Abstract
Laparoscopic liver resection (LLR) for tumors in the posterosuperior liver [segment (S) 7 and deep S6] is a challenging clinical procedure. This area is located in the bottom of the small subphrenic space (rib cage), with the large and heavy right liver on it when the patient is in the supine position. Thus, LLR of this area is technically demanding because of the handling of the right liver which is necessary to obtain a fine surgical view, secure hemostasis and conduct the resection so as to achieve an appropriate surgical margin in the cage. Handling of the right liver may be performed by the hand-assisted approach, robotic liver resection or by using spacers, such as a sterile glove pouch. In addition, the operative field of posterosuperior resection is in the deep bottom area of the subphrenic cage, with the liver S6 obstructing the laparoscopic caudal view of lesions. The use of intercostal ports facilitates the direct lateral approach into the cage and to the target area, with the combination of mobilization of the liver. Postural changes during the LLR procedure have also been reported to facilitate the LLR for this area, such as left lateral positioning for posterior sectionectomy and semi-prone positioning for tumors in the posterosuperior segments. In our hospital, LLR procedures for posterosuperior tumors are performed via the caudal approach with postural changes. The left lateral position is used for posterior sectionectomy and the semi-prone position is used for S7 segmentectomy and partial resections of S7 and deep S6 without combined intercostal ports insertion. Although the movement of instruments is restricted in the caudal approach, compared to the lateral approach, port placement in the para-vertebra area makes the manipulation feasible and stable, with minimum damage to the environment around the liver.
Keywords: Hepatectomy, Laparoscopic surgery, Liver cancer, Posture, Prone position
Core tip: Laparoscopic liver resection for posterosuperior tumors is technically challenging because this area is located in the bottom of the small subphrenic cage, overlaid by the right liver. Thus, obtaining a fine surgical view is difficult and manipulation of the right liver is required to ensure hemostasis and obtainment of an appropriate surgical margin. The right liver may be handled by the hand-assisted approach, robotic liver resection, or a spacer-based approach. Intercostal ports can facilitate a direct lateral approach into the cage and postural changes may help. We successfully apply semi-prone positioning in the caudal approach without intercostal ports.
INTRODUCTION
Laparoscopic liver resection (LLR) for liver tumors has rapidly expanded worldwide since the first report of its successful application 25 years ago[1]. Indeed, in 2014, the 2nd International Consensus Conference on LLR (ICCLLR) reported that minor LLR (involving two or less segments, mainly partial resection of the antero-lateral segments and the left lateral sectionectomy) is now standard practice[2]. However, it also reported that major LLR remains an innovative procedure and recommended its continued, albeit cautious, introduction. Although reports of major LLR are increasing, there are several conditions that still represent technical challenges to the procedure[3,4] and preclude its preference over the procedures of living-donor liver resection[5] and liver resection with reconstruction of the vessels[6]. LLR for tumors in the posterosuperior liver [segment (S) 7 and deep S6, around the bare area of the liver] is one of the last frontiers to be discussed in this field.
The surgical difficulty of each LLR depends upon a variety of factors, ranging from the style and extent of liver resection to the tumor condition (size, location and proximity to major vessels) and the background liver condition (liver functional reserve, fibrosis, steatosis, deformity and adhesion after previous resection[7]). A novel difficulty score for LLR has been proposed and is calculated by adding an applicable score for the extent of liver resection, tumor location, liver function, tumor size and tumor proximity to major vessels[8]. In this scoring system, a tumor located in S7 earns the highest score, due to the difficulties presented by its location.
Several researchers have reported on the significant technical challenges of LLRs for posterosuperior liver tumors[9-12]. These tumors are located in the bottom of the subphrenic rib cage, overlaid by the large and heavy right liver when the patient is in the supine position. To manipulate the liver during open liver resection (OLR), a surgeon opens the subphrenic cage with a large subcostal incision, lifts up the costal arch, and physically picks up the liver with his/her left hand after dissecting the retro-peritoneal attachments (Figure 1). In LLR, however, there are no instruments as nimble as the surgeon’s left hand and, moreover, no anterior space without the abdominal wall incision. Therefore, LLR of this area is technically demanding in the handling of the large and heavy right liver in the small subphrenic rib cage; obtaining a fine surgical view is very difficult, as is the manipulation to ensure hemostasis and obtainment of appropriate surgical margin.
Figure 1.
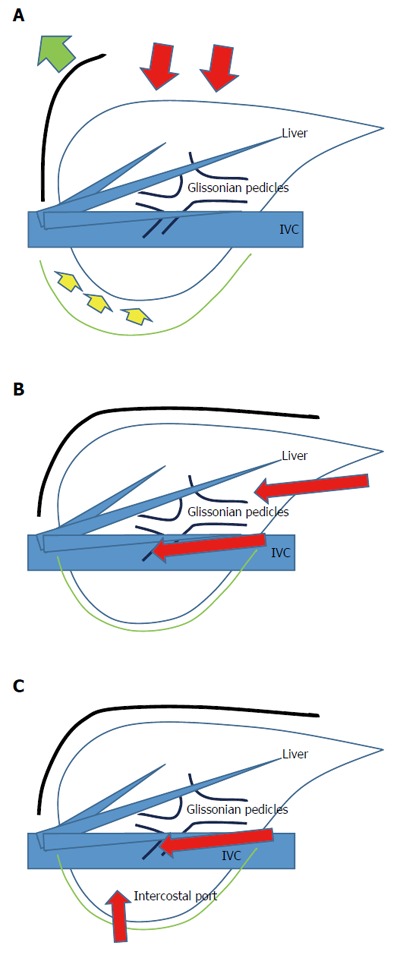
Liver resection (A), laparoscopic liver resection (regular caudal approach, B) and laparoscopic liver resection (lateral approach, C). Red arrows indicate the directions of the view and manipulation in each approach. A: In the open approach, the subcostal cage containing the liver is opened with a large subcostal incision, and instruments are used to lift the costal arch. The liver is dissected and mobilized (lifted) from the retroperitoneum; B: In the regular laparoscopic caudal approach, the laparoscope and forceps are placed into the subcostal cage from caudal direction, and surgery is performed with minimal alteration and destruction of the associated structures; C: In the laparoscopic lateral approach, the intercostal (transdiaphragmatic) ports combined with total mobilization of the liver from the retroperitoneumcan allow the direct lateral approach into the cage and to the posterosuperior tumors.
We previously reported a caudal approach posterior sectionectomy in the left lateral position[13], in which the novel concept of the caudal approach in LLR was presented for the first time in the literature worldwide. In this Editorial, various approaches to LLR for tumors located in the posterosuperior liver are discussed and our caudal approach with postural changes is presented.
LLR APPROACHES FOR TUMORS LOCATED IN THE POSTEROSUPERIOR LIVER
In contrast to OLR, LLR targeting tumors in the posterior section of the liver involves sectionectomy or right hepatectomy more frequently than segmentectomy or partial resection[14-17]. The reason for this tendency is the fact that the straightforward transection plane of the liver from its caudal edge to the diaphragm in right hepatectomy or posterior sectionectomy is easier to be handled in an LLR caudal approach, both for the aspects of view and manipulation. The sight of the laparoscope from the caudal direction views the transection plane for the S7 segmentectomy or partial resection deep in the small subphrenic space behind the liver, with S6 posing an obstacle in the way of the lesions. Since surgeons need to create a precisely curved or angulated transection plane in the space, the parenchyma-preserving S7 segmentectomy or partial resection of the posterosuperior tumors is technically more difficult than the posterior sectionectomy or right hepatectomy.
Retaining adequate functional reserve of the liver after resection is another important objective of LLR, especially in patients with impaired liver function, such as is present in patients with chronic liver diseases (CLD), as well as for its oncological efficacy, as in patients with hepatocellular carcinoma (HCC)[18]. There are several reports which describe different approaches to conquering this particular problem in LLR for posterosuperior tumors[19-23].
How to handle the heavy right liver in LLR
To solve the problem of handling the right liver in LLR - wherein the surgeon’s left hand is not able to reach behind the liver, as it is in OLR - the hand-assisted approach[19], robotic liver resection (RLR)[20], and the approach using spacers, such as the sterile glove pouch[21], have been proposed for LLR of posterior lesions.
Herman et al[19] reported that in laparoscopic posterior sectionectomy the hand-assisted approach helps with visualization, mobilization, pedicle control and parenchymal transection, while still providing the benefits of laparoscopy. However, how to achieve the hand usage and hand-port positioning without disruption of the operative view and maximize manipulation of the organ in the small subphrenic cage should be further discussed in regards to LLR for posterosuperior tumors in S7 segmentectomy and partial resections.
Patriti et al[20] reported that RLR, with its greater maneuverability, as compared to LLR, and powerful third-arm to maintain a stable operative field in the right posterior section, is as safe and feasible as OLR. They also mentioned that in RLR they could apply the same proportion of segmentectomy or less to sectionectomy or more as in OLR, different from the regular LLR as mentioned above[24], with the same short-term outcome. However, they reported that the morbidity of RLR was similar to that of OLR, although it is expected to be less as it is a minimally invasive procedure. This finding was related to their experience of a higher rate of pulmonary complication, which may have been related to their usage of an intercostal port in the RLR for the posterior section. Intercostally-inserted powerful robotic arms and ports may cause damage to the chest wall and pleura, and may lead to a higher incidence of pulmonary complication. Furthermore, using a powerful robotic arm for compression of the right liver may cause damage to the liver parenchyma itself. When the pulmonary complications were excluded from that study, there was less morbidity for the RLR than the OLR.
Bin et al[21] reported that the liver exposure achieved by means of the sterile glove pouch applied as a spacer led to shortened operative time, decreased bleeding and reduced levels of post-operative alanine aminotransferase/aspartate aminotransferase in right liver surgery, including S7 segmentectomy. The usage of spacers, such as the sterile glove pouch, may help in posterosuperior resections; specifically, their use, without disturbance of the operative view and to facilitate manipulation in the small subphrenic cage, should be established in LLR for posterosuperior lesions in S7 segmentectomy and partial resections.
Approaches to obtain good and stable access to the posterosuperior liver in LLR: lateral approach
Without the subcostal incision, there is no space in the anterior direction toward the subphrenic small cage in LLR (Figure 1). The operative field for posterosuperior resection lies in the deep bottom area of the cage (in the supine position), and with the liver S6 obscuring the lesions in the laparoscopic caudal view. Therefore, another important objective of LLR for posterosuperior liver is to achieve good and stable access to the posterosuperior area, through which the liver and tumors may be safely and efficiently handled and a well-opened transection plane can be acquired.
An intercostal (transdiaphragmatic) port with the combination of total mobilization of the liver from the retroperitoneum facilitates the direct lateral approach into the cage[22,25,26]. This is different from the thoracoscopic approach that is often employed for lesions in S8[27-29]. Incision on the diaphragm, adjacent to the tumor, can provide direct exposure into the pleural cavity to reach the S8 tumor via the thoracoscopic approach and without the dissection of the liver attachments; however, intercostal ports achieve direct lateral access into the abdominal cavity and to the S7 tumor but with retroperitoneal dissection of the liver. For the S8 tumors, the endoscopes are placed in the pleural cavity, and for the S7 tumors the endoscopes are placed in the abdominal cavity (Figure 1).
Ogiso et al[22] reported that “the optimization of laparoscopic visualization and access directly affects procedural precision and efficiency”. A good view facilitated by the transdiaphragmatic laparoscope was found to be helpful in identifying the structures and avoiding inadvertent injury. In addition, straight access of instruments to the operative target through the transdiaphragmatic ports was found to allow better control of bleeding and to facilitate generation of precisely curved or angulated transections, as planned. The authors also reported that cranial, large or deep tumors in S7 which necessitate deep partial resection or segmentectomy and tumors close to critical structures are resected more successfully via the intercostal lateral approach than via the regular caudal approach. However, like in RLR, placements of intercostal ports transdiaphragmatically was noted to have risk of causing pleural effusion and other pulmonary complications, especially for vulnerable HCC/CLD patients.
Approaches to obtain good and stable access to the posterosuperior liver in LLR: postural change
As mentioned above, surgical view and organ manipulation is restricted in the regular caudal approach for LLR. In order to solve this problem, postural changes can be applied. Postural changes in LLR had been reported by several studies, and include the left lateral position for posterior sectionectomy[12] and semi-prone position for tumors located in the posterosuperior segments[30-32]. Ikeda et al[23] reported on their employment of the semi-prone position in LLR with the use of intercostal ports for tumors in the anterosuperior and posterior segments. Among the several advantages of this positioning over the supine position were the easy visualization of the right side of the hilar plate, facilitated by the adjacent organs having fallen down (by gravity) to the lower left, and the convenient encircling and ligation of the portal pedicles of the right liver. The weight of the liver facilitates the mobilization of the liver itself, ultimately creating a space above the liver. This event also eases the use of intercostal ports. Furthermore, since the posterior section is positioned higher than the inferior vena cava (IVC), less intraoperative bleeding occurs and the irrigation fluids and blood flow down to the lower left side, facilitating a good view of the operative field.
OUR APPROACH: CAUDAL APPROACH WITH POSTURAL CHANGES
The above-mentioned various approaches are summarized in Table 1. They are categorized into two groups of optional approaches for (A) handling of the right liver (above the surgical field) and acquiring of a stable surgical field; and (B) acquiring access to the posterosuperior lesion in the rib cage. It is important to note here that our surgeons are particularly concerned about the potential damage that intercostal ports may cause to the pleura and chest wall, especially for our HCC/(sometimes severe) CLD patients, as these may lead to pleural effusion and other pulmonary complications. Therefore, our approach was developed by first selecting the caudal approach for its facilitation of access to the posterosuperior lesion. In the caudal approach to the posterosuperior LLR, postural changes from a left lateral position to a semi-prone position are employed to maximize the laparoscopic-specific advantage and to minimize any disturbance to the view of the surgical field and any risk of damage to the liver and surrounding environment, such as the chest wall. The clinical introduction of this new approach was met with improved peri-operative outcomes for posterosuperior LLR cases; since then, the successful outcomes have reached a level similar to those of other-area LLRs performed in our institute (data not shown).
Table 1.
Approaches to laparoscopic liver resection for tumors located in the posterosuperior lesion
| (A) Options for handling the right liver and acquiring a stable surgical field |
| Hand-assisted laparoscopic surgery |
| Hand usage and hand-port positioning without causing disturbance of the operative view and allowing for manipulation in the small subphrenic cage should be further discussed |
| Robotic liver resection |
| Intercostally-inserted powerful robotic arms and ports may cause damage to the chest wall and pleura and may be responsible for the reported higher incidence of pulmonary complications |
| Specific spacers (i.e., sterile glove pouch) |
| Spacer usage without causing disturbance of the operative view and allowing for manipulation in the small subphrenic cage should be established |
| Postural change (semi-left lateral - left lateral - semi-prone)1 |
| Laparoscopic surgery facilitates the use of postural changes |
| (B) Options for access to posterosuperior lesions in the rib cage (Figure 1) |
| Lateral approach (using intercostal ports) |
| Caudal approach (using laparoscopic-specific caudal view)1 |
| Thoracoscopic liver resection (for segment 8, but not segment 7) |
Used in our approach.
We had previously reported on use of the caudal approach posterior sectionectomy in the left lateral position[12]. In that report, the novel concept of the caudal approach in LLR was posed for the first time in the literature worldwide, and several papers followed[33,34]. For hemi-hepatectomies, resection of the anterolateral segments, as well as of S8 and cranial 4 and 1 resection of LLR, the supine to semi-lateral positioning with tilting of the operating table during surgery had been employed in our hospital. However, the boundary plane between the anterior and posterior sections, which represents the cutting plane for posterior sectionectomy, is horizontal in the supine position. Although the cutting plane should be well opened in the small subphrenic space in order to achieve a successful laparoscopic posterior sectionectomy, gravity obstructs the exposure of the cutting plane in that position. On the other hand, one of the advantages of LLR is a clear view from the caudal and the dorsal directions (Figure 1).
We also had developed a new procedure that facilitates exposure of the cutting plane in a pure laparoscopic posterior sectionectomy, which is a caudal approach with parenchymal transection prior to mobilization of the liver conducted under the laparoscopic caudal view with the patient in the left lateral position. In that procedure, the cutting plane is well-opened between the retroperitoneal-fixed resected liver and the remnant liver, which has fallen downwards by gravity (Figure 2). The decreased venous pressure in the right hepatic vein, positioned vertically upwards from the IVC, leads to decreased intraoperative bleeding. Moreover, an oncological benefit similar to that as the anterior approach in open right hepatectomy can be obtained. As mentioned above, however, the transection of S7 segmentectomy or posterosuperior partial resection should be performed in the deep small subphrenic space when S6 of the liver is an obstacle to lesions under the laparoscopic caudal view, even when the patient is in the left lateral position. Therefore, we employed the semi-prone position only for S7 segmentectomy and partial resections of S7 and deep S6, but not for S6 or 8 segmentectomy and partial resection of S8 or shallow S6.
Figure 2.
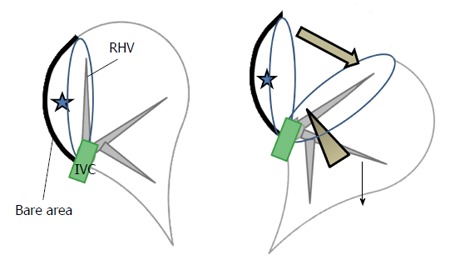
Schema of the caudal approach for laparoscopic posterior sectionectomy with prior transection without mobilization in the left lateral position. Stars denote the tumor in the posterior section of the liver. The left image shows the organ pre-transection, and the right image shows the organ during transection. The transection was performed in one direction from the caudal edge of the liver, with exposure of the inferior vena cava (IVC) and the right hepatic vein on the cutting plane (arrow head, right). This procedure facilitates exposure of the cutting plane (thick arrow, right) between the retroperitoneal-fixed resected liver and the remnant liver, which has fallen downward by gravity (thin arrow, right). The decreased venous pressure in the right hepatic vein, vertically standing up from the IVC, leads to decreased intraoperative bleeding. Finally, the oncological benefit is the same as the anterior approach in open right hepatectomy.
In contrast to the report from Ikeda et al[23], our semi-prone LLR are performed using the caudal approach, without intercostal ports, to accommodate the caution needed to avoid damage to the chest wall and pleura that may otherwise lead to pleural effusion and pulmonary complications. Moreover, our S7 partial resection is often performed with only partial dissection of the retroperitoneum. A key aim of our LLR is to carry out minimum dissection of the attachments and adhesions around the liver, in order to minimize the risk of damage to the environment around the liver. Most of our LLR patients have HCC/CLD and, occasionally, severe CLD patients undergo LLR[35]; this is partially due to the well-recognized situation in Japan involving a shortage of cadaver donors. Damage to the proximal environment associated with liver resection, such as dissection-related destruction of collateral venous/lymphatic flows, can easily lead to massive ascites and liver failure.
Here, we describe our approach to posterosuperior tumors. Patients are first placed in semi-prone position, with the port arrangements as shown in Figure 3. With the gravity-assisted movement of the colon, small intestine and also S6 of the liver, downward to the left, Morrison’s pouch becomes well-opened, facilitating port insertion to the area from the back near the vertebra (Figure 3). The gravity-assisted movement of the S6 also makes direct access to S7 possible from the caudal side. For the S7 segmentectomy, a good view and access to the right part of the hilar plate, posterior and S7 Glissonian pedicles are established upon flipping up of the liver S6 and the gallbladder in the left upward direction (Figure 4). In cases in which a large tumor is lodged into the subphrenic space, prior parenchymal transection for mobilization is performed to acquire good view and manipulation of the transection plane that has become well-opened by gravity, similar to our procedure for posterior sectionectomy in the left lateral position. However, regular S7 segmentectomy and partial resection of S7 and deep S6 are performed after liver mobilization from the retroperitoneum, which is easily achieved via gravity (Figure 5). After mobilization, there is adequate space above the liver and it is possible to perform stable handling of the instruments and removal of tumors in regions up to the root of the right hepatic vein (Figure 5).
Figure 3.
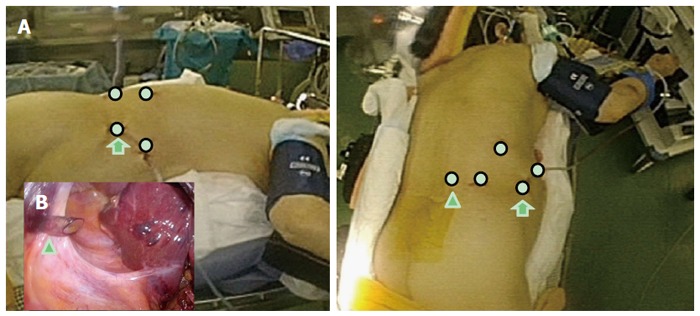
Schema of the settings for semi-prone positioning and port placement. A: The patient is placed in the semi-prone position (left-lateral view, right-caudal view). The white circles indicate the locations for the ports; the arrowhead indicates the port in the para-vertebra area; the arrows indicate the port mainly used for laparoscope; B: The arrowhead indicates the port in the para-vertebra area, as seen under the view of laparoscope inside the abdomen. Morrison’s pouch is well-opened in the semi-prone position, with the gravity-assisted movement of the organs, and the port is easily inserted into the area.
Figure 4.
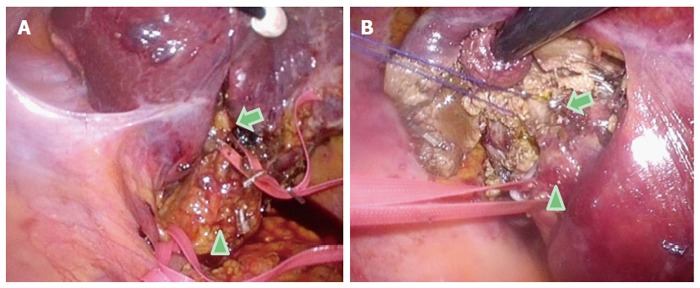
Intraoperative findings of Glissonian pedicles during the semi-prone position caudal approach for segment 7 segmentectomy. For the segment (S) 7 segmentectomy, a good view and access to the right part of the hilar plate, posterior and the S7 Glissonian pedicles are established upon flipping-up of the liver S6 and of the gallbladder in the left upward direction. A: The arrowhead indicates the hepatoduodenal ligament encircled with tape; the arrow indicates the posterior branch of the Glissonian pedicle encircled with tape in the Rouviere’s sulcus; B: The arrowhead indicates the posterior branch of the Glissonian pedicle encircled with tape; the arrow shows the S7 branch of the Glissonian pedicle encircled with string.
Figure 5.
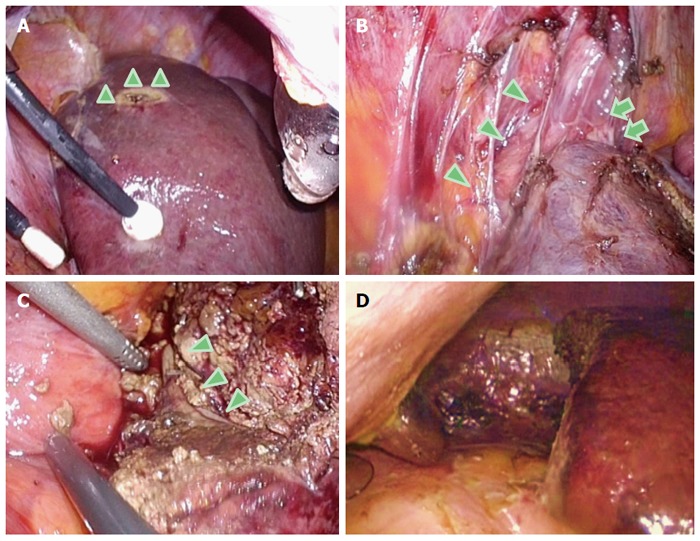
Intraoperative findings in the caudal approach for S7 segmentectomy in the semi-prone position. A: Dissection and mobilization are easily performed, since the liver is hanging down from the retroperitoneum. The arrowheads indicate the tension on the retroperitoneum; B: The inferior vena cava (arrowheads) and the root of the right hepatic vein (arrows) are easily recognized and dissected after the liver mobilization from the right dorsal side; C: The right hepatic vein (arrowheads) is exposed on the transection plane in the S7 segmentectomy. This procedure is safely performed on the well-opened transection plane; D: After completion of the resection, a clear and stable view of the subphrenic area is obtained.
As of the writing of this article, all of the LLR procedures performed in our institute are carried out by the caudal approach with postural changes. The left lateral position is employed for posterior sectionectomy and the semi-prone position is employed for S7 segmentectomy and partial resections of S7 and deep S6. For tumors in S8 and shallow S6, the supine to semi-lateral positioning is employed. Movement of instruments is restricted in our semi-prone caudal approach, as compared with the lateral approach LLR for posterosuperior tumors. However, port placement, as described above and especially that in which the port is placed in the para-vertebra area, makes the manipulation feasible and stable, while posing a minimum risk of damage to the environment around the liver.
CONCLUSION
LLR for posterosuperior tumor is technically demanding for obtaining a fine surgical view and manipulation that is sufficient to ensure hemostasis and obtainment of an adequate surgical margin. Handling of the large right liver may be performed by either the hand-assisted approach, RLR, or the approach using spacers. The use of intercostal ports allows for a direct lateral approach into the subphrenic rib cage, while applying postural changes allows for a stable view and space enough for manipulation in the target area. In our institute, we apply the caudal approach with the patient in the semi-prone position for LLR, without the use of intercostal ports, for treatment of posterosuperior tumors; this approach facilitates stable S7 segmentectomy and partial resection, with minimal risk of damage to the environment around the liver.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The author declares no conflict of interest related to this publication.
Peer-review started: August 12, 2016
First decision: September 12, 2016
Article in press: October 31, 2016
P- Reviewer: Li ZF, Waisberg J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–958. [PubMed] [Google Scholar]
- 2.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 3.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Isetani M. How Far Can We Go with Laparoscopic Liver Resection for Hepatocellular Carcinoma? Laparoscopic Sectionectomy of the Liver Combined with the Resection of the Major Hepatic Vein Main Trunk. Biomed Res Int. 2015;2015:960752. doi: 10.1155/2015/960752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho CM, Wakabayashi G, Nitta H, Takahashi M, Takahara T, Ito N, Hasegawa Y. Total laparoscopic limited anatomical resection for centrally located hepatocellular carcinoma in cirrhotic liver. Surg Endosc. 2013;27:1820–1825. doi: 10.1007/s00464-012-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauchy F, Schwarz L, Scatton O, Soubrane O. Laparoscopic liver resection for living donation: where do we stand? World J Gastroenterol. 2014;20:15590–15598. doi: 10.3748/wjg.v20.i42.15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machado MA, Makdissi FF, Surjan RC, Mochizuki M. Laparoscopic resection of hilar cholangiocarcinoma. J Laparoendosc Adv Surg Tech A. 2012;22:954–956. doi: 10.1089/lap.2012.0339. [DOI] [PubMed] [Google Scholar]
- 7.Isetani M, Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S. Pure laparoscopic hepatectomy as repeat surgery and repeat hepatectomy. World J Gastroenterol. 2015;21:961–968. doi: 10.3748/wjg.v21.i3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745–753. doi: 10.1002/jhbp.166. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery. 1996;120:468–475. doi: 10.1016/s0039-6060(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 10.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko H. Laparoscopic hepatectomy: indications and outcomes. J Hepatobiliary Pancreat Surg. 2005;12:438–443. doi: 10.1007/s00534-005-1028-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery. 2008;144:32–38. doi: 10.1016/j.surg.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Tomishige H, Morise Z, Kawabe N, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R, Isetani M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg. 2013;5:173–177. doi: 10.4240/wjgs.v5.i6.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 15.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, Marvin M, Ravindra KV, Mejia A, Lainas P, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 17.Cho JY, Han HS, Yoon YS, Shin SH. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg. 2009;144:25–29. doi: 10.1001/archsurg.2008.510. [DOI] [PubMed] [Google Scholar]
- 18.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392. doi: 10.3748/wjg.v20.i39.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman P, Krüger JA, Perini MV, Coelho FF, Lupinacci RM. Laparoscopic hepatic posterior sectionectomy: a hand-assisted approach. Ann Surg Oncol. 2013;20:1266. doi: 10.1245/s10434-012-2750-3. [DOI] [PubMed] [Google Scholar]
- 20.Patriti A, Cipriani F, Ratti F, Bartoli A, Ceccarelli G, Casciola L, Aldrighetti L. Robot-assisted versus open liver resection in the right posterior section. JSLS. 2014;18 doi: 10.4293/JSLS.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bin J, Binghai Z, Sanyuan H. Liver Exposure Using Sterile Glove Pouch During Laparoscopic Right Liver Surgery in Hepatocellular Carcinoma Patients. World J Surg. 2016;40:946–950. doi: 10.1007/s00268-015-3343-7. [DOI] [PubMed] [Google Scholar]
- 22.Ogiso S, Conrad C, Araki K, Nomi T, Anil Z, Gayet B. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg. 2015;262:358–365. doi: 10.1097/SLA.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T, Toshima T, Harimoto N, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y. Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc. 2014;28:2484–2492. doi: 10.1007/s00464-014-3469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troisi RI, Patriti A, Montalti R, Casciola L. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Robot. 2013;9:160–166. doi: 10.1002/rcs.1495. [DOI] [PubMed] [Google Scholar]
- 25.Gumbs AA, Gayet B. Video: the lateral laparoscopic approach to lesions in the posterior segments. J Gastrointest Surg. 2008;12:1154. doi: 10.1007/s11605-007-0455-x. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz L, Aloia TA, Eng C, Chang GJ, Vauthey JN, Conrad C. Transthoracic Port Placement Increases Safety of Total Laparoscopic Posterior Sectionectomy. Ann Surg Oncol. 2016;23:2167. doi: 10.1245/s10434-016-5126-2. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Aoki T, Kato T. Video-assisted thoracoscopic surgery: hepatectomy for liver neoplasm. World J Surg. 2011;35:1050–1054. doi: 10.1007/s00268-011-0999-5. [DOI] [PubMed] [Google Scholar]
- 28.Aikawa M, Miyazawa M, Okamoto K, Toshimitsu Y, Okada K, Ueno Y, Yamaguchi S, Koyama I. Thoracoscopic hepatectomy for malignant liver tumor. Surg Endosc. 2014;28:314. doi: 10.1007/s00464-013-3128-8. [DOI] [PubMed] [Google Scholar]
- 29.Teramoto K, Kawamura T, Takamatsu S, Noguchi N, Nakamura N, Arii S. Laparoscopic and thoracoscopic partial hepatectomy for hepatocellular carcinoma. World J Surg. 2003;27:1131–1136. doi: 10.1007/s00268-003-6936-5. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda T, Yonemura Y, Ueda N, Kabashima A, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, et al. Pure laparoscopic right hepatectomy in the semi-prone position using the intrahepatic Glissonian approach and a modified hanging maneuver to minimize intraoperative bleeding. Surg Today. 2011;41:1592–1598. doi: 10.1007/s00595-010-4479-6. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda T, Mano Y, Morita K, Hashimoto N, Kayashima H, Masuda A, Ikegami T, Yoshizumi T, Shirabe K, Maehara Y. Pure laparoscopic hepatectomy in semiprone position for right hepatic major resection. J Hepatobiliary Pancreat Sci. 2013;20:145–150. doi: 10.1007/s00534-012-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Hondt M, Yoshihara E, Vansteenkiste F, Steelant PJ, Van Ooteghem B, Pottel H, Devriendt D, Van Rooy F. Laparoscopic parenchymal preserving hepatic resections in semiprone position for tumors located in the posterosuperior segments. Langenbecks Arch Surg. 2016;401:255–262. doi: 10.1007/s00423-016-1375-6. [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21:723–731. doi: 10.1002/jhbp.139. [DOI] [PubMed] [Google Scholar]
- 34.Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, Scatton O. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg. 2015;261:1226–1231. doi: 10.1097/SLA.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 35.Morise Z, Sugioka A, Kawabe N, Umemoto S, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J Endosc Surg. 2011;4:143–146. doi: 10.1111/j.1758-5910.2011.00081.x. [DOI] [PubMed] [Google Scholar]


