Abstract
The temperature-sensitive hemagglutinin (Tsh) is an autotransporter protein secreted by avian-pathogenic Escherichia coli strains that colonize the respiratory tract and lead to airsacculitis, pericarditis, and colisepticemia. It is synthesized as a 140-kDa precursor protein, whose processing results in a 106-kDa passenger domain (Tshs) and a 33-kDa β-domain (Tshβ). The presence of a conserved 7-amino-acid serine protease motif within Tshs classifies the protein in a subfamily of autotransporters, known as serine protease autotransporters of the Enterobacteriaceae. In this study, we report that purified Tshs is capable of adhering to red blood cells, hemoglobin, and the extracellular matrix proteins fibronectin and collagen IV. We also demonstrate that Tshs exerts proteolytic activity against casein, and we provide experimental evidence demonstrating that serine 259 is essential for the protease function. However, this residue is not required for adherence to substrates, and its replacement by an alanine does not abolish binding activity. In summary, our results demonstrate that Tsh is a bifunctional protein with both adhesive and proteolytic properties.
Gram-negative bacteria have evolved several transport systems for translocation of their proteins to the cell surface or the extracellular environment. Among them, the autotransporter or type V secretion system is unique in that its substrates are capable of directing their own secretion across the outer membrane. A typical autotransporter is synthesized as a precursor protein comprising three different domains: a N-terminal signal peptide, an internal passenger domain, and a C-terminal domain (β-domain). Following passage through the inner membrane by the Sec-translocase, the signal peptide is cleaved and the protein is released into the periplasm, where it may undergo structural modifications. The β-domain then inserts into the outer membrane, where it is thought to form a β-barrel with a central hydrophilic pore. Through this pore, the passenger domain is secreted to the cell surface in an unfolded or partially folded state. Thereafter, it may remain attached to the bacterium or be released to the external milieu by proteolytic cleavage (16, 18, 19, 24, 27).
Proteins identified to date that are secreted by the type V pathway are virulence factors, and although their β-domains are highly conserved, their passenger domains can function as adhesins, proteases, hemagglutinins, toxins, or mediators of intracellular motility, depending on the particular niche occupied by the pathogen (18, 24). The serine protease autotransporters of the Enterobacteriaceae (SPATEs) are good examples of the autotransporter family. These molecules are characterized by a conserved serine protease motif, GDSGS, located in their passenger domains (8, 18, 19, 27). The first serine represents the catalytic site (1), and the presence of conserved histidine and aspartate residues has proven essential for protease activity (12). They have a high degree of sequence homology, ranging between 40 and 58% identity (18). Phylogenetic analyses indicate that their passenger domains have a common ancestral root (3). However, their proteolytic specificities differ significantly and are in each case limited to a narrow substrate spectrum (8). Several SPATE proteins have been identified to date, such as the Shigella extracellular protein A (SepA) (3) and the protein involved in intestinal colonization (Pic) (17) proteins from Shigella flexneri; the secreted autotransporter toxin (Sat) from uropathogenic Escherichia coli strains (14); enteropathogenic E. coli secreted protein C (EspC) (37) and E. coli secreted protein P (EspP) (5) from enteropathogenic and enterohemorrhagic E. coli, respectively; the plasmid-encoded toxin (Pet) from enteroaggregative E. coli (11); and the temperature-sensitive hemagglutinin (Tsh) from avian-pathogenic E. coli (APEC) and uropathogenic E. coli (15, 32). All of these proteins are involved in the virulence of the bacterium, although the exact mechanism of their action and contribution to disease development still remain vague (18). Some of the SPATEs, such as EspC, EspP, Sat, and Pet, have been reported to function as cytotoxins on different types of cells (8, 14, 25). EspC and Pet have also been found to cleave spectrin, pepsin, and human coagulation factor V (8, 39). The last two substrates are proteolytically processed by EspP as well (5). Sat, on the other hand, cleaves spectrin and human coagulation factor V (8), whereas Pic displays mucinolytic activity (8, 17).
Tsh was the first SPATE to be described (32) and has served as a model for the study of the secretion and function of these proteins. Tsh is secreted by strains of APEC and is primarily responsible for infections that cause agglutination of bird erythrocytes, leading to airsacculitis and colisepticemia (7, 32). It is synthesized as a 140-kDa precursor that undergoes cleavage of its 52-amino-acid signal sequence in the periplasm. As a next step, the 33-kDa C-terminal domain (residues 1101 to 1377 of the pro-protein) inserts into the outer membrane to mediate translocation of the passenger domain to the cell exterior. Once surface localized, the secreted 106-kDa domain (residues 53 to 1100 of the precursor) remains temporarily bound to the cell envelope, possibly mediating adherence during the early stages of infection, before it is released into the extracellular environment (36). It was recently reported that uropathogenic E. coli also secretes a Tsh autotransporter protein (15).
The reported hemagglutination activity of Tsh, along with the presence of the serine protease motif within its sequence, led us to the hypothesis that Tsh may be a bifunctional protein with both adhesive and proteolytic activities. In the present study, we show that purified Tshs binds with high affinity to red blood cells (RBCs), consistent with its ability to agglutinate RBCs. We demonstrate that it can also adhere to purified hemoglobin, in agreement with the high degree of homology between Tsh and E. coli Hbp hemoglobinase. Furthermore, our results indicate that Tshs binds with great efficiency to extracellular matrix proteins. In addition, we show that serine 259, which corresponds to the first serine of the proteolytic motif, is essential for substrate cleavage but does not affect significantly the protein's binding ability.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The Tsh autotransporter was purified from the recombinant laboratory E. coli K-12 strain χ6212/pYA232/pYA3551 (6) expressing the functional Tsh protein. The mutagenized Tsh that contains an alanine in place of the serine-259 of the putative active site was purified from the recombinant E. coli RW193/pYA3432 strain (36). Plasmid pYA3551 was constructed by inserting the 4.2-kb EcoRI-BamHI fragment that corresponds to the tsh gene from plasmid pYA3418 (36) into the EcoRI-BamHI restriction sites of the pYA3332 plasmid (provided by Roy Curtiss III, Washington University). E. coli strains were grown in Luria broth medium at 37°C. The antibiotic concentrations used were 10 μg of tetracycline per ml and 100 μg of ampicillin per ml.
Protein purification.
E. coli strains χ6212/pYA232/pYA3551 and RW193/pYA3432 expressing wild-type Tshs and Tshs that has serine-259 mutated to alanine (TshsS259A) respectively, were grown overnight at 37°C in 5 ml of Luria broth medium containing tetracycline in the first case and ampicillin in the second case. Each culture was transferred in 1 liter of fresh Luria broth medium with the appropriate antibiotic and incubated at 37°C. At an optical density of 0.6 at 600 nm, synthesis of Tsh was induced for 3 h by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The culture supernatant was collected after centrifugation (Beckman J2-21M centrifuge, rotor JA-10) at 4,000 rpm for 30 min (all centrifugation steps were carried out at 4°C) and subjected to ammonium sulfate precipitation. At a concentration of 60% of 3.8 M (NH4)2SO4, wild-type and mutated Tshs were precipitated by centrifugation in the J2-21M centrifuge at 9,000 rpm for 30 min. The pellet was dissolved in 5 ml of 20 mM Tris-HCl buffer (pH 8.0) and dialyzed against the same buffer by using 10,000-molecular-weight-cutoff dialysis cassettes (Pierce, Rockford, Ill.). The protein concentration of the obtained Tsh-containing sample was measured by the Bradford assay. The protein sample (0.423 mg/ml) was centrifuged (Eppendorf 5417R centrifuge, rotor F45-30-11) at 10,000 × g for 10 min and applied to a 5-ml Hitrap Q Fast Flow anion-exchange column (Amersham Biosciences Corp., Piscataway, N.J.), equilibrated with 20 mM Tris-HCl buffer (pH 8.0). Proteins were eluted using a linear ionic strength gradient ranging from 5 mM to 1 M NaCl, and their presence was monitored by observing the optical density at 280 nm. The flow rate was 1 ml/min, and 4-ml fractions were collected. Proteins were visualized by silver staining after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide). Estimation of the protein purity and yield was carried out by densitometric analysis, using ImageJ software (http://rsb.info.nih.gov/ij/).
Enzyme-linked immunosorbent assay (ELISA) with RBCs.
Sheep RBCs (Sigma, St. Louis, Mo.) were coated onto a 96-well plate in a dilution of 1:50 in phosphate-buffered saline (PBS). Fifty microliters of purified Tshs or TshsS259A, each diluted in 20 mM Tris-HCl (pH 8.0) to a final concentration of 10 μg/ml, was added to separate wells, and the plate was incubated for 3 h at 37°C. Unbound protein was washed away with PBS, and 50 μl of rabbit anti-Tsh antibody was added to each well, diluted 1:2,000 in PBS containing 0.2% bovine serum albumin (BSA). Incubation was carried out for 2 h at 37°C. A secondary anti-rabbit immunoglobulin G (IgG) antibody conjugated to alkaline phosphatase (Sigma) was used at a dilution of 1:10,000 in PBS-0.2% BSA. The reaction took place for 1 h at 37°C. After the final wash, binding was detected by incubating wells with 100 μl of an alkaline phosphatase substrate solution (Pierce). The reaction was stopped with 100 μl of 2 N NaOH. The absorbance was measured at 405 nm, and the data were analyzed by Softmax software version 2.02. Three independent experiments were performed, with four wells for every reaction in each experiment, and the results were averaged. Reactions in which the same amount of bovine serum albumin (Sigma) was incubated with the RBCs along with reactions that consisted of buffer incubated with the erythrocytes and reactions that had not undergone the primary antibody incubation step were used as negative controls. Buffer was used to blank the spectrophotometer, and the measured background value was subtracted from the presented results.
ELISA with proteins.
BSA, bovine mucin, and turkey hemoglobin (Sigma) prepared at a concentration of 50 μg/ml in PBS were coated onto separate wells of a 96-well plate. The wells were blocked with 150 μl of PBS-0.25% BSA-0.05% Tween 20 for 1 h at 37°C. Then 100 μl of Tshs diluted in PBS to a final concentration of 100 μg/ml was added to each well, and the wells were incubated for 1 h at 37°C. After being washed three times with PBS-0.5% Tween 20, the wells were incubated for 1 h at 37°C with 100 μl of rabbit anti-Tsh antibody in a dilution of 1:3,000 in PBS-0.25% BSA-0.05% Tween 20. A secondary anti-rabbit IgG antibody conjugated to horseradish peroxidase (Bio-Rad Laboratories, Inc., Hercules, Calif.) was added to each well, diluted 1:20,000 in PBS-0.25% BSA-0.05% Tween 20. Incubation was carried out for 1 h at 37°C. Substrate solution for horseradish peroxidase (Pierce) was used for 30 min at room temperature to estimate binding. The reaction was stopped by addition of 1 M H2SO4, and absorbance was measured at 450 nm. Data were analyzed by Softmax software version 2.02. Three independent experiments were performed, with four wells for every reaction in each experiment, and the values obtained were averaged. The negative control was set by using PBS-0.25% BSA-0.05% Tween 20 instead of Tshs to incubate with the subject proteins. Buffer was used to blank the spectrophotometer, and the blank was subtracted from the presented values.
ELISA with ECM proteins.
Adherence of Tshs to collagen IV (mouse), fibronectin (human), and laminin (mouse) was measured by using 96-well plates coated with the subject ECM proteins, obtained from BD Biosciences (Bedford, Mass.). The blocking step was performed for 1 h at room temperature with 200 μl of PBS-0.2% BSA in each well. The wells were washed twice with PBS and incubated with 100 μl of purified Tshs diluted in 20 mM Tris-HCl (pH 8.0) to final concentrations of 100, 50, 10, 1, and 0.5 μg/ml, for 2 h at 37°C. Unbound protein was washed away with PBS, and 100 μl of rabbit anti-Tsh antibody diluted 1:2,000 in PBS-0.2% BSA was added to each well. The plate was incubated for 2 h at 37°C, and excess antibody was washed away with PBS. A 100-μl volume of anti-rabbit IgG antibody conjugated to horseradish peroxidase (Pierce) was added to each well at a dilution of 1:10,000 in PBS-0.2% BSA. Incubation was carried out for 1 h at 37°C. The wells were washed three times with PBS and incubated with 200 μl of horseradish peroxidase substrate solution (Pierce). After oxidation with 150 μl of 1 M H2SO4, absorbance was measured at 450 nm. Data were analyzed by using Softmax software version 2.02. BSA (100 μg/ml) incubated with the ECM proteins in place of Tshs was used as a negative control. Reactions that had not undergone the primary antibody incubation step were used to ensure that there was no cross-reaction of the secondary antibody. Three independent experiments were carried out with each reaction in triplicate, and the measured values were averaged. Buffer was used to blank the spectrophotometer, and the measured background was subtracted from the presented values.
Proteolytic assay.
Proteolytic activity was detected using a casein derivative that is heavily labeled with the pH-insensitive green fluorescent BODIPY FL dye (Molecular Probes Inc., Eugene, Oreg.) as a substrate. Proteolytic cleavage of casein releases fluorescent-labeled peptides, and the increase in green fluorescence can be measured in a microplate reader. Trypsin (Sigma) and purified Tshs (15 μg each), each diluted in a digestion buffer consisting of 10 mM Bis-Tris (pH 6.0) to a final volume of 100 μl, were added to separate triplicate wells of a 96-well plate. Then 100 μl of casein substrate prepared at 10 μg/ml in digestion buffer was added to each well. Incubation was carried out at 37°C for 16 h, with the samples protected from light. Fluorescence was measured in microplate fluorometer FLX800 from Bio-Tec (St. Louis, Mo.) with standard fluorescein filters (excitation, 485 ± 20 nm; emission, 528 ± 20 nm). Data were analyzed using KC4 software version 3.0. Reactions in which casein was incubated with digestion buffer were used as a negative control. Three independent experiments were carried out, with three wells for every reaction in each experiment, and the values presented correspond to the means, with the background measurement subtracted.
Bioinformatic analyses.
The protein domains of Tshs were analyzed using the 3D-PSSM folding recognition algorithm (www.sbg.bio.ic.ac.uk).
Statistical analyses.
Data are presented as mean values with a 95% confidence interval. The different test groups were compared by a one-way analysis of variance, followed by pairwise comparisons using Tukey's honest significant difference test. Data were log-transformed to correct for heterogeneity of the variances where necessary. The Student t test was used when comparing only two groups, as is the case for the ELISA with hemoglobin, mucin, and BSA. A P value below 5% was considered indicative of statistical significance. Analyses were performed using the R software (http://cran.stat.ucla.edu/).
RESULTS
Purified Tshs binds RBCs.
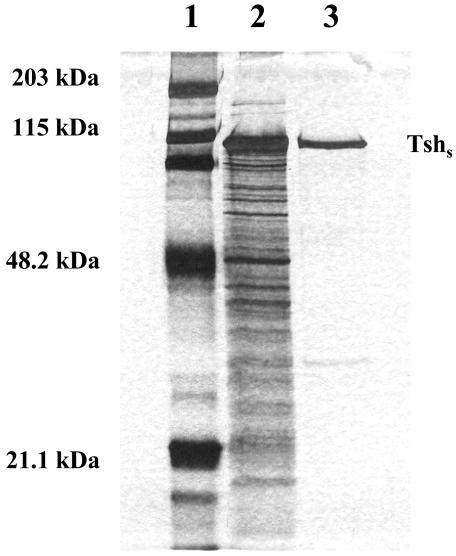
Tsh has been previously reported to agglutinate erythrocytes while still in contact with the extracellular surface of the bacterial cell (32, 36). However, culture supernatant containing the secreted domain of Tsh does not cause agglutination of erythrocyte cells (36). We sought to determine whether Tshs purified from the culture supernatant of E. coli χ6212/pYA232/pYA3551 strain could interact with RBCs in an ELISA. Tshs was isolated from the secreted proteins by ammonium sulfate precipitation and anion-exchange chromatography with a linear gradient of increasing ionic strength (Fig. 1). The protein purity corresponded to 96%, with one contaminant (less than 4% of the total protein) of approximately 30 kDa, which most probably does not represent product of proteolytic cleavage, since it does not react with anti-Tsh antibody (data not shown). The insignificant amount of this smaller protein compared to the high concentration of Tshs makes it highly impossible that this protein could interfere with the observed results. The recovered Tshs in the pure fraction was estimated to be 55% of the total Tshs in the unpurified sample, a significant yield for such a high percentage of purity. This fraction of purified Tshs was used for incubation with sheep RBCs coated onto a 96-well flat-bottom plate. Binding was monitored with a polyclonal antiserum raised against Tshs. The obtained values revealed a significant degree of adhesion of Tshs to the RBCs compared to the negative control, which consisted of RBCs incubated with buffer instead of protein (Fig. 2). No appreciable binding was measured for BSA, which was used as an additional negative control. Reactions that lacked primary anti-Tsh antibody were set up to ensure that there was no cross-reaction of the secondary antibody. The results indicate that Tshs is an adhesin that retains its binding ability even after release from the bacterial cell.
FIG. 1.
SDS-PAGE profile of purified Tshs secreted by E. coli. The culture supernatant was collected, subjected to ammonium sulfate precipitation, and dialyzed against Tris buffer (pH 8.0). The obtained Tsh-containing sample (0.423 mg/ml) was applied to a Hitrap Q Fast Flow 5-ml Sepharose column. Proteins were eluted using a linear ionic strength gradient ranging from 5 mM to 1 M NaCl, and their presence was monitored at 280 nm. The flow rate was 1 ml/min. The fractions were resolved by SDS-PAGE (10% polyacrylamide) and silver stained. Lanes: 1, molecular mass marker; 2, total protein sample prior to chromatography; 3, purified Tshs fraction.
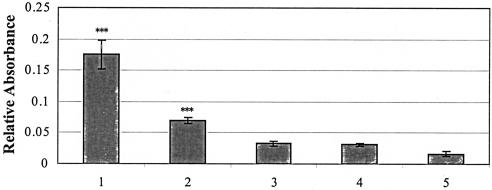
FIG. 2.
Adherence of Tshs and TshsS259A to RBCs. RBCs were coated on a 96-well microplate and incubated with 0.5 μg of purified Tshs or TshsS259A. Binding was detected by an indirect ELISA using an alkaline phosphatase-conjugated anti-rabbit IgG antibody. Buffer incubated with RBCs and reactions that lacked the primary antibody incubation step were used as negative controls. BSA incubated with the erythrocytes under the same conditions served as an additional negative control. Lanes: 1, RBCs with Tshs; 2, RBCs with TshsS259A; 3, negative control with RBCs and buffer; 4, negative control with RBCs and BSA; 5, negative control with RBCs and Tshs but no primary antibody. Three independent experiments were performed with four wells for each reaction. Values are the mean absorbances, with a 95% confidence interval. Statistical analysis was performed on log-transformed data. ***, P < 0.001 (compared to the controls).
Tshs binds to avian hemoglobin and ECM proteins.
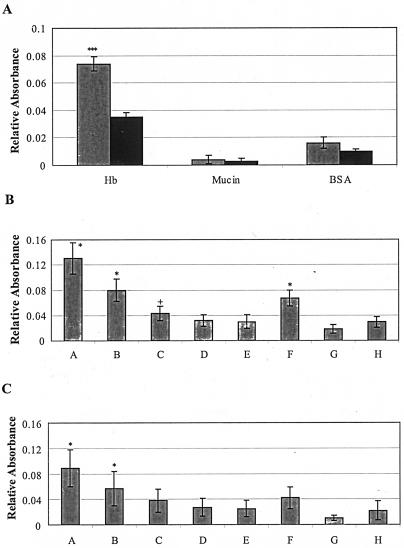
Aiming to gain a better understanding of the role of Tshs in APEC pathogenicity, we were prompted to identify proteins that could serve as binding targets promoting bacterial infection. The high degree of identity between Tsh and Hbp, a hemoglobin protease secreted by the human-pathogenic E. coli strain EB1 (30), along with the previously reported hemagglutinin activity (32) and the hereby determined ability of purified Tshs to adhere to RBCs, made hemoglobin an appealing substrate to test. On the other hand, the abundance of mucin on the mucosal surface, where it is considered to have a protective function against bacterial invasion, along with the fact that Pic, a close homologue of Tsh, has been shown to possess mucinolytic activity (17), directed our interest to this glycoprotein as well. Both mucin and hemoglobin were used in a binding assay to test for interactions with Tshs. After incubation of the subject proteins with Tshs, binding was detected with rabbit anti-Tsh antibody. Samples without Tshs were used as negative controls for each reaction. The secreted domain of Tsh adhered significantly to hemoglobin, exhibiting a strong signal that was more than twofold higher than the one obtained with the negative control without Tshs treatment (Fig. 3A). No significant binding was detected for mucin under the conditions tested.
FIG. 3.
Adherence of Tshs to hemoglobin and ECM proteins. (A) Tshs binds to hemoglobin. BSA, mucin, and hemoglobin were each coated onto a 96-well microplate. Purified Tshs was added to each well. Binding was detected with a horseradish peroxidase-conjugated anti-rabbit IgG antibody. The negative control reactions did not contain Tshs. Hb, hemoglobin with Tshs (left) and negative control with hemoglobin and buffer (right); mucin, mucin with Tshs (left) and negative control with mucin and buffer (right). BSA, BSA with Tshs (left) and negative control with BSA and buffer (right). Three independent assays were carried out with four wells for each reaction in every experiment, and the average values, with the background values subtracted, are presented with a 95% confidence interval. ***, P < 0.001 compared to the control (Student's t test). (B and C) Adherence of purified Tshs and TshsS259A to ECM proteins. Binding to collagen IV (B) and fibronectin (C) was detected by ELISA at an absorbance of 450 nm. Lanes A through E correspond to decreasing concentrations of purified Tshs incubated with the ECM protein in each case. Three independent experiments were carried out with each reaction in triplicate, and the values presented correspond to the means of the measured absorbance with the background value subtracted. Lanes: A, 10 μg of Tshs; B, 5 μg of Tshs; C, 1 μg of Tshs; D, 0.1 μg of Tshs; E, 0.05 μg of Tshs; F, 10 μg of TshsS259A; G; 10 μg of Tshs without primary antibody; H, 10 μg of BSA. *, P < 0.05 compared to the controls (Tukey's honest significant difference test on an analysis of variance model). Absorbances increase linearly with concentration (E to A) (linear regression, P < 0.001). Values indicated by + become significant at the 90% confidence level.
The fact that Tsh was isolated from an APEC strain that infects the avian respiratory tract (32), along with previously reported results from in vivo infection studies (7), prompted us to test whether the secreted domain of Tsh is involved in the colonization of the respiratory cells by the bacterium. To address this question, we examined the ability of purified Tshs to bind to ECM proteins. Different concentrations of Tshs samples, ranging from 100 to 0.5 μg/ml, were incubated separately with purified collagen IV, fibronectin, and laminin that had already been coated onto the wells of a 96-well ELISA microplate. The obtained results are summarized in Fig. 3B and C and reveal a dose-dependent binding of Tshs to both collagen IV and fibronectin (lanes A to E). No binding was detected for laminin (data not shown).
Purified Tshs cleaves casein, and serine-259 is required for its proteolytic activity.
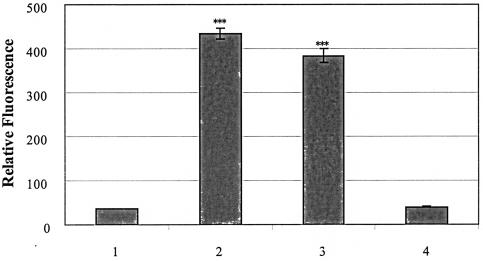
It has been previously reported that autotransporters possessing a serine protease motif exhibit protease activity, which is restricted to a limited number of substrates in each case (8). We assayed Tshs for its ability to cleave casein and tested the role of serine-259, the first serine of the motif in Tsh, as a putative proteolytically active site. Purified Tshs was incubated overnight with a highly fluorescent casein substrate. Trypsin, a well-known serine protease with a great variety of reported substrates including casein, was used as a positive control in the assay. Measurements of the released fluorescence revealed a high degree of casein cleavage by Tshs compared to that of trypsin (Fig. 4). Thus, casein is a protein substrate of Tshs and particularly one that is cleaved by Tshs with high efficiency.
FIG. 4.
Tshs is a serine protease that cleaves casein. Purified wild-type Tshs and mutant TshsS259A proteins (15 μg each) were incubated separately with fluorescently labeled casein in a digestion buffer of 10 mM Bis-Tris (pH 6.0). An equal amount of trypsin, incubated with the same volume of casein solution and the required volume of digestion buffer, was used as a positive control. The negative control consisted of casein solution incubated with digestion buffer. Incubation was performed at 37°C for 16 h, with the samples protected from light. Lanes: 1, negative control with casein and buffer; 2, positive control with casein and trypsin; 3, casein with Tshs; 4, casein with TshsS259A. All reactions, for both samples and controls, were performed in triplicate, and the results of three independent experiments were averaged. The mean absorbances are presented with a 95% confidence interval. ***, P < 0.001 (compared to the negative control).
To evaluate the role of serine-259 on the proteolytic function of Tshs, we isolated protein from the culture supernatant of an E. coli strain expressing a mutated tsh gene that encodes an alanine in place of the putative active serine at position 259 of the precursor Tsh. TshsS259A was purified as described in Materials and Methods. Its proteolytic activity was assessed after incubation with fluorescence-labeled casein under the same conditions as those used for the wild-type protein. The obtained results showed that TshsS259A exhibits no proteolytic activity (Fig. 4), indicating that the mutated serine is critical for the protein to act as a protease.
TshsS259A can function as an adhesin.
The fact that serine-259 is essential for the proteolytic activity of Tshs prompted us to test if this residue is also required for the adhesive function of the protein. Purified TshsS259A was screened for its ability to bind to RBCs in an ELISA. Reaction mixtures containing wild-type Tshs incubated with the RBCs were included as a positive control. Strong binding was measured for the mutated protein compared to the wild-type one, as shown in Fig. 2. This shows that serine-259 is not required for adhesion to host cells and that its absence does not significantly affect the binding interaction. These data suggest that the site required for adherence may be independent of the site responsible for proteolytic activity of Tshs.
To further extend these findings, we tested for the effects of mutated serine-259 on the adherence of Tshs to ECM proteins. TshsS259A was incubated with collagen IV and fibronectin under the same conditions as the ones used for wild-type Tshs (Fig. 3B and C, lanes F). The obtained measurements indicate that the mutagenized Tshs can still bind to both fibronectin and collagen IV but that the strength of the observed binding interaction is decreased. These results are in good agreement with the ones presented above and further strengthen the assumption that the adhesive and proteolytic functions of Tshs lie in different domains of the protein.
DISCUSSION
Autotransporters comprise a family of virulence factors (18, 24) characterized by the unique ability to promote their own secretion across the outer membrane of the bacterial envelope (16, 19, 35, 38). Tsh is a representative member of this group of proteins and is involved in the extraintestinal infections caused by APEC strains. APEC strains invade the respiratory tracts of chicken and turkeys and cause inflammation that leads to airsacculitis, pericarditis, perihepatitis, and septicemia (7, 32). Their virulence lies in unique chromosomal and plasmid regions, which are absent in E. coli K-12, and such a specialized DNA sequence is the tsh gene (4).
Tsh is synthesized as a 140-kDa precursor protein composed of a cleavable N-terminal signal peptide, a 106-kDa passenger domain, and a 33-kDa C-terminal domain (36). It was initially reported as a hemagglutinin for avian erythrocytes, acting in a temperature-dependent manner and while still in contact with the bacterial surface (32, 36). This function suggested that Tsh might be a putative adhesin that mediates colonization of the respiratory tract by the bacterial pathogen during the early stages of infection, a hypothesis that was further supported by the results of in vivo infection studies (7). The latter demonstrated that Tsh is responsible for the development of lesions and deposition of fibrin in the avian air sacs and also showed that its presence accelarates the progression of the infection (7). Furthermore, although the optimal temperature for the hemagglutination activity of Tsh is 26°C (32), the protein is still present at higher temperatures, implying that it may play multiple roles in the infectious process (36).
We performed in vitro studies to assess the ability of Tsh to act as an adhesin. Since the main function of an autotransporter is carried out by its passenger domain (18, 19, 24, 27), we tested purified Tshs for binding to RBCs in an ELISA. The obtained results are in good agreement with the previously reported hemagglutination activity of the surface-localized Tsh (32) and indicate that the secreted protein domain itself is sufficient for adherence to the RBCs. Furthermore, these data demonstrate that Tshs remains in a binding-competent state even after release to the extracellular medium. This suggests that Tshs contributes to disease development not only while still on the bacterial surface but even after being secreted to the external environment, which implies that the protein may be important for later stages of the infection as well.
To extend these findings, we looked for binding interactions of Tshs with substrates that could be putative targets, with the aim of elucidating the mechanism of action of Tsh and its link to APEC pathogenicity. Earlier studies identified an autotransporter protein from pathogenic E. coli strain EB1 that is identical to Tsh in terms of sequence, with the exception of two amino acid residues at positions 209 and 842. This close Tsh homologue, known as Hbp, was reported to degrade hemoglobin and bind its heme portion (30). The high degree of homology between Tsh and Hbp, along with the previously reported hemagglutination activity of Tsh (32), prompted us to test Tshs for its ability to adhere to hemoglobin. We observed a strong binding interaction, indicative of efficient adherence of Tshs to hemoglobin. Taking into consideration that Tshs is also capable of interacting with the RBCs, these data could suggest that upon binding to the erythrocytes, the protein enters the cells and gains access to hemoglobin. Alternatively, binding of Tshs to hemoglobin may take place after release of the latter to the extracellular medium due to erythrocyte lysis, which might involve the proteolytic properties of Tshs (see below) as well. Further studies need to be carried out to gain a better understanding of the in vivo effects of these binding interactions. We also tested for interactions of Tshs with mucin, since Tsh is secreted by a bacterium that invades the mucosal surface. In this case, however, the obtained signal corresponded to insignificant adherence under the conditions tested.
To gain a better insight into the virulence of Tsh, we screened for its ability to adhere to ECM proteins. These proteins are closely associated with endothelial and epithelial cells and form a mesh that functions not only to mechanically support the tissues but also to protect them against bacterial invasion (29, 33, 40). ECM components constitute binding targets for many bacterial pathogens, such as streptococci, staphylococci, enterococci, and yersiniae, and interaction with these host compounds is crucial for the infection process (9, 22, 26, 34). It has been previously shown that Hap, an adhesive autotransporter secreted by Haemophilus influenzae (21), is also capable of binding with high affinity to selected components of the ECM (13). The fact that Hap is closely related to Tsh in terms of sequence homology provided further motivation for examining interactions between Tsh and major ECM proteins of the respiratory epithelium, such as collagen IV, fibronectin, and laminin (13, 33). The binding assays revealed a strong binding interaction of purified Tshs with fibronectin and collagen IV. These data suggest an involvement of Tsh in colonization of the host respiratory tract by APEC strains. Adherence to the host cells enables the microorganism to withstand mechanical defense responses generated by the immune response system. Therefore, Tsh may play a critical role in the early stages of infection. Given that Tshs is a serine protease (see below), it is also possible that adherence to the ECM proteins is followed by their degradation, permitting the bacterium to penetrate to deeper host tissue compartments and thus enhancing bacterial dispersal and protection against immune responses. Further studies need to be carried out before we can gain a better understanding of the adherence properties of the protein in the infection process.
The presence of a serine protease motif, GDSGSPL, within the passenger domain of Tsh (32) prompted us to study the proteolytic activity of the protein. This motif is conserved among many reported proteases of the autotransporter family, such as IgA proteases from Neisseria gonorrhoeae and Haemophilus influenzae (31), Hap from H. influenzae (20), and the SPATEs, a subfamily of autotransporters secreted by the Enterobacteriaceae (18). The first serine of this motif is the putative active site, and its substitution renders the enzyme inactive (1, 31). Two additional residues, a conserved histidine and aspartate, are also important for proteolytic activity (12). Sequence alignments revealed that these three residues, in the case of Tsh, correspond to S259, H124, and D152. Despite the homology between the secreted domain of Tsh and that of IgA protease, the two proteins do not seem to have the same substrate specificity, since Tshs cannot cleave human or chicken IgA (36). Furthermore, Tsh does not require the serine protease motif for procession and secretion of the surface localized mature protein (36), in contrast to both IgA and Hap, which employ an autoproteolytic mechanism for release to the extracellular milieu (20).
Recent studies showed that Tshs exhibits mucinolytic activity (8). In the present study, we show that Tshs can cleave casein, providing further evidence for its proteolytic function. We also demonstrate that serine-259 is absolutely required for enzymatic activity since the latter is abolished when this residue is replaced by alanine. Taken together, these results demonstrate that Tsh is a typical serine protease, with the first serine of the GDSGSPL motif being the catalytically active site. Further studies need to be carried out to clarify how the proteolytic activity is linked to the pathogenicity of the host bacterium.
The results presented above indicate that the passenger domain of Tsh is a bifunctional protein that can act both as an adhesin and as a protease. We sought to determine whether the two functions of Tshs are related or carried out independently. We have demonstrated that serine-259 is indispensable for the proteolytic function of Tshs. We have also investigated whether this residue is required for the adhesin properties of Tshs as well. Our findings show that absence of serine-259 does not annul the ability of the protein to bind to its substrates, and thus this residue, although critical for the proteolytic activity, is not crucial for the binding interactions of Tshs. From these results, it appears that the proteolytic and adhesive functions of Tshs are carried out by distinct domains of the protein and do not depend on each other. However, a decrease in the binding efficiency is observed for TshsS259A, implying that substitution of serine-259 may have affected the ability of the protein to fold properly, resulting in a slightly altered binding pocket that can still recognize and adhere to its targets but with reduced affinity. Future work is needed to verify whether these two functions of Tshs are indeed independent.
Analysis using the 3D-PSSM folding recognition algorithm (23) indicates the presence of two distinct structural domains within the Tshs sequence. (i) The first domain corresponds to the amino-terminal moiety of Tshs (residues 53 to 708) and has structural similarity to the C-terminal domain of the alkaline protease of Pseudomonas aeruginosa (2). (ii) The second domain corresponds to the carboxy-terminal moiety of Tshs (residues 709 to 1100) and has structural similarity to the Bordetella pertussis P.69 adhesin (10). The structures of both the P. aeruginosa alkaline protease and the B. pertussis P.69 adhesin are characterized by the extended presence of β-strand conformation. The C-terminal domain of P. aeruginosa alkaline protease consists of a 21-strand β-sandwich. The P.69 adhesin, the only autotransporter with a solved crystal structure (10), folds into a novel 16-strand parallel β-helix with a V-shaped cross section and represents the largest β-helix known to date. Interestingly, the carboxyl-terminal moiety of Tshs that has structural similarity to the P.69 adhesin does not include a 90-amino-acid sequence that has been shown to be necessary for folding of the passenger domain in several other autotransporters (28). It will be interesting to determine whether the amino- and carboxy-terminal domains of Tshs can function independently of each other when they are not linked in the same polypeptide. Further studies are under way in our laboratory to determine the structure-function relationship in the Tsh autotransporter protein.
Acknowledgments
We thank Joseph St. Geme for his helpful suggestions during this study and his critical review of the manuscript, Steven Blanke for his critical review of the manuscript, Maria Hadjifrangiskou for proofreading the manuscript, Yuejin Li for performing the hemoglobin-binding assay, and Vanessa Vazquez for her assistance on the Tsh protein isolation. We also gratefully acknowledge Ricardo Azevedo for his help in the statistical analysis of the data.
This study was supported by a Welch Foundation Award (E-1548) and a research grant from the U.S. Department of Agriculture.
Editor: J. B. Bliska
REFERENCES
- 1.Bachovchin, W. W., A. G. Plaut, G. R. Flentke, M. Lynch, and C. A. Kettner. 1990. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J. Biol. Chem. 265:3738-3743. [PubMed] [Google Scholar]
- 2.Baumann, U., S. Wu, K. M. Flaherty, and D. B. McKay. 1993. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 12:3357-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjelloun-Touimi, Z., M. S. Tahar, C. Montecucco, P. J. Sansonetti, and C. Parsot. 1998. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology 144:1815-1822. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P. K., and R. Curtiss III. 1996. Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 93:11149-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 6.Curtiss, R., III, J. E. Galan, K. Nakayama, and S. M. Kelly. 1990. Stabilization of recombinant avirulent vaccine strains in vivo. Res. Microbiol. 141:797-805. [DOI] [PubMed] [Google Scholar]
- 7.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta, P. R., R. Cappello, F. Navarro-Garcia, and J. P. Nataro. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 70:7105-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 10.Emsley, P., I. G. Charles, N. F. Fairweather, and N. W. Isaacs. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90-92. [DOI] [PubMed] [Google Scholar]
- 11.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink, D. L., L. D. Cope, E. J. Hansen, and J. W. St. Geme III. 2001. The Haemophilus influenzae Hap autotransporter is a chymotrypsin clan serine protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492-39500. [DOI] [PubMed] [Google Scholar]
- 13.Fink, D. L., B. A. Green, and J. W. St. Geme III. 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 15.Helmer, S. R., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 17.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 20.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 21.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 22.Hussain, M., K. Becker, C. von Eiff, J. Schrenzel, G. Peters, and M. Herrmann. 2001. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J. Bacteriol. 183:6778-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 24.Loveless, B. J., and M. H. Saier, Jr. 1997. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol. Membr. Biol. 14:113-123. [DOI] [PubMed] [Google Scholar]
- 25.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Hook, and B. E. Murray. 2000. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman, C., and C. Stathopoulos. Autotransporter and two-partner secretion: delivery of large-size virulence factors by gram-negative bacterial pathogens. Crit. Rev. Microbiol, in press. [DOI] [PubMed]
- 28.Oliver, D. C., G. Huang, E. Nodel, S. Pleasance, and R. C. Fernandez. 2003. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol. Microbiol. 47:1367-1383. [DOI] [PubMed] [Google Scholar]
- 29.Ortega, N., and Z. Werb. 2002. New functional roles for non-collagenous domains of basement membrane collagens. J. Cell Sci. 115:4201-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen, K., J. Reinholdt, and M. Kilian. 1992. A comparative genetic study of serologically distinct Haemophilus influenzae type 1 immunoglobulin A1 proteases. J. Bacteriol. 174:2913-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman, J. 1996. Extracellular matrix and lung inflammation. Immunol. Res. 15:163-178. [DOI] [PubMed] [Google Scholar]
- 34.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lutticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the Lral adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stathopoulos, C., D. R. Hendrixson, D. G. Thanassi, S. J. Hultgren, J. W. St. Geme III, and R. Curtiss III. 2000. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microbes Infect. 2:1061-1072. [DOI] [PubMed] [Google Scholar]
- 36.Stathopoulos, C., D. L. Provence, and R. Curtiss III. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanassi, D. G., and S. J. Hultgren. 2000. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr. Opin. Cell Biol. 12:420-430. [DOI] [PubMed] [Google Scholar]
- 39.Villaseca, J. M., F. Navarro-Garcia, G. Mendoza-Hernandez, J. P. Nataro, A. Cravioto, and C. Eslava. 2000. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 68:5920-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687-694. [DOI] [PubMed] [Google Scholar]