structure and body function, whereas there were a limited number of outcomes examining activities and participation. This review highlights the need for a core set of outcomes for RCTs in exercise training for this population.
INTRODUCTION
As the acute morbidity and mortality associated with solid organ transplantation continues to improve, interventions that improve quality of life and long-term health outcomes are needed. Exercise training has several important health benefits for solid organ transplant (SOT) recipients, such as improving maximal aerobic capacity (VO2 peak), body composition and quality of life[1]. Exercise and physical activity also have potential effects for mitigating long-term complications post-transplant and side-effects of immunosuppressant medication such as reducing blood pressure, controlling blood glucose[2], managing weight gain[3], improving muscle[4] and bone strength[5], and reducing fatigue[6-8]. A limitation of the current literature on exercise for SOT is the inability to combine outcomes from studies due to the wide range of reported outcomes. In a systematic review of exercise training in SOT recipients conducted in 2012 by Didsbury et al[1], the authors included 15 randomized controlled trials (RCTs) with 28 unique outcomes. The majority of outcomes were related to cardiovascular parameters (VO2 peak, blood pressure, cholesterol), with fewer studies examining body composition, frailty indicators or quality of life. The authors were therefore hampered in their ability to conduct meta-analyses, which limited the conclusions of their comprehensive review.
The inability to synthesize data from studies in the field of SOT is of particular concern, as this is a small population and studies on exercise training are often conducted at single transplant centres with relatively small sample sizes. In order to gain greater statistical power to draw conclusions, studies need to be combined using knowledge synthesis approaches, which require common outcomes. Inconsistencies in the reporting of outcomes can affect the conclusions of systematic reviews and may contribute to reporting bias[9]. Therefore, in order to facilitate standard reporting of key outcomes across studies, the development of core outcomes sets for clinical trials is gaining more attention[10,11].
The International Classification of Functioning, Disability and Health (ICF) is an established framework developed by the World Health Organization and is commonly used in rehabilitation. The ICF is designed to describe health and health-related status from biological, personal and societal perspectives[12]. The framework classifies human function into four domains: Body functions; body structures; activities and participation; and environmental factors[12]. These domains match well with the goals of exercise training and physical rehabilitation programs; specifically to identify, measure and treat physical impairments (body function and structure); to reverse or normalize activity limitations; and to enhance participation in all settings[13]. Using the ICF to map the outcomes of the current literature on exercise training in SOT recipients will assist in classifying the breadth of outcomes that have been used in the studies to date and also in identifying any domains that are understudied in this population. This information can provide a starting point for developing a core set of standard outcomes[10] for clinical trials of exercise and physical rehabilitation in SOT recipients.
The objectives of this systematic review were to identify the outcome measures that have been used in RCTs of exercise training in SOT recipients and to link these outcomes to the ICF framework.
MATERIALS AND METHODS
Data sources and search strategy
This systematic review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement[14]. A librarian designed and performed electronic literature searches of Medline from inception until May 2016. The search was then adapted for EMBASE, CINAHL, Cochrane, Scopus, and Web of Science and run on these databases. Search terms included organ transplantation, transplant recipients, graft recipient, heart, lung, kidney, pancreas, liver, exercise, exercise therapy, rehab, rehabilitation, resistance training, physical education, training, physical activity, and physical exertion (Table 1). The searches were limited to RCTs, published in English, and in humans. One investigator (Stacey Konidis) also conducted hand searches of the reference lists of all the studies that met the inclusion criteria to identify additional relevant articles.
Table 1.
Electronic search strategy used in MEDLINE
| Search # | Keywords and number of records identified |
| Search #1 | Organ transplantation (110179) |
| Search #2 | Transplantation conditioning (7738) |
| Search #3 | Transplant recipients (195) |
| Search #4 | “Transplant recipient$” (27594) |
| Search #5 | 1 or 2 or 3 or 4 (122169) |
| Search #6 | Exercise/or Exercise Therapy/or exercise$ (192344) |
| Search #7 | Rehab$/or rehabilitation (151761) |
| Search #8 | Resistance training/or “physical education and training”/or training (181282) |
| Search #9 | “Physical activity” (47446) |
| Search #10 | Physical exertion (11451) |
| Search #11 | 6 or 7 or 8 or 9 or 10 (474657) |
| Search #12 | 5 and 11 (2399) |
| Search #13 | Heart or lung or kidney or pancreas or liver (1433618) |
| Search #14 | 12 and 13 (2200) |
| Search #15 | Limit 14 to humans (2156) |
| Search #16 | Limit 14 to animals (76) |
| Search #17 | 15 not 16 (2121) |
| Search #18 | Limit 17 to randomized controlled trial (60) |
Criteria for including studies in the review
We selected all RCTs that investigated the effect of exercise training in SOT recipients. We included trials that compared the effects of exercise training programs to standard care as well as trials that compared two or more different exercise training programs in SOT recipients. In the case of multiple publications of the same study, we considered all of them if the outcomes measures were different. We excluded studies that did not have an isolated exercise intervention group (i.e., those that examined the effect of a drug combined with exercise). We also excluded non-English articles and conference abstracts. One investigator (Stacey Konidis) reviewed the study titles and abstracts to determine potential study eligibility. When this investigator was uncertain, a second reviewer (Tania Janaudis-Ferreira) was consulted. Two investigators independently reviewed the full texts of the articles to determine eligibility (Stacey Konidis and Tania Janaudis-Ferreira).
Data extraction and synthesis
Two reviewers (Stacey Konidis and Cecile Beaurepaire) performed the data extraction and tabulation. A third reviewer (Tania Janaudis-Ferreira) double-checked the extracted data. Outcome measures were abstracted using a standard form and imported into a spreadsheet, sorted into primary and secondary outcomes and classified according to four domains of the ICF (body functions, body structures, activities and participation, and environmental factors). Information about the exercise interventions and patient populations were also retrieved. Considering the purpose of this review, study quality or risk of bias assessments of the included studies were not deemed to be necessary.
RESULTS
Literature search
The electronic and hand searches led to the identification of 522 articles. After excluding 109 duplicates, there were 413 articles left for title and abstract screening. Following the study title and abstract screening, 366 were considered to be unrelated to the objectives of the review. Of the 47 articles that remained for full-text analysis, 12 were excluded. This left a total of 35[2-5,15-45] articles for inclusion in this review. The study flow and reasons for exclusion are shown in Figure 1.
Figure 1.
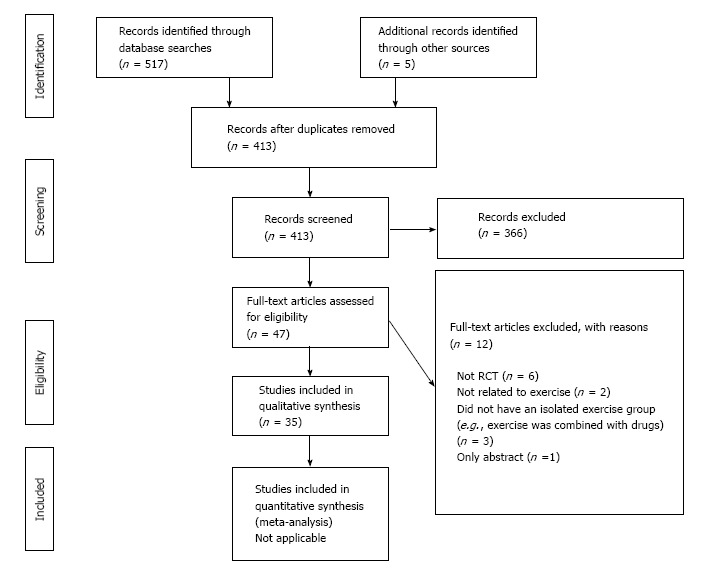
PRISMA 2009 flow diagram. From: Moher et al[14]. For more information, visit www.prisma-statement.org.
Review of studies and outcome domains assessed
The studies included were designed to compare the effects of exercise training programs to usual care or to another exercise training program and reported on transplantation of heart (n = 21), kidney (n = 9), lung (n = 3), and liver (n = 2). A total of 1313 patients were randomized in the 35 studies. Description of the exercise programs and other details about the studies is presented in Table 2.
Table 2.
Description of studies
| Ref. | Country | Year | Organ | Time-post transplant (wk) | Treatment duration (wk) | Randomized patients1 | Exercise intervention | Comparison |
| Braith et al[5] | United States | 1996 | Heart | > 8 | 24 | 16 | Lumbar extension 1 d/wk; variable resistance exercises 2 d/wk | Usual care |
| Braith et al[4] | United States | 1998 | Heart | > 8 | 24 | 162 | Lumbar extension 1 d/wk; variable resistance exercises 2 d/wk | Usual care |
| Kobashigawa et al[15] | United States | 1999 | Heart | > 2 | 26 | 27 | Individualized cardiac rehabilitation (strengthening, flexibility, and moderate aerobic exercises) 1-3 d/wk | Usual care (unstructured therapy at home) |
| Painter et al[16] | United States | 2002 | Kidney | 4-8 | 48 | 167 | Independent home-based exercise 4 d/wk | Usual care |
| Mitchell et al[17] | United States | 2003 | Lung | > 8 | 26 | 16 | Lumbar extension resistance exercise 1 d/wk and walking program | Usual care (walking program) |
| Painter et al[18] | United States | 2003 | Kidney | > 4 | 48 | 96 | Independent home-based exercise 4 d/wk | Usual care |
| Braith et al[19] | United States | 2005 | Heart | > 8 | 24 | 15 | Variable resistance exercises 2 d/wk | Usual care |
| Juskowa et al[20] | Poland | 2006 | Kidney | > 0.5 | 4-5 | 69 | Strength exercise training 7 d/wk | Usual care |
| Krasnoff et al[3] | United States | 2006 | Liver | > 8 | 40 | 151 | Cardiovascular exercise training 3 d/wk | Usual care |
| Bernardi et al[21] | Italy | 2007 | Heart | > 24 | 24 | 26 | Stationary bicycle; 30 min/5 d per week | Usual care |
| Karapolat et al[22] | Turkey | 2007 | Heart | Mean 14-17 | 8 | 38 | Hospital-based exercise program (flexibility, stretching, aerobic, strengthening, breathing, relaxation) 3 d/wk | Home-based exercise program (flexibility, stretching, aerobic, strengthening, breathing, relaxation) 3 d/wk |
| Braith et al[23] | United States | 2008 | Heart | > 8 | 12 | 20 | Aerobic treadmill exercise | Usual care |
| Karopola et al[24] | Turkey | 2008 | Heart | Mean 14-17 | 8 | 383 | Hospital-based exercise program (flexibility, stretching, aerobic, strengthening, breathing, relaxation) 3 d/wk | Home-based exercise program (flexibility, stretching, aerobic, strengthening, breathing, relaxation) 3 d/wk |
| Pierce et al[25] | United States | 2008 | Heart | > 8 | 12 | 20 | Aerobic exercise training | Usual care |
| Wu et al[26] | Taiwan | 2008 | Heart | > 52 | 8 | 37 | Resistance and aerobic training 3 d/wk | Usual care |
| Haykowsky et al[27] | Canada | 2009 | Heart | > 26 | 12 | 23 | Aerobic 5 d/wk and strength training 2 d/wk | Usual care |
| Mandel et al[28] | United States | 2009 | Liver | 6-12 | 12 | 50 | Targeted lower body resistance strengthening exercise 3-4 d/wk | Usual care (walking program) |
| Hermann et al[29] | Denmark | 2011 | Heart | > 52 | 8 | 27 | Aerobic interval training program 3 d/wk | Usual care |
| Ihle et al[30] | Germany | 2011 | Lung | > 52 | 4 | 60 | Inpatient rehabilitation (exercise training 4 d/wk and aerobic session 5 d/wk) | Outpatient physiotherapy |
| Christensen et al[31] | Denmark | 2012 | Heart | Mean 84 | 8 | 4 | High-intensity aerobic interval training 3 d/wk | Usual care |
| Langer et al[2] | Belgium | 2012 | Lung | 1-6 | 12 | 40 | Aerobic and resistance training 3 d/wk | Usual care |
| Nytrøen et al[32] | Norway | 2012 | Heart | 52-416 | 52 | 52 | High-intensity aerobic interval training 3 d/wk | Usual care |
| Rustad et al[33] | Norway | 2012 | Heart | 52-416 | 12 | 52 | High-intensity aerobic interval training 3 d/wk | Usual care |
| Kawauchi et al[34] | Brazil | 2013 | Heart | < 1 | to hospital discharge | 22 | 10-phase incremental exercise program (breathing, active resistance exercises, aerobic exercises, stretching) | Institution exercise routine (breathing, stretching walking) 5 d/wk |
| Kouidi et al[35] | Greece | 2013 | Kidney | > 52 | 26 | 24 | Aerobic exercise and strength training 4 d/wk | Usual care |
| Nytrøen et al[36] | Norway | 2013 | Heart | 52-416 | 52 | 525 | High-intensity aerobic interval training 3 d/wk | Usual care |
| Dall et al[37] | Denmark | 2014 | Heart | > 52 | 12 (5 mo washout) | 17 | High-intensity aerobic interval training 3 d/wk | Moderate biking exercise 3 d/wk |
| Monk-Hansen et al[38] | Denmark | 2014 | Heart | > 52 | 8 | 30 | High intensity training 3 d/wk | Usual care |
| Pascoalino et al[39] | Brazil | 2015 | Heart | > 52 | 12 | 42 | Endurance exercise training 3 d/wk | Usual care |
| Pooranfar et al[40] | Iran | 2013 | Kidney | 104-156 | 10 | 44 | Aerobic and resistance training 3 d/wk | Usual care |
| Riess et al[41] | Canada | 2013 | Kidney | > 26 | 12 | 31 | Endurance and strength training 2 d/wk | Usual care |
| Tzvetanov et al[42] | United States | 2014 | Kidney | > 4 | 52 | 17 | Resistance exercise training 2 d/wk (as well as behaviour and nutrition) | Usual care |
| Dall et al[43] | Denmark | 2015 | Heart | > 52 | 12 (5 mo washout) | 176 | High-intensity aerobic interval training 3 d/wk | Moderate biking exercise 3 d/wk |
| Greenwood et al[44] | England | 2015 | Kidney | < 52 | 12 | 60 | Home-based aerobic training and resistance training 3 d/wk | Usual care |
| Karelis et al[45] | Canada | 2015 | Kidney | 6-8 | 16 | 24 | Resistance training 3 d/wk (once a week in hospital and 2 × /week at home) | Usual care (no exercise) |
Does not add to 1313 since some patients included in more than one study;
Same patients as Braith 1996;
Same patients as Karolopat 2007;
Same patients as Hermann 2011;
Same patients as Nytrøen 2012;
Same patients as Dall 2014. BMD: Bone mineral density; HR: Heart rate; BP: Blood pressure; HRQOL: Health-related quality-of-life; CVD: Cardiovascular disease; BMI: Body mass index; METs: Metabolic unit of task; HRRe: Heart rate reserve; HRR1: Heart rate recovery; CRI: Chronotropic response index; CRP: C-reactive protein; IL-6: Interleukin-6; TNF-a: Tumor necrosis factor-alpha; sICAM-1: Intercellular adhesion molecule-1; 6MWD: 6 minute walk distance; FVC: Forced vital capacity; HRV: Heart rate variability; BRS: Baroreflex sensitivity.
Table 3 outlines the outcome measures that were used in each study. In total, there were 126 outcome measures. Of the 126 outcome measures, 62 were used as primary outcome measures in at least one study. The most commonly occurring primary outcomes were peak VO2 (n = 13), SF-36 (n = 8), and muscle strength (n = 7).
Table 3.
List of outcome measures by study
| Ref. | Year | Organ group | Primary outcome measures | Secondary outcome measures |
| Braith et al[5] | 1996 | Heart | Bone mineral density (body and regional: Femur neck, lumbar vertebra) | Bone mineral content Total bone calcium Acute rejection episodes |
| Braith et al[4] | 1998 | Heart | Body mass Fat-free mass Fat mass Muscle strength (upper and lower body) | Percent body fat Acute rejection episodes |
| Kobashigawa et al[15] | 1999 | Heart | Blood pressure (peak and resting) Heart rate (peak and resting) Anaerobic threshold Exercise duration (to exhaustion) Peak ventilation Peak VO2 Peak workload Ventilatory equivalent for carbon dioxide and oxygen | Muscle strength (lower limb) |
| Painter et al[16] | 2002 | Kidney | Body mass index Body weight Fat mass/body fat Lean tissue mass Percent body fat Blood pressure (peak) Muscle strength (quadriceps) Peak ventilation Peak VO2 SF-36 | Self-reported activity level (frequency, type, length, and intensity of exercise) Blood creatinine Blood urea nitrogen levels Hematocrit Hemoglobin Bone mineral density Peak workload Rating of perceived exertion (Borg) Peak respiratory exchange ratio Immunosuppression use (type, dose) |
| Mitchell et al[17] | 2003 | Lung | Bone mineral density (lumbar spine) | Acute rejection episodes Muscle strength (lumbar extensor) |
| Painter et al[18] | 2003 | Kidney | Cholesterol (TC, HDL) Body mass index Total CVD risk (Framingham) Blood pressure Peak workload (METs) | Blood lipids Incidence of diabetes Smoking status |
| Braith et al[19] | 2005 | Heart | Muscle composition (fiber types) Muscle metabolic enzyme activity | Muscle strength (upper and lower body) |
| Juskowa et al[20] | 2006 | Kidney | Blood lipids Cholesterol (TC, HDL, LDL) Body mass index | Blood calcium level Blood creatinine Blood electrolytes Blood glucose Blood phosphorus Blood protein levels (albumin, fibrinogen, total protein level) Enzyme levels (alanine transferase, alkaline phosphatase, aspartate aminotransferase) Folate concentrations Hemoglobin Interleukin-18 Total-homocysteine Vitamin B12 Blood pressure Muscle strength (upper limbs) Peak expiratory flow |
| Krasnoff et al[3] | 2006 | Liver | Body mass index Body weight Bone mineral content Bone mineral density Fat mass/body fat Lean tissue mass Percent body fat Muscle strength (quadriceps) Peak VO2 SF-36 Peak respiratory exchange ratio Nutritional intake (Block-95 - calories/day; protein, carb and fat calories) | Rating of perceived exertion (Borg) |
| Bernardi et al[21] | 2007 | Heart | Baroceptor control of blood pressure Baroceptor control of heart rate | Blood pressure; Heart rate Neck pressure RR interval Anaerobic threshold CO2 production Exercise duration (to exhaustion) Peak ventilation Peak VO2; Peak workload Ventilatory equivalent for CO2 and oxygen |
| Karapolat et al[22] | 2007 | Heart | Peak VO2 Beck depression inventory SF-36 State-trait anxiety inventory | |
| Braith et al[23] | 2008 | Heart | Endothelial function (flow-mediated dilation) | Blood glucose Blood lipids Cholesterol (TC, HDL, LDL) Oxidative stress-induced lipid peroxidation Plasma norepinephrine Serum metabolic and hematologic indicators Body mass Acute rejection episodes Blood pressure (resting and peak) Brachial artery diameter Exercise duration (to exhaustion) Peak VO2 |
| Karapolat et al[24] | 2008 | Heart | Chronotropic response index Heart rate recovery Heart rate reserve Peak VO2 | Duke Treadmill Score |
| Pierce et al[25] | 2008 | Heart | C-reactive protein Interleukin-6 Serum metabolic profile Soluble cell adhesion molecules (sICAM-1) Tumour necrosis factor-alpha Muscle vasodilation (forearm and calf) | Blood glucose Cholesterol (TC, HDL, LDL) Cytomegalovirus IgG status White blood cell levels Acute rejection episodes Blood pressure (resting) Heart rate (peak and resting) Exercise duration (to exhaustion) Rating of perceived exertion (Borg) Peak respiratory exchange ratio |
| Wu et al[26] | 2008 | Heart | Muscle endurance (quadriceps) Muscle strength (quadriceps) Peak VO2 World Health Organization Questionnaire on Quality of Life - BREF | Daily physical activity Blood pressure Heart rate (resting and peak) Nutritional intake (caloric intake questionnaire) Peak ventilation Peak workload Rating of perceived exertion (Borg) |
| Haykowsky et al[27] | 2009 | Heart | Peak VO2 | Lean tissue mass (total and leg) Blood pressure (peak) Endothelial function (endothelial-dependent vasodilation, endothelial-independent vasodilation, reactive hyperemia index) Heart rate (peak) Left ventricular systolic function Muscle strength (upper and lower body) Peak power output Peak respiratory exchange ratio |
| Mandel et al[28] | 2009 | Liver | 6MWD Muscle strength (lower body) Chronic liver disease questionnaire (CLDQ) SF-36 (physical function/limitations) | |
| Hermann et al[29] | 2011 | Heart | Peak VO2 | Blood creatinine Blood glucose; Blood lipids Blood protein levels (adiponectin, MR-proANP, NT-proBNP, provasopressin/copeptin) Cholesterol Hemoglobin High sensitive C-reactive protein Interleukin-6 Serum insulin Tumour necrosis factor-alpha Body mass index; Body weight Hip-waist ratio Blood pressure (resting) Brachial artery diameter Endothelial function (flow-mediated vasodilation, nitroglycerin-induced vasodilation) Heart rate (resting) Peak power output |
| Ihle et al[30] | 2011 | Lung | 6MWD Peak VO2 SF-36 St. George’s Respiratory Questionnaire | Heart rate (peak and resting) Anaerobic threshold Oxygen uptake at anaerobic threshold Peak workload Peak respiratory exchange ratio Ventilatory reserve and capacity |
| Christensen et al[31] | 2012 | Heart | Hospital Anxiety and Depression Scale | Peak VO2 |
| Langer et al[2] | 2012 | Lung | SF-36 Daily walking time (time spend in different postures: sedentary, standing, walking) | Daily steps Movement intensity Time spent in moderate intense activities Blood lipids Body weight Bone mineral density Blood pressure 6MWD Muscle strength (quadriceps and handgrip) Peak workload Mood status SF-36 Forced expiratory volume Respiratory muscle force Incidence of morbidity (diabetes, hyperlipidemia, hypertension, osteoporosis) |
| Nytrøen et al[32] | 2012 | Heart | Peak VO2 | Blood lipids Blood protein levels (NT-proBNP) C-reactive protein Interleukin-6, 8 and 10 levels Body mass index; Body weight; % body fat Chronotropic response index Glycemic control parameters Blood pressure (peak and resting) Heart rate (peak and resting) Heart rate recovery and reserve Stroke volume (O2 pulse; resting and peak) Anaerobic threshold Exercise duration (to exhaustion) Muscle strength (quadriceps and hamstrings) Peak ventilation Rating of perceived exertion (Borg) SF-36 Visual Analog Scale (subjective difference in HRQoL) Peak respiratory exchange ratio |
| Rustad et al[33] | 2012 | Heart | Echocardiographic parameters (rest and during exercise; systolic and diastolic parameters) Peak VO2 | Biochemical parameters Blood pressure Cardiac allograft vasculopathy (coronary angiography) Cardiac output Heart rate (resting and peak) Stroke volume Peak workload Peak respiratory exchange ratio |
| Kawauchi et al[34] | 2013 | Heart | 6MWD Forced vital capacity Respiratory muscle force/strength | Muscle strength (upper and lower limbs) Maximum expiratory/inspiratory pressure |
| Kouidi et al[35] | 2013 | Kidney | Baroreflex sensitivity Heart rate variability parameters (SDNN, rMSSD, pNN50, LF, HF, LF/HF) | Baroreflex effectiveness index Blood pressure (peak and resting) Heart rate (peak and resting) Exercise duration (to exhaustion) Peak ventilation Peak VO2 |
| Nytrøen et al[36] | 2013 | Heart | Cardiac allograft vasculopathy (intravascular ultrasound and virtual histology) | Blood creatinine Blood glucose Blood lipids C-reactive protein Cholesterol (TC, HDL, LDL) Hemoglobin Interleukin-6, 8 and 10 levels Body mass index Body water (total) Body weight Bone mass Lean tissue mass Percent body fat Visceral fat scale Basal metabolic rate Glycemic control parameters Metabolic age Muscle strength (quadriceps and hamstrings) Peak VO2 |
| Dall et al[37] | 2014 | Heart | Peak VO2 | Body weight Blood pressure Heart rate (peak and resting) Heart rate recovery Heart rate reserve CO2 production Peak ventilation Peak workload Peak respiratory exchange ratio |
| Monk-Hansen et al[38] | 2014 | Heart | Echocardiography parameters (systolic and diastolic function) | Body mass index Blood pressure Heart rate (peak and resting) Peak VO2 Peak workload |
| Pascoalino et al[39] | 2015 | Heart | Arterial stiffness (carotid-femoral pulse wave velocity) Blood pressure (ambulatory; peak and resting) | Plasma norepinephrine Heart rate (peak and resting) Anaerobic threshold CO2 production Exercise duration (to exhaustion) Peak VO2 Peak respiratory exchange ratio Respiratory compensation point |
| Pooranfar et al[40] | 2013 | Kidney | Blood lipids Cholesterol (TC, HDL, LDL) Sleep quality and quantity questionnaire (self-report; Pittsburgh Sleep Quality Index) | |
| Riess et al[41] | 2013 | Kidney | Peak VO2 | Cholesterol (TC, HDL) Lean tissue mass Total CVD risk (Framingham) Arterial pressure (mean) Arterial stiffness (pulse wave velocity) Arteriovenous oxygen difference (a-vO2) Blood pressure (ambulatory; peak and resting) Cardiac output Heart rate (peak); Stroke volume Systemic vascular endurance Muscle strength (lower body) Peak workload SF-36 Peak respiratory exchange ratio |
| Tzvetanov et al[42] | 2014 | Kidney | Glomerular filtration rate SF-36 Adherence to training and follow-up Employment status | Blood creatinine; Blood glucose; Blood lipids Cholesterol (TC, HDL, LDL) Hemoglobin Body mass index Body weight Bone mineral content Lean tissue mass Percent body fat Arterial stiffness (carotid-femoral pulse wave velocity Blood pressure Carotid intima-media thickness Muscle strength |
| Dall et al[43] | 2015 | Heart | Blood glucose Blood protein levels (adiponectin, orosomucoid, YLK 40) Interleukin-6 Serum insulin Tumour necrosis factor-alpha Arterial stiffness (augmentation index) Endothelial function (reactive hyperemia index) Hospital Anxiety and Depression Scale SF-36 | Body weight Homeostasis model assessment Heart rate (peak) Peak VO2 Peak respiratory exchange ratio |
| Greenwood et al[44] | 2015 | Kidney | Muscle strength (quadriceps) | Arterial stiffness (pulse wave velocity) Blood pressure (peak and resting) Heart rate (peak and resting) STS-60 Peak VO2 Body mass index; Body weight Waist girth Glomerular filtration rate high-sensitivity C-reactive protein interleukin-6 Fetuin A Tumor necrosis factor-alpha tumor necrosis factor receptors 1 and 2 SF-36 Duke Activity Status Index |
| Karelis et al[45] | 2015 | Kidney | World Health Organization-5 Well-Being Index Muscle strength index Adherence to training and follow-up (feasibility) | Body weight Body height Body mass index Waist girth Hip girth Fat mass/body fat Lean tissue mass Cholesterol (TC, HDL, LDL) Blood glucose Blood pressure Peak VO2 |
SF-36: Short-form 36; TC: Total cholesterol; HDL: High-density lipoprotein fraction of cholesterol; LDL: Low-density lipoprotein fraction of cholesterol; RR-interval: Inter-beat interval (heart rate); BREF: A shorter version of the original; rMSSD: Root-mean-square of successive NN interval differences; pNN50: Percentage value of NN50 count; LF: Low-frequency components; HF: High-frequency components; CVD: Cardio-vascular disease; STS-60: Sit-to-stand 60.
Each outcome measure was linked to an ICF domain and the list is shown in Table 4. The majority of outcomes fell into the body function domain (n = 93). Fourteen outcome measures were linked to the activities and participation, 5 to body structures, 2 to environmental factors and 2 described outcomes were unclassified in the ICF. Frailty indicators such as grip strength (n = 1), fatigue (n = 0) or gait speed (6-minute-walk) (n = 3) were rarely used. Ten multi-dimensional questionnaires were used in the studies reviewed.
Table 4.
International Classification of Functioning, Disability and Health outcome classifications
| ICF component | Domain | Category | Outcome measures | Count primary1 | Organ group |
| Body Function | Global mental functions | b134 | Sleep quality and quantity | 1 | Kidney |
| b152 | Mood status | 0 | Lung | ||
| Functions of the cardiovascular system (heart functions) | b410 | Cardiac output | 0 | Heart, kidney | |
| b410 | Carotid intima-media thickness | 0 | Kidney | ||
| b410 | Echocardiographic parameters | 2 | Heart | ||
| b410 | Endothelial function | 2 | Heart | ||
| b410 | Left ventricular systolic function | 0 | Heart | ||
| b410 | RR interval | 0 | Heart | ||
| b410 | Stroke volume | 0 | Heart, kidney | ||
| b410 | Systemic vascular endurance | 0 | Kidney | ||
| Functions of the cardiovascular system (heart rate) | b4100 | Heart rate | 1 | Heart, kidney, lung | |
| b4100 | Heart rate recovery | 1 | Heart | ||
| b4100 | Heart rate reserve | 1 | Heart | ||
| b4100 | Heart rate variability | 1 | Kidney | ||
| Functions of the cardiovascular system | b410-429 | Baroceptor control of blood pressure | 1 | Heart | |
| b410-429 | Baroceptor control of heart rate | 1 | Heart | ||
| b410-429 | Baroflex effectiveness index | 0 | Kidney | ||
| b410-429 | Baroflex sensitivity | 1 | Kidney | ||
| b410-429 | Chronotropic response index | 1 | Heart | ||
| b410-429 | Total CVD risk | 1 | Kidney | ||
| b410-429 | Cardiac allograft vasculopathy | 1 | Heart | ||
| Functions of the cardiovascular system (blood vessel | b415 | Arterial stiffness | 3 | Heart, kidney | |
| functions) | b415 | Brachial artery diameter | 0 | Heart | |
| Functions of the cardiovascular system (blood | b420 | Arterial pressure | 0 | Kidney | |
| pressure functions) | b420 | Blood pressure | 4 | Heart, kidney, lung | |
| b420 | Neck pressure | 0 | Heart | ||
| Functions of the cardiovascular system (oxygen-carrying functions of the blood) | b4301 | Arteriovenous oxygen difference | 0 | Kidney | |
| Functions of the hematological and immunological | b430-439 | Biochemical parameters | 0 | Heart | |
| systems | b430-439 | Blood calcium level | 0 | Kidney | |
| b430-439 | Blood creatinine | 0 | Heart, kidney | ||
| b430-439 | Blood electrolytes | 0 | Kidney | ||
| b430-439 | Blood glucose | 1 | Heart, kidney | ||
| b430-439 | Blood lipids | 2 | Heart, kidney, lung | ||
| b430-439 | Blood phosphorus | 0 | Kidney | ||
| b430-439 | Blood protein levels | 1 | Heart, kidney | ||
| b430-439 | Blood urea nitrogen levels | 0 | Kidney | ||
| b430-439 | C-reactive protein | 1 | Heart | ||
| b430-439 | Cholesterol | 3 | Heart, kidney | ||
| b430-439 | Folate concentrations | 0 | Kidney | ||
| b430-439 | Hematocrit | 0 | Kidney | ||
| b430-439 | Hemoglobin | 0 | Heart, kidney | ||
| b430-439 | High sensitive C-reactive protein | 0 | Heart | ||
| b430-439 | Interleukin levels | 2 | Heart, kidney | ||
| b430-439 | Plasma norepinephrine | 0 | Heart | ||
| b430-439 | Soluble cell adhesion molecules | 1 | Heart | ||
| b430-439 | Total-homocysteine | 0 | Kidney | ||
| b430-439 | Tumour necrosis factor-alpha | 2 | Heart | ||
| B430-439 | Tumor necrosis factor receptor | 0 | Kidney | ||
| b435 | Cytomegalovirus IgG status | 0 | Heart | ||
| b435 | White blood cell levels | 0 | Heart | ||
| b435 | Acute rejection episodes | 0 | Heart, lung | ||
| Functions of the respiratory system (respiration functions) | b440 | Forced expiratory volume | 0 | Lung | |
| functions) | b440 | Forced vital capacity | 1 | Heart | |
| b440 | Maximum expiratory/inspiratory pressure | 0 | Heart | ||
| b440 | Peak expiratory flow | 0 | Kidney | ||
| b440 | Peak respiratory exchange ratio | 1 | Heart, kidney, liver, lung | ||
| b440 | Respiratory compensation point | 0 | Heart | ||
| b440 | Ventilatory reserve and capacity | 0 | Lung | ||
| Functions of the respiratory system (respiration rate) | b4400 | CO2 production | 0 | Heart | |
| b4400 | Oxygen uptake at anaerobic threshold | 0 | Lung | ||
| b4400 | Peak ventilation | 2 | Heart, kidney | ||
| b4400 | Peak VO2 | 13 | Heart, kidney, liver, lung | ||
| b4400 | Ventilatory equivalent for carbon dioxide and oxygen | 1 | Heart | ||
| Functions of the respiratory system (respiratory muscle functions) | b445 | Respiratory muscle force/strength | 1 | Heart, lung | |
| Functions of the cardiovascular system (general physical endurance) | b4550 | Rating of perceived exertion | 0 | Heart, kidney, liver | |
| Functions related to the digestive, metabolism and the endocrine system | b530 | Body mass index | 4 | Heart, kidney, liver | |
| endocrine system | b530 | Body weight/mass | 3 | Heart, kidney, liver, lung | |
| b530 | Fat mass/body fat | 3 | Heart, kidney, liver | ||
| b530 | Fat-free mass | 1 | Heart | ||
| b530 | Hip girth | 0 | Kidney | ||
| b530 | Hip-waist ratio | 0 | Heart | ||
| b530 | Lean tissue mass | 2 | Heart, kidney, liver | ||
| b530 | Percent body fat | 2 | Heart, kidney, liver | ||
| b530 | Visceral fat scale | 0 | Heart | ||
| b530 | Waist girth | 0 | Kidney | ||
| General metabolic functions, unspecified | b5400 | Basal metabolic rate | 0 | Heart | |
| b5400 | Metabolic age | 0 | Heart | ||
| General metabolic functions, other, specified | B5408 | Maximal metabolic units | 1 | Kidney | |
| Functions related to metabolism and the endocrine system | b540-559 | Enzyme levels | 0 | Kidney | |
| system | b540-559 | Fetuin A | 0 | Kidney | |
| b540-559 | Oxidative stress-induced lipid peroxidation | 0 | Heart | ||
| b540-559 | Serum insulin | 1 | Heart | ||
| b540-559 | Serum metabolic and/or hematologic profile | 1 | Heart | ||
| b540-559 | Vitamin B12 | 0 | Kidney | ||
| b540-559 | Glycemic control parameters | 0 | Heart, kidney | ||
| b540-559 | Muscle metabolic enzyme activity | 1 | Heart | ||
| b545 | Body water | 0 | Heart | ||
| b545 | Homeostasis model assessment | 0 | Heart | ||
| Functions of the genitourinary and reproductive functions (urinary functions) | b610-639 | Glomerular filtration rate | 1 | Kidney | |
| Neuromusculoskeletal and movement-related functions (muscle power functions) | b730 | Peak workload/power output | 1 | Heart, kidney, lung | |
| b730 | Muscle strength | 7 | Heart, kidney, liver, lung | ||
| b730-b749 | Muscle vasodilation | 1 | Heart | ||
| b740 | Muscle endurance | 1 | Heart | ||
| Body structure | Structures related to movement - additional musculoskeletal structures related to movement (bones) | s7700 | Bone mass | 0 | Heart |
| s7700 | Bone mineral content | 1 | Heart, kidney, liver | ||
| s7700 | Bone mineral density | 3 | Heart, kidney, liver, lung | ||
| s7700 | Total bone calcium | 0 | Heart | ||
| s7702 | Muscle composition (fibre types) | 1 | Heart | ||
| Activities and participation | Mobility - walking and moving | d410 | STS-60 | 0 | Kidney |
| participation | Mobility - walking and moving (walking) | d450 | Daily steps | 0 | Lung |
| d450 | Daily walking time | 1 | Lung | ||
| d450 | 6 Minute Walk Distance | 3 | Heart, liver, lung | ||
| d450 | Anaerobic threshold | 1 | Heart, lung | ||
| Mobility - walking and moving | d450-469 | Daily physical activity | 0 | Heart | |
| d450-469 | Movement intensity | 0 | Lung | ||
| d450-469 | Self-reported activity level | 0 | Kidney | ||
| d450-469 | Time spent in moderate intense activities | 0 | Lung | ||
| d450-469 | Duke Treadmill Score | 0 | Heart | ||
| d450-469 | Exercise duration | 1 | Heart, kidney | ||
| Managing diet and fitness | d5701 | Caloric intake | 0 | Heart | |
| d5701 | Nutritional intake | 1 | Liver | ||
| Major life areas (work and employment) | d840-859 | Employment status | 1 | Kidney | |
| Environmental factors | Products or substances for personal consumption, other specified | e1108 | Smoking status | 0 | Kidney |
| Drugs | e1101 | Immunosuppression use | 0 | Kidney | |
| Questionnaires | DASI | 0 | Kidney | ||
| Quality of Life Profile for Chronic Diseases Questionnaire | 1 | Lung | |||
| SF-36 | 8 | Heart, kidney, liver, lung | |||
| St. George’s Respiratory Questionnaire | 1 | Lung | |||
| State-Trait Anxiety Inventory | 1 | Heart | |||
| Beck Depression Inventory | 1 | Heart | |||
| Hospital Anxiety and Depression Scale | 2 | Heart | |||
| Visual Analog Scale (change in HRQoL) | 0 | Heart | |||
| WHOQOL-BREF | 2 | Heart, kidney | |||
| Not covered by ICF | Chronic Liver Disease Questionnaire | 1 | Liver | ||
| Incidence of morbidity | 0 | Kidney, lung | |||
| Adherence to training and follow-up | 2 | Kidney |
Count Primary: Count of studies that used this measure as a primary measure. RR-interval: Inter-beat interval (heart rate); CVD: Cardio-vascular disease; STS-60: Sit-to-stand 60; SF-36: Short-form 36; HRQoL: Health-related quality of life; WHOQOL-BREF: A shorter version of the original World Health Organization Quality of Life Questionnaire; DASI: Duke Activity Status Index.
DISCUSSION
Physical rehabilitation in SOT patients strives to minimize the impairments associated with prolonged chronic illness, allowing individuals to improve their ability to carry out daily tasks and activities and to participate in life roles. When selecting outcome measures to use in clinical trials of SOT recipients, it is important to capture changes across all domains that are relevant to the primary goals of the physical rehabilitation intervention. We have used the ICF categories to classify the outcome measures used in RCTs of exercise interventions after SOT. From this systematic review, we have learned that the outcome measures used in these RCTs vary widely. This finding is in line with the results of similar systematic reviews conducted in other populations (e.g., individuals with critical illness, post-surgery and stroke)[11] Some of the studies focused on multiple primary outcomes and others used just two or three. In total, 62 different primary outcomes were used with the most common being peak VO2 (n = 13) and the SF-36 (n = 8). Most of the outcomes used fell into the body functions domain (n = 93) with very few in the activities and participation domain (n = 14). Few studies included outcomes that are also considered frailty indicators. These are important outcomes as frailty is present in many SOT recipients and can have a negative impact on transplant outcomes[6-8].
As we did, Disdbury et al[1] found that the most commonly used outcome measure was VO2 peak. However, this is an expensive test that requires complex equipment as well as expertise from a professional to interpret the results. Functional exercise capacity tests that are more relevant to patients’ activities and participation in daily life and less costly to administer should be considered.
Disdbury et al[1] were unable to merge data on health-related quality-of-life (HRQoL) measures since so many different questionnaires were used. We found that 11 of the RCTs analyzed used multi-dimensional questionnaires as an outcome measure with several using more than one. These questionnaires each cover many different ICF categories. For instance, Cieza and Stucki[46] have linked individual questions from the short-form-36 (SF-36) questionnaire to ICF domains and found that this questionnaire incorporates at least 21 ICF codes. Linking individual items on HRQoL questionnaires could help researchers select a questionnaire that covers many ICF codes and that would be most suited to be part of the core set of outcome measures recommended, thus making it possible to meaningfully merge data from multiple studies.
A core set of outcome measures to be used in all of these populations would be helpful to minimize and standardize the number of outcomes used in this patient group. While it is important to conduct a comprehensive assessment, the use of a large number of outcome measures can be burdensome for both patients and evaluators. Ideally, the core set of variables should cover all four domains of the ICF, i.e., they need to cover all aspects of the health condition. Furthermore, the core set of variables needs to include outcomes that are common to all organ groups. Many of the issues that affect physical function and exercise capacity are common across the transplant types despite each SOT having its own unique characteristics and challenges[47]. Some of the pre-transplant issues that limit physical function are specific to the failing organ, but the physiological changes associated with severe chronic disease, deconditioning and nutritional depletion are common to all groups[48]. Post-transplant issues that limit physical function vary depending on the phase of recovery, but include things such as extended hospital and intensive care stay, prolonged sedentary time, immunosuppressant medications and episodes of organ rejection[48]. Outcome measures that relating to these commonalities and to increasing physical function would be suitable for inclusion in the core set of variables. However, there are some organ specific issues that may be important to address differently among the groups (e.g., the effects of exercise in the denervation of the heart after transplant or the effects of exercise on early onset of diabetes after kidney transplant) and researchers should be encouraged to include secondary outcomes to address them.
The selection of outcome measures should reflect the length of time since the transplant and whether the course of recovery has been complicated. For example, the main goal of physical rehabilitation for acute phase post-transplant is usually to improve basic mobility and activities of daily living while rehabilitation for long-term recipients is generally focused on improving their exercise capacity and levels of physical activity to prevent cardiovascular complications. When considering appropriate outcomes, is also important to take into account their psychometric properties[49]. Knowing the validity of the outcomes in the transplant population can help researchers with sample size calculations for interventional studies and justify the use of the selected primary outcomes.
None of the studies reviewed included an economic evaluation of the exercise programs and the potential cost savings if SOT recipients experience less long-term cardiovascular disease and fewer hospital readmission related to frailty and physical disability. Although robust economic studies can be challenging, they may be important to convince healthcare funders that exercise programs can be cost-effective and have a positive impact on transplant outcomes and survival. Exercise programs also need to be more readily available for transplant recipients as lack of availability of post-transplant exercise programs has been identified for example in Canada[50].
Limitations
A limitation of this systematic review is the inclusion of only RCTs. There are other studies on exercise training in SOT recipients that use different research designs, especially observational studies using pre-post designs that were not included. We chose this strategy because RCTs are of the highest quality of study design. We assumed that investigators conducting RCTs have chosen their outcomes carefully and that this group of studies is representative of all rehabilitation trials in transplant recipients. We have also limited our search to studies published in English, which may have reduced our sample size.
There is little standardization in outcome measures used in RCTs of exercise interventions in SOT recipients. Outcome measures for clinical trials should also be selected based on their psychometric properties, stage post transplantation and severity of impairments of the patient population. Further research is needed to develop consensus on a standardized core set of outcomes to measure the effectiveness of such interventions. The ICF framework can be used to select appropriate outcomes that cross all domains and that would be appropriate to all SOT recipients.
COMMENTS
Background
Over 30 randomized controlled trials (RCTs) have been conducted to examine the effectiveness of exercise training on outcomes in solid organ transplant (SOT) recipients. However, the synthesis of findings across studies has been limited by the lack of similar outcomes across studies. The objectives of this systematic review were to identify the outcome measures that have been used in RCTs of exercise training in SOT recipients and to link these outcomes to the International Classification of Functioning, Disability and Health (ICF) framework.
Research frontiers
Between 1996 and 2015 more than 30 RCTs were published on the effects of exercise training in SOT recipients. Taken together, the results of these RCTs show that exercise training improves maximal aerobic capacity, muscle strength, body composition, cardiopulmonary variables and quality of life. There is little evidence for the effect of exercise in physical activity and participation in SOT recipients. In a systematic review of exercise training in SOT recipients conducted in 2012 by Didsbury et al, the authors included 15 RCTs with 28 unique outcomes. The majority of outcomes were related to cardiovascular parameters (VO2 peak, blood pressure, cholesterol), with fewer studies examining body composition, frailty indicators or quality of life. The authors were therefore hampered in their ability to conduct meta-analyses, which limited the conclusions of their comprehensive review.
Innovations and breakthroughs
There are numerous studies examining the role of exercise training to improve outcomes following SOT. Exercise training has several important health benefits for SOT recipients, such as improving maximal aerobic capacity (VO2 peak), body composition and quality of life. A limitation of the current literature on exercise for SOT is the inability to combine outcomes from studies due to the wide range of reported outcomes.
Applications
This systematic review suggests that there is a need to develop consensus on a standardized core set of outcomes to measure the effectiveness of exercise interventions in SOT. A standardized core set of outcomes would facilitate standard reporting of key outcomes across studies.
Terminology
The ICF is an established framework developed by the World Health Organization and is commonly used in rehabilitation. The ICF is designed to describe health and health-related status from biological, personal and societal perspectives. The framework classifies human function into four domains: body functions; body structures; activities and participation; and environmental factors. These domains match well with the goals of exercise training and physical rehabilitation programs; specifically to identify, measure and treat physical impairments (body function and structure); to reverse or normalize activity limitations; and to enhance participation in all settings.
Peer-review
It is a well written review concerning several domains to assess the function outcome of patients with organ transplants subjected to exercise training. It is very helpful for the readers.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
Data sharing statement: The original tables are available from the corresponding author at tania.janaudis-ferreira@mcgill.ca.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: July 1, 2016
First decision: September 5, 2016
Article in press: October 24, 2016
P- Reviewer: Kelesidis T, Kin T, Pan SC, Shi YJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, Wong G. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation. 2013;95:679–687. doi: 10.1097/TP.0b013e31827a3d3e. [DOI] [PubMed] [Google Scholar]
- 2.Langer D, Burtin C, Schepers L, Ivanova A, Verleden G, Decramer M, Troosters T, Gosselink R. Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant. 2012;12:1584–1592. doi: 10.1111/j.1600-6143.2012.04000.x. [DOI] [PubMed] [Google Scholar]
- 3.Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896–1905. doi: 10.1111/j.1600-6143.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 4.Braith RW, Welsch MA, Mills RM, Keller JW, Pollock ML. Resistance exercise prevents glucocorticoid-induced myopathy in heart transplant recipients. Med Sci Sports Exerc. 1998;30:483–489. doi: 10.1097/00005768-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Braith RW, Mills RM, Welsch MA, Keller JW, Pollock ML. Resistance exercise training restores bone mineral density in heart transplant recipients. J Am Coll Cardiol. 1996;28:1471–1477. doi: 10.1016/s0735-1097(96)00347-6. [DOI] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365. doi: 10.1136/bmj.c365. [DOI] [PubMed] [Google Scholar]
- 10.Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, Tugwell P. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Available from: http://www.comet-initiative.org/
- 12.World Health Organization. Towards a common language for functioning, disability and health: ICF. World Health Organisation, 2002. Available from: http://www.who.int/classifications/icf/en/
- 13.Gilchrist LS, Galantino ML, Wampler M, Marchese VG, Morris GS, Ness KK. A framework for assessment in oncology rehabilitation. Phys Ther. 2009;89:286–306. doi: 10.2522/ptj.20070309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobashigawa JA, Leaf DA, Lee N, Gleeson MP, Liu H, Hamilton MA, Moriguchi JD, Kawata N, Einhorn K, Herlihy E, et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med. 1999;340:272–277. doi: 10.1056/NEJM199901283400404. [DOI] [PubMed] [Google Scholar]
- 16.Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, Tomlanovich SL, Ascher NL. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74:42–48. doi: 10.1097/00007890-200207150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell MJ, Baz MA, Fulton MN, Lisor CF, Braith RW. Resistance training prevents vertebral osteoporosis in lung transplant recipients. Transplantation. 2003;76:557–562. doi: 10.1097/01.TP.0000076471.25132.52. [DOI] [PubMed] [Google Scholar]
- 18.Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, Tomlanovich SL, Ascher NL. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42:362–369. doi: 10.1016/s0272-6386(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 19.Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, Aranda JM. Effect of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005;95:1192–1198. doi: 10.1016/j.amjcard.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Juskowa J, Lewandowska M, Bartłomiejczyk I, Foroncewicz B, Korabiewska I, Niewczas M, Sierdziński J. Physical rehabilitation and risk of atherosclerosis after successful kidney transplantation. Transplant Proc. 2006;38:157–160. doi: 10.1016/j.transproceed.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi L, Radaelli A, Passino C, Falcone C, Auguadro C, Martinelli L, Rinaldi M, Viganò M, Finardi G. Effects of physical training on cardiovascular control after heart transplantation. Int J Cardiol. 2007;118:356–362. doi: 10.1016/j.ijcard.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Karapolat H, Eyigör S, Zoghi M, Yagdi T, Nalbangil S, Durmaz B. Comparison of hospital-supervised exercise versus home-based exercise in patients after orthotopic heart transplantation: effects on functional capacity, quality of life, and psychological symptoms. Transplant Proc. 2007;39:1586–1588. doi: 10.1016/j.transproceed.2007.01.079. [DOI] [PubMed] [Google Scholar]
- 23.Braith RW, Schofield RS, Hill JA, Casey DP, Pierce GL. Exercise training attenuates progressive decline in brachial artery reactivity in heart transplant recipients. J Heart Lung Transplant. 2008;27:52–59. doi: 10.1016/j.healun.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Karapolat H, Eyigor S, Zoghi M, Yagdi T, Nalbantgil S, Durmaz B, Ozbaran M. Effects of cardiac rehabilitation program on exercise capacity and chronotropic variables in patients with orthotopic heart transplant. Clin Res Cardiol. 2008;97:449–456. doi: 10.1007/s00392-008-0648-7. [DOI] [PubMed] [Google Scholar]
- 25.Pierce GL, Schofield RS, Casey DP, Hamlin SA, Hill JA, Braith RW. Effects of exercise training on forearm and calf vasodilation and proinflammatory markers in recent heart transplant recipients: a pilot study. Eur J Cardiovasc Prev Rehabil. 2008;15:10–18. doi: 10.1097/HJR.0b013e3282f0b63b. [DOI] [PubMed] [Google Scholar]
- 26.Wu YT, Chien CL, Chou NK, Wang SS, Lai JS, Wu YW. Efficacy of a home-based exercise program for orthotopic heart transplant recipients. Cardiology. 2008;111:87–93. doi: 10.1159/000119695. [DOI] [PubMed] [Google Scholar]
- 27.Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734–739. doi: 10.1111/j.1600-6143.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 28.Mandel DW. Comparison of Targeted Lower Extremity Strengthening and Usual Care Progressive Ambulation in Subjects Post-Liver Transplant: A Randomized Controlled Trial. Available from: http://xueshu.baidu.com/s?wd=paperuri:(15d7def06167aa5c5228841b35d7d3a7)&filter=sc_long_sign&sc_ks_para=q%3DComparison+of +Targeted+Lower+ Extremity+Strengthening+and+ Usual+Care+Progressive+Ambulation+in+Subjects+Post-Liver+Transplant%3A+A+Randomized+Controlled+Trial&tn=SE_baiduxueshu_c1gjeupa&ie=utf-8&sc_us=11096312547240 331258.
- 29.Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11:536–541. doi: 10.1111/j.1600-6143.2010.03403.x. [DOI] [PubMed] [Google Scholar]
- 30.Ihle F, Neurohr C, Huppmann P, Zimmermann G, Leuchte H, Baumgartner R, Kenn K, Sczepanski B, Hatz R, Czerner S, et al. Effect of inpatient rehabilitation on quality of life and exercise capacity in long-term lung transplant survivors: a prospective, randomized study. J Heart Lung Transplant. 2011;30:912–919. doi: 10.1016/j.healun.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: a randomized, controlled trial. J Heart Lung Transplant. 2012;31:106–107. doi: 10.1016/j.healun.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Nytrøen K, Rustad LA, Aukrust P, Ueland T, Hallén J, Holm I, Rolid K, Lekva T, Fiane AE, Amlie JP, et al. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12:3134–3142. doi: 10.1111/j.1600-6143.2012.04221.x. [DOI] [PubMed] [Google Scholar]
- 33.Rustad LA, Nytrøen K, Amundsen BH, Gullestad L, Aakhus S. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: a randomised controlled trial. Eur J Prev Cardiol. 2014;21:181–191. doi: 10.1177/2047487312469477. [DOI] [PubMed] [Google Scholar]
- 34.Kawauchi TS, Almeida PO, Lucy KR, Bocchi EA, Feltrim MI, Nozawa E. Randomized and comparative study between two intra-hospital exercise programs for heart transplant patients. Rev Bras Cir Cardiovasc. 2013;28:338–346. doi: 10.5935/1678-9741.20130053. [DOI] [PubMed] [Google Scholar]
- 35.Kouidi E, Vergoulas G, Anifanti M, Deligiannis A. A randomized controlled trial of exercise training on cardiovascular and autonomic function among renal transplant recipients. Nephrol Dial Transplant. 2013;28:1294–1305. doi: 10.1093/ndt/gfs455. [DOI] [PubMed] [Google Scholar]
- 36.Nytrøen K, Rustad LA, Erikstad I, Aukrust P, Ueland T, Lekva T, Gude E, Wilhelmsen N, Hervold A, Aakhus S, et al. Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32:1073–1080. doi: 10.1016/j.healun.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Dall CH, Snoer M, Christensen S, Monk-Hansen T, Frederiksen M, Gustafsson F, Langberg H, Prescott E. Effect of high-intensity training versus moderate training on peak oxygen uptake and chronotropic response in heart transplant recipients: a randomized crossover trial. Am J Transplant. 2014;14:2391–2399. doi: 10.1111/ajt.12873. [DOI] [PubMed] [Google Scholar]
- 38.Monk-Hansen T, Dall CH, Christensen SB, Snoer M, Gustafsson F, Rasmusen H, Prescott E. Interval training does not modulate diastolic function in heart transplant recipients. Scand Cardiovasc J. 2014;48:91–98. doi: 10.3109/14017431.2013.871058. [DOI] [PubMed] [Google Scholar]
- 39.Pascoalino LN, Ciolac EG, Tavares AC, Castro RE, Ayub-Ferreira SM, Bacal F, Issa VS, Bocchi EA, Guimarães GV. Exercise training improves ambulatory blood pressure but not arterial stiffness in heart transplant recipients. J Heart Lung Transplant. 2015;34:693–700. doi: 10.1016/j.healun.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Pooranfar S, Shakoor E, Shafahi M, Salesi M, Karimi M, Roozbeh J, Hasheminasab M. The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transplant Med. 2014;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- 41.Riess KJ, Haykowsky M, Lawrance R, Tomczak CR, Welsh R, Lewanczuk R, Tymchak W, Haennel RG, Gourishankar S. Exercise training improves aerobic capacity, muscle strength, and quality of life in renal transplant recipients. Appl Physiol Nutr Metab. 2014;39:566–571. doi: 10.1139/apnm-2013-0449. [DOI] [PubMed] [Google Scholar]
- 42.Tzvetanov I, West-Thielke P, D’Amico G, Johnsen M, Ladik A, Hachaj G, Grazman M, Heller R, Fernhall B, Daviglus M. A novel and personalized rehabilitation program for obese kidney transplant recipients. Transplant Proc. 2014;46:3431–3437. doi: 10.1016/j.transproceed.2014.05.085. [DOI] [PubMed] [Google Scholar]
- 43.Dall CH, Gustafsson F, Christensen SB, Dela F, Langberg H, Prescott E. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: A randomized, crossover trial. J Heart Lung Transplant. 2015;34:1033–1041. doi: 10.1016/j.healun.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood SA, Koufaki P, Mercer TH, Rush R, O’Connor E, Tuffnell R, Lindup H, Haggis L, Dew T, Abdulnassir L, et al. Aerobic or Resistance Training and Pulse Wave Velocity in Kidney Transplant Recipients: A 12-Week Pilot Randomized Controlled Trial (the Exercise in Renal Transplant [ExeRT] Trial) Am J Kidney Dis. 2015;66:689–698. doi: 10.1053/j.ajkd.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Karelis AD, Hébert MJ, Rabasa-Lhoret R, Räkel A. Impact of Resistance Training on Factors Involved in the Development of New-Onset Diabetes After Transplantation in Renal Transplant Recipients: An Open Randomized Pilot Study. Can J Diabetes. 2015;40:382–388. doi: 10.1016/j.jcjd.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Cieza A, Stucki G. Content comparison of health-related quality of life (HRQOL) instruments based on the international classification of functioning, disability and health (ICF) Qual Life Res. 2005;14:1225–1237. doi: 10.1007/s11136-004-4773-0. [DOI] [PubMed] [Google Scholar]
- 47.Williams TJ, McKenna MJ. Exercise limitation following transplantation. Compr Physiol. 2012;2:1937–1979. doi: 10.1002/cphy.c110021. [DOI] [PubMed] [Google Scholar]
- 48.Mathur S, Janaudis-Ferreira T, Wickerson L, Singer LG, Patcai J, Rozenberg D, Blydt-Hansen T, Hartmann EL, Haykowsky M, Helm D, et al. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235–2245. doi: 10.1111/ajt.12874. [DOI] [PubMed] [Google Scholar]
- 49.Cleemput I, Dobbels F. Measuring patient-reported outcomes in solid organ transplant recipients: an overview of instruments developed to date. Pharmacoeconomics. 2007;25:269–286. doi: 10.2165/00019053-200725040-00002. [DOI] [PubMed] [Google Scholar]
- 50.Trojetto T, Elliott RJ, Rashid S, Wong S, Dlugosz K, Helm D, Wickerson L, Brooks D. Availability, characteristics, and barriers of rehabilitation programs in organ transplant populations across Canada. Clin Transplant. 2011;25:E571–E578. doi: 10.1111/j.1399-0012.2011.01501.x. [DOI] [PubMed] [Google Scholar]


