Abstract
Proteases, enzymes catalyzing the hydrolysis of peptide bonds, are present at high concentrations in the gastrointestinal tract. Besides their well-known role in the digestive process, they also function as signaling molecules through the activation of protease-activated receptors (PARs). Based on their chemical mechanism for catalysis, proteases can be classified into several classes: serine, cysteine, aspartic, metallo- and threonine proteases represent the mammalian protease families. In particular, the class of serine proteases will play a significant role in this review. In the last decades, proteases have been suggested to play a key role in the pathogenesis of visceral hypersensitivity, which is a major factor contributing to abdominal pain in patients with inflammatory bowel diseases and/or irritable bowel syndrome. So far, only a few preclinical animal studies have investigated the effect of protease inhibitors specifically on visceral sensitivity while their effect on inflammation is described in more detail. In our accompanying review we describe their effect on gastrointestinal permeability. On account of their promising results in the field of visceral hypersensitivity, further research is warranted. The aim of this review is to give an overview on the concept of visceral hypersensitivity as well as on the physiological and pathophysiological functions of proteases herein.
Keywords: Proteases, Proteinase-activated receptors, Protease inhibitors, Visceral hypersensitivity, Visceral pain, Irritable bowel syndrome, Inflammatory bowel diseases
Core tip: Proteases are enzymes catalyzing the hydrolysis of peptide bonds. They are present at high levels in the gastrointestinal tract and they execute a large variety of physiological and pathophysiological functions. In the last decade, it became clear that proteases fulfill an important role in visceral pain, a major symptom in patients with inflammatory bowel diseases and/or irritable bowel syndrome. These review articles aim at providing an overview of the diverse roles of proteases in both health and disease states related to the gastrointestinal functions, with the emphasis on visceral pain in this review.
INTRODUCTION
Abdominal pain is a key feature of two major gastrointestinal disorders, inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). On the one hand, IBD, such as ulcerative colitis (UC) and Crohn’s disease (CD), are characterized by acute flares of inflammation followed by periods of remission[1]. On the other hand, IBS is a functional disorder defined by the presence of altered bowel habits and abdominal pain, in the absence of an organic cause[2]. The diagnosis of IBS is based on the Rome IV criteria: recurrent abdominal pain for at least 3 d/mo in the last 3 mo associated with two or more of the following symptoms: (1) improvement with defecation; (2) onset associated with a change in frequency of stool; and (3) onset associated with a change in form (appearance) of stool[2,3]. Currently four IBS subtypes are defined based on the predominant stool pattern: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS (IBS-M) and unsubtyped IBS (IBS-U)[2]. Both IBD and IBS have an increasing prevalence; IBD affects up to 1.5 million people in the United States of America and 2.2 million Europeans[4], whereas IBS has a worldwide prevalence of 11.2%[5]. Although IBD and IBS are regarded as two different diseases, they seem to be related: the prevalence of IBS-like symptoms in IBD patients with an active disease and in IBD patients in remission amounts to 44% and 35%, respectively[6,7]. Besides being highly prevalent, these disorders have a considerable impact through their chronic character, as well as a negative influence on the quality of life and an important socio-economic impact[8,9]. Furthermore, the current treatment options are mostly focusing on the reduction of inflammation for IBD or the motility disturbances for IBS. Only a few therapies aim directly at diminishing the abdominal pain. Remarkably, only 24% of the IBS patients report complete relief of abdominal pain after treatment which is mostly only a symptomatic treatment of the most explicit motility-related symptom[8]. Therefore, further research in this area is of utmost importance. However, the search for new treatment targets is hampered due to the incomplete understanding of the pathogenesis of visceral pain.
Visceral hypersensitivity, or an increased pain perception in the bowel, is an important factor underlying the abdominal pain in IBS[10]. Visceral hypersensitivity occurs via a disturbance of the sensitization pathways that might be located at different levels. At the peripheral level, chemical, mechanical and thermal information is registered by primary afferent neurons. The cell bodies of these neurons are located in the dorsal root ganglia (DRGs)[11]. Centrally, they connect with secondary afferent neurons in the dorsal horn of the spinal cord or in the brain stem. From there, the signal is transduced to different cerebral areas involved in the somatosensory sensation of (pain) signals arising from the bowel. Within the central nervous system, signals of peripheral afferent nerves are modulated via descending pathways, resulting in facilitation or inhibition of the impulse conduction[12]. For a complete overview of the neuroanatomy of lower gastrointestinal pain disorders, we like to refer the interested readers to a review by Vermeulen et al[13].
During gastrointestinal inflammation, the continuous release of inflammatory mediators can give rise to the sensitization of peripheral afferent nerves, thus contributing to the development of visceral hypersensitivity. Besides being an important well-known trigger for IBD, (microscopic) inflammation has been demonstrated to play a role in IBS as well. As already mentioned, an increased prevalence of IBS-like symptoms is present in IBD patients. Moreover, a disease state denominated as post-infectious IBS has been described; it is seen in 3%-35% of the patients that experienced an acute gastroenteritis related to water contamination[14]. These observations suggest a strong association between gastrointestinal inflammation on the one hand and the onset of visceral hypersensitivity on the other hand, in which case the hypersensitivity persists even after complete resolution of the inflammation.
At the peripheral level, inflammatory cells, e.g., mast cells, T-cells and neutrophils, are activated. Upon activation, they release excessive amounts of inflammatory mediators such as histamine, serotonin, several cytokines and proteases. These mediators, in turn, can sensitize peripheral afferent neurons, thus contributing to visceral hypersensitivity. The role of most of these inflammatory mediators in visceral hypersensitivity has been investigated extensively[7].
However, the role of proteases is not fully elucidated yet. Therefore, the aim of this review is to focus on proteases and their physiological role as well as their role in the pathophysiology of visceral hypersensitivity. In an accompanying paper by Van Spaendonk et al[15], we discussed the role of proteases in intestinal permeability.
VISCERAL HYPERSENSITIVITY
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by altered bowel habits and abdominal pain. This visceral pain - pain originating from internal organs such as the gut - is typically vague, diffuse, poorly localized and often associated with referred pain[16]. Visceral hypersensitivity is the main mechanism underlying this abdominal pain[17]. Hypersensitivity refers to the increased sensation of stimuli: both allodynia (pain evoked by stimuli that are normally not painful) and hyperalgesia (increased response to a painful stimulus) are present[18]. In both basic and clinical research, visceral hypersensitivity is commonly investigated by colorectal balloon distensions using a barostat[19]. Compared to healthy individuals, patients suffering from visceral hypersensitivity report discomfort at lower colonic distension pressures (lower pain threshold) and increased pain in response to standardized stimuli[18]. Currently, the pathophysiology of visceral hypersensitivity has not been fully elucidated yet, but several mechanisms, such as inflammation, psychosocial factors and/or sensorimotor alterations, are thought to be involved. The latter can be located both at the central and/or peripheral level along the anatomical afferent information pathway from the viscera towards the brain. Moreover, an important role for peripheral as well as central sensitization of the afferent visceral neuron pathways is proposed[20].
The mast cell is thought to fulfill an important task in the development of abdominal pain in IBS patients[21], since an association was found between mast cell infiltration in the bowel wall and the frequency and severity of abdominal pain[22]. The mast cell is an important immune cell that can be activated by cytokines, antigens and neuropeptides. This activation is followed by a degranulation of the cell, releasing vasoactive and pro-inflammatory mediators[17]. Histamine, an important mast cell mediator, has already been shown to play an important role in visceral hypersensitivity: we demonstrated a role for histamine in a rat model for post-inflammatory visceral hypersensitivity, mediated by histamine H1 and H4 receptors[23]. Also in humans, a recent clinical trial with the H1 receptor-antagonist ebastine showed promising results in IBS patients resulting in a phase II trial (www.clinicaltrials.gov)[24]. Apart from histamine, mast cells release many other mediators such as cytokines, growth factors, leukotrienes, prostaglandins, serotonin and several proteases[25]. In this review, we will focus on the role of this last group of mediators, the proteases, and their role in visceral hypersensitivity. In the accompanying paper the role in intestinal permeability is addressed.
PROTEASES
Proteases are enzymes catalyzing the hydrolysis of peptides and/or proteins, thereby releasing amino acids or peptides. They represent up to 2% of the human genome and are present at particularly high levels in the gastrointestinal tract[26]. Proteases execute a large variety of physiological functions. They are vital for processes such as blood coagulation, cell growth and migration, tissue arrangement, activation of zymogens, protein catabolism and the release of hormones and pharmacologically active peptides from precursor proteins. Apart from that, they are involved in pathological processes such as inflammation and tumor growth and metastasis[27,28].
Based on the position of the peptide bond that can be cleaved, proteases are subdivided into two major groups: exopeptidases and endopeptidases. Exopeptidases catalyze the cleavage of the terminal or the penultimate peptide bond of the protein, releasing a single amino acid or a dipeptide, respectively[29,30]. Since peptide chains have both an amino- and a carboxy-terminus, exopeptidases can be further divided into amino- and carboxypeptidases[29]. Aminopeptidases could release a single amino acid, a dipeptide or a tripeptide from the N-terminus, while carboxypeptidases can liberate an amino acid or a dipeptide from the C-terminus[31]. Unlike exopeptidases, endopeptidases catalyze the cleavage of nonterminal peptide bonds within the molecule, thus releasing larger peptides instead of single amino acids, dipeptides or tripeptides[29,30]. The concept of exo- and endopeptidases is represented in a simplified way in Figure 1.
Figure 1.
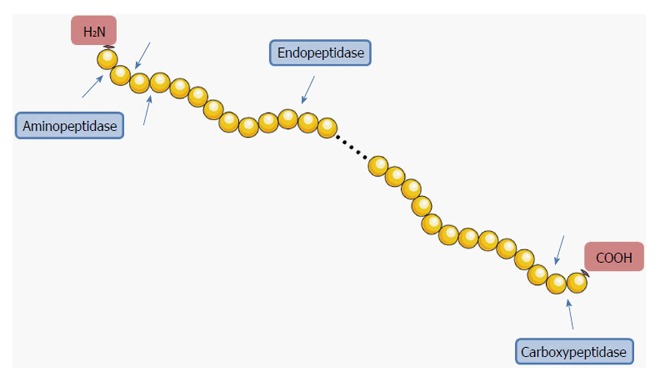
Simplified representation of the concept of endo- and exopeptidases. Endopeptidases cleave internal peptide bonds. Exopeptidases cleave terminal peptide bonds; they can be subdivided into amino- and carboxypeptidases according to the position of the cleavage of the peptide bond. Aminopeptidases cleave at amino (NH2) terminal bonds, while carboxypeptidases cleave at carboxy (COOH) terminal bonds. Image constructed using the Servier Image Bank.
Due to their huge diversity in action and structure, proteases can be classified in other ways as well[28]. For example, it is possible to classify the enzymes according to their evolutionary relatedness with reference to structure, the type of reaction catalyzed or the pH optimum of the enzyme[28]. However, they are usually categorized according to their catalytic type, based upon the presence of different nucleophiles in the molecular structure of the enzyme[29-33]. The major advantage of using a catalytic type based classification is that proteases of the same catalytic type usually respond to the same protease inhibitors[33]. Historically, four major groups could be distinguished in this classification, based upon the functional group present at the active site: serine, cysteine, aspartic and metalloproteases[30,32]. More recently, threonine, glutamate and asparagine proteases have been added as classes. However, glutamate and asparagine proteases have not been found in humans or other mammals so far[33]. The seven catalytic classes are depicted in Figure 2. In the last decades, proteases have come into the picture as a new target for drug development. An overview of the protease inhibitors approved for clinical use can be found in a Nature review by Turk[34]. Concerning visceral hypersensitivity, serine proteases are thought to be an important class[35]. Some examples of serine protease inhibitors that were already tested in clinical trials are summarized in Table 1, thereby emphasizing the widespread indications. Proteases can also act as signaling molecules; they regulate cell functions by modulating protease-activated receptors (PARs). PARs belong to a family of cell-surface signaling proteins called G protein-coupled receptors (GPCRs) and consist of seven transmembrane domains with three intracellular and three extracellular protein loops[26,36], as visualized in Figure 3. So far, four PARs have been described: PAR1, PAR2, PAR3 and PAR4[37].
Figure 2.
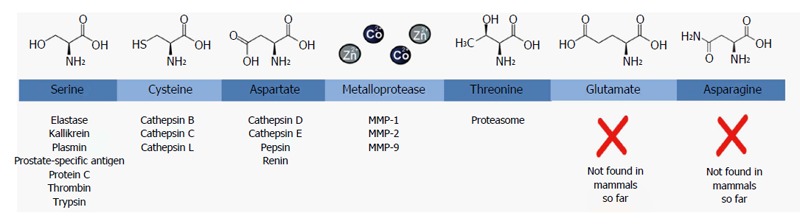
Classification of proteases based on the chemical structure of their active site. For each class, the chemical structure of the core residue in their active site is shown on top and a few examples with medical relevance of proteases belonging to that family are displayed below. MMP: Matrix metalloprotease.
Table 1.
Serine protease inhibitors (mammalian): examples of clinical applications in different organ systems
| Category | Indication | Serine protease inhibitor (target) | Status | Ref. |
| Cardiovascular | ACS | Bivalirudin (thrombin) | Approved | [73] |
| AF | Rivaroxaban (factor Xa) | Approved | [74] | |
| Edoxaban (factor Xa) | Approved | [75] | ||
| VTE | Dabigatran (thrombin) | Approved | [76] | |
| Dermatology | Herpes zoster | Argatroban (thrombin) | Clinical - phase II | [77] |
| Oral leukoplakia | BBIC (broad specificity) | Clinical - phase II | [78] | |
| Hematology | Heparin-induced thrombocytopenia | Argatroban (thrombin) | Approved | [79] |
| Fondaparinux (factor Xa) | Approved | |||
| Oncology | Pancreatic cancer: CTx | Upamostat (uPA) | Clinical - phase II | [80] |
| Nafamostat mesilate (broad specificity) | Clinical - phase II | [81] | ||
| Lung cancer: RTx | Ulinastatin (broad specificity) | Clinical - phase unknown | [82] | |
| Colorectal cancer: CTx | Talabostat (fibroblast activating protein) | Clinical - phase II | [83] | |
| Esophageal cancer: Sx | Ulinastatin (broad specificity) | Clinical - phase unknown | [84] | |
| Pneumology | Asthma | APC 366 (mast cell tryptase) | Clinical - phase II | [85] |
| α1 antitrypsin deficiency | α1 antitrypsin (broad specificity) | Approved | [86] | |
| Cystic fibrosis | α1 antitrypsin (broad specificity) | Clinical - phase II | [87] | |
| Endocrinology | Diabetes | Gliptins (DPP-IV)1 | Approved | [88] |
| Surgery | NA | Aprotinin (broad specificity) | Approved | [89] |
| Gabexate mesilate (broad specificity) | Clinical - phase III | [90] | ||
| Nafamostat mesilate (broad specificity) | Clinical - phase IV | [91] | ||
| Sivelestat (neutrophil elastase) | Clinical - phase unknown | [92] |
DPP-IV is a non-classical serine protease belonging to family S9: prolyl oligopeptidase (novel class). Literature search in Pubmed (last updated Sept 19 2016) with MeSH terms serine protease inhibitor - dpp-4 inhibitor - clinical trials - English - human. ACS: Acute coronary syndrome; A: Atrial fibrillation; BBIC: Bowman Birk inhibitor concentrate; BHR: Bronchial hyperresponsiveness; cf.: Compared to; CTx,: Chemotherapy; DPP-IV: Dipeptidylpeptidase IV; EAR: Early asthmatic response; GP: Glycoprotein IIb/IIIa inhibitor; ICH: Intracranial hemorrhage; LAR: Late asthmatic response; PA: Protease activity; PI: Protease inhibitor; POC: Postoperative complications; RTx: Radiotherapy; SIRS: Systemic inflammatory respiratory syndrome; Sx: Surgery; uPA: Urokinase plasminogen activator; VTE: Venous thromboembolism.
Figure 3.
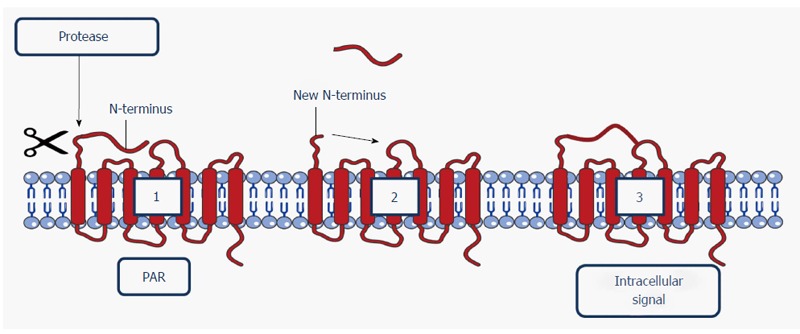
Schematic representation of the activation of a protease-activated receptor. A protease cleaves the N-terminal domain (1), releasing a new N-terminus (2). The new N-terminus binds to the receptor as a tethered ligand, providing an intracellular signal (3). Image constructed using the Servier Image Bank. N-terminus: Amino-terminus; PAR: Protease-activated receptor.
PARs have been found in various cell types throughout the whole gastrointestinal tract. Remarkably, a great overlap can be detected in the cell types expressing PAR1 and PAR2. PAR1 and PAR2 have already been described in enterocytes, neurons, fibroblasts, mast cells, smooth muscle cells, endothelium and immune cells. On the other hand, PAR4 has only been identified in enterocytes, neurons, endothelium and immune cells. Furthermore, PAR3 has been identified in stomach and small intestine but the exact cell types still need to be defined. For a complete overview of the location of the different PARs in the gastrointestinal tract, we would like to refer to a review by Vergnolle[38].
PARs can transduce signals by canonical activation. The activation process starts with a protease recognizing one of the extracellular domains of the receptor, situated on the N-terminus (i.e., the canonical site of the PAR receptor). Some proteases, such as thrombin, subsequently bind to this domain. For other proteases, e.g., trypsin or tryptase, it is not required to establish a stable bond in order to cleave the receptor. When the receptor has been activated, the N-terminal domain will be cleaved by proteolysis at the recognition site, thus exposing a new N-terminal sequence. This sequence acts as a tethered ligand that binds domains situated in the second extracellular loop of the receptor, thus initiating common signaling pathways, such as the G-protein- and/or β-arrestin-dependent pathways (e.g., PAR2 activation by trypsin)[26,39]. The activation process is visualized in Figure 3.
Apart from canonical activation, PAR signaling pathways can be initiated in several other ways. Biased agonism is a second possibility; proteases cleave at sites distinct from the canonical sites, thereby activating unique and biased signaling pathways (e.g., PAR2 activation by activated protein C)[39]. Proteases are also able to induce the opposite effect; this third manner is called proteolytic disarming. In that case, proteases can remove or destroy tethered ligands leading to the termination of PAR activation (e.g., PAR2 disarming by cathepsin-G)[37,39]. A fourth manner is the non-tethered ligand activation, suggesting that the formation of tethered ligands is not essential for PAR activation (e.g., PAR2 activation by elastase). Finally, it is possible to activate PARs via so-called PAR-activating peptides (PAR-APs), synthetic peptides corresponding to the first five or six amino acids of the tethered ligand sequence. This form of artificial activation bypasses the proteolytic cleaving process. PAR-APs are capable of inducing common as well as biased signaling pathways (e.g., PAR2 activation by SLIGKV-NH2)[39,40]. Since these peptides are specific for a single receptor, they are very important pharmacological tools to investigate the physiology and physiopathology of PARs[37].
Currently, thrombin is regarded as the main activator for PAR1, PAR3 and PAR4, as is the serine protease trypsin for PAR2 and PAR4. Although some proteases can activate different PARs, every protease has a preference for one specific receptor. For example, thrombin has the highest potency for PAR1, a lower potency for PAR3, and even weaker for PAR4[26]. For an overview of the characteristics of the four PARs, their activating proteases and agonists/antagonists, we refer to a detailed and excellent review by Vergnolle[38].
PROTEASES, PROTEASE-ACTIVATED RECEPTORS AND PROTEASE INHIBITORS IN VISCERAL HYPERSENSITIVITY
Proteases
So far, the expression and activity of proteases in the colon and feces of IBS patients have not been investigated intensively. A higher expression of the serine proteases tryptase[22,41-45] and trypsin[41,42] as well as the cysteine protease calpain-8[46] was described in colonic biopsy samples of IBS patients compared to healthy controls. However, no changes in tryptase expression (protein level) could be detected in fecal samples of IBS patients[47,48]. Serine protease activity was increased in IBS patients compared to healthy controls in both colon and feces in several studies[41,48-50]. Likewise, an elevated serine protease activity was observed in colonic samples in a post-infectious IBS mouse model[51]. Taken together all the studies described above, an important role for serine proteases in IBS cannot be denied. However, the origin of those serine proteases was unclear for a long time. Recently, the Spiller group published a paper demonstrating the human (and not bacterial) origin of the most abundant fecal serine proteases in IBS-D patients[52]. Cysteine protease activity was elevated in fecal samples of IBS-C patients[53], although this alteration could not be observed in colonic samples of post-infectious IBS mice[51]. Recently, concerns have been raised about the tests used to determine the protease activity. Many of these tools suffer from a lack of selectivity for individual proteases and most of them detect several enzymes. For example, when determining tryptase-activity, mostly trypsin-like activity is quantified due to the lack of specificity of the substrates used[54]. Therefore, with regard to a detailed study of the role of proteases in e.g., visceral hypersensitivity, the improvement of chemical tools to assess the activity of specific proteases is of utmost importance[54].
Protease-activated receptors
Proteases are thought to influence visceral sensitivity through protease-activated receptors (PARs). Remarkably, after the activation of these receptors, the effects on pain are not the same for all PARs. When PAR1 and PAR4 are activated, antinociceptive effects are observed, while the activation of PAR2 induces pronociceptive effects. An overview of the studies described in the following paragraph is shown in Table 2. The antinociceptive properties of PAR1 activation are demonstrated in different animal models showing a decrease in carrageenan-induced visceral hyperalgesia in rats and capsaicin-evoked visceral pain in mice after an intraplantar administration with the PAR1-agonists TFFLR-NH2 and thrombin[55,56]. In mice, PAR4 activation seems to inhibit visceral hypersensitivity as well[49,57]. In parallel, PAR4 expression is lowered in the colon of IBS patients[42,58]. The PAR4-agonist AYPGKF-NH2 was able to reduce visceral hypersensitivity after an intracolonic administration in sub-inflammatory doses, while higher doses showed pro-inflammatory effects in mice[57].
Table 2.
Preclinical studies investigating the effects of protease-activated receptor-targeting molecules on visceral hypersensitivity
| PAR | Agonist/antagonist | Species (hypersensitivity model) | Study type | Effect | Ref. |
| PAR-1 | Agonist (thrombin, TFLLR-NH2) | Rat (carrageenan) | In vivo | ↓ hyperalgesia | [56] |
| PAR-1 | Agonist (TFLLR-NH2) | Mice (capsaicin) | In vivo | ↓ hyperalgesia | [55] |
| PAR-2 | Agonist (SLIGRL-NH2) | Mice (PAR2-agonist) | In vivo | ↑ hyperalgesia | [59] |
| PAR-2 | Agonist (SLIGRL-NH2, trypsin) | Rat (PAR2-agonist) | In vivo | ↑ hyperalgesia | [60] |
| PAR-2 | Agonist (SL-NH2, trypsin, typtase) | Guinea pig submucosal neurons (PAR2-agonist) | Ex vivo | ↑ neuron excitability | [61] |
| PAR-2 | Agonist (SLIGRL-NH2, Tc-NH2, trypsin, tryptase) | Mice, rat (PAR2-agonist) | KO | ↑ hyperalgesia, absent in KO | [62] |
| PAR-2 | Agonist (2-furoyl-LIGRL-NH2) | Mice (capsaicin) | KO | ↑ hyperalgesia, absent in KO | [63] |
| PAR-2 | Antagonist (ENMD-1068) | Mice (IBS-supernatant) | KO | ↓ hypersensitivity, absent in KO | [41] |
| PAR-2 | / | Mice DRG (IBS-D supernatant) | KO | ↑ neuron excitability, absent in KO | [64] |
| PAR-4 | Agonist (PAR-4-AP, Cat-G) | Mice (IBS-D supernatant) | In vivo | ↓ hypersensitivity | [49] |
| PAR-4 | Agonist (AYPGKF-NH2) | Mice (PAR2-agonist, TRPV4-agonist) | In vivo | ↓ hypersensitivity | [57] |
DRG: Dorsal root ganglia; IBS: Irritable bowel syndrome; KO: Knock-out; PAR: Protease-activated receptor; TRPV: Transient receptor potential vanilloid channels.
In sharp contrast to PAR1 and PAR4, the activation of PAR2 results in a pronociceptive effect. This was firstly demonstrated by Kawabata et al[59] and Coelho et al[60] who confirmed the presence of visceral hypersensitivity in rats after the administration (intracolonic/intraplantar) of the PAR2-activating peptide SLIGRL-NH2 or trypsin. An increased Fos-expression[60] and the presence of PAR2 mRNA in the dorsal root ganglia (DRG)[59] confirmed these results. An ex vivo study reconfirmed these observations: the application of several PAR2-agonists, such as trypsin, mast cell tryptase and SL-NH2, induced hyperexcitability of submucosal neurons in the ileum of guinea pigs[61]. The next step in this research included the use of experimental knock-out (KO) models. Similar to the studies described above, visceral hyperalgesia was observed in wild-type (WT) mice after the administration (intracolonic/intraplantar) of PAR2-activating peptides such as 2-furoyl-LIGRL-NH2 and trypsin. However, these effects were reduced in PAR2-KO mice[62,63]. The effects described above were confirmed using IBS-patient supernatant, which is a well-known alternative stimulus for visceral pain in experimental animal models. Murine sensory neurons in culture were sensitized after the addition of IBS patient supernatant, while this effect was absent in neurons from the KO mice lacking PAR2. Furthermore, this supernatant caused visceral hypersensitivity in WT mice, but not in mice treated with a PAR2-antagonist or in PAR2-KO mice[41]. Also, the IBS-D supernatant was able to enhance the neuronal excitability of colonic DRGs in WT but not in PAR2-KO mice, again demonstrating the importance of PAR2[64]. Based on these literature data we can conclude that the effects of proteases on visceral pain following PAR activation is dependent on the type of receptor involved: PAR1 and PAR4 evoke antinociceptive effects while the activation of PAR2 results in pronociception.
Protease inhibitors
So far, research groups in the field of visceral hypersensitivity have mainly focused on PAR-knockout experiments, while protease inhibitors have been investigated to a lesser extent. In this paragraph, an overview of the studies exploring the effects of protease inhibitors in visceral hypersensitivity, is given. All protease inhibitors, with their respective targets, are listed in Table 3. Nafamostat mesilate or FUT-175 is a broad specificity serine protease inhibitor. In mice, visceral hypersensitivity induced by the intracolonic infusion of IBS-D fecal supernatants, could be suppressed when the supernatant was pre-incubated with nafamostat mesilate[65]. Similar results were observed by the group of Cenac et al[41] who used a similar, but slightly different experimental design. They used the supernatant of biopsies of IBS patients instead of fecal samples and apart from a decrease in visceral hypersensitivity, they also observed less sensitization of murine neurons after a pre-incubation with nafamostat mesilate. We recently demonstrated a positive effect of a single intraperitoneal injection of nafamostat mesilate in a trinitrobenzenesulfonic acid (TNBS)-induced rat model for both acute and post-inflammatory visceral hypersensitivity[66,67]. Furthermore, the newly developed serine protease inhibitor benzyl N-1-[bis(4-acetamidophenoxy)phosphoryl]-2-(4-carbamimidamidophenyl)ethyl-carbamate [UAMC-0050, patent WO2007045496 (A1)] showed anti-nociceptive properties as well, both in an acute and in a post-inflammatory setting[66,67]. Camostat mesilate, another serine protease inhibitor with structural properties similar to nafamostat mesilate showed analogous results. Intragastric pre-treatment with camostat mesilate decreased hypersensitivity in rats with visceral hypersensitivity induced by acute restraint stress as well as spinal c-Fos expression (an indirect marker of neuronal activity) and fecal protease activity[68,69]. Also in an acute TNBS colitis model, which is a preclinical model for IBD, positive results were observed on visceral hypersensitivity after treatment with different protease inhibitors. Moussa et al[70] found a decrease in visceral sensitivity, fecal protease activity and PAR2 expression in acute TNBS colitis rats treated with a fermented soy germ extract, containing phytoestrogens (isoflavones) and serine protease inhibitors (Bowman-Birk Inhibitor). However, the effects on visceral sensitivity were completely reversed by simultaneous treatment with an estrogen receptor antagonist, suggesting that the effects were mostly attributed to the phytoestrogens. The same group also demonstrated the positive effect of phytoestrogens on hypersensitivity in another animal model: this time, stress-induced hypersensitivity in female rats could be prevented by a treatment with either estradiol benzoate or fermented soy germ extract. Again this time, the positive effects were abolished after concomitant administration of an estrogen receptor antagonist[71].
Table 3.
Serine protease inhibitors investigated in experimental visceral hypersensitivity models
| Inhibitor name | Target(s) | Ref. |
| Aprotinin | Chymotrypsin, elastase, KLK, plasmin, PA, trypsin, urokinase, XIIa | [49] |
| Bowman-Birk inhibitor | Chymotrypsin, trypsin | [70,71] |
| Camostat mesilate (FOY-305) | Trypsin, matriptase, prostasin, plasmin, tPA, uPA, Xa, IXa, thrombin, tissue factor, complement factors, tryptase, HNE, KLK | [68,69] |
| Cathepsin-G inhibitor | Cathepsin G | [49] |
| Nafamostat mesilate (FUT-175) | Tryptase, trypsin, C1r, C1s, thrombin, kallikrein, plasmin | [37,65-67] |
| UAMC-0050 | Tryptase, matriptase, KLK4, KLK8, uPA | [66,67,93] |
| Soybean trypsin inhibitor (SBTI) | Trypsin, chymotrypsin, plasmin, kallikrein, Xa | [49] |
C1r: Complement component 1r; C1s: Complement component 1s; HNE: 4-hydoxynonenal; KLK: Kallikrein; PA: Plasminogen activator; tPA: Tissue plasminogen activator; uPA: Urokinase plasminogen activator.
In sharp contrast to the hypersensitivity seen in mice after an intracolonic infusion with fecal IBS-D supernatant, fecal supernatant of UC patients evoked hyposensitivity to colorectal distension. In IBS-D, PAR2 is stimulated due to the increased fecal serine protease activity, resulting in hypersensitivity. In addition, the activation of PAR4, by adding Cathepsin-G (Cat-G) to the supernatant, reversed this effect. Hyposensitivity could be observed after the infusion of a UC supernatant most likely because PAR4 (activated by Cat-G) is predominantly activated. However after the inhibition of PAR4 or Cat-G, hypersensitivity appeared and the addition of the serine protease inhibitors aprotinin/SBTI normalized sensitivity[49]. Based on these studies by Annaházi et al[49] the importance of the equilibrium between the activation of PAR2 and PAR4 in visceral sensitivity was clearly shown. To summarize this paragraph, the limited amount of data available regarding protease inhibitors and visceral pain show promising results. However, only a few broad specificity inhibitors were investigated and in a majority of the studies, a preventive treatment scheme was used. Thus, this topic needs to be further explored.
CONCLUSION
The pharmacological treatment of gastrointestinal disorders such as IBD and IBS remains a challenge and until today, mainly focuses on symptomatic control. One of the biggest challenges is the management of visceral hypersensitivity, which is seen as the mechanism behind abdominal pain. In the last decades, many possible pharmacological targets have been proposed, but unfortunately an effective, causative treatment is still lacking. Therefore, further elucidating the pathophysiology of visceral hypersensitivity and eventually discovering new possible pharmacological targets is of great importance. Recently, serine proteases have come into the picture as a promising new pharmacological target for visceral pain. Up until now, research has focused mainly on PAR-agonists/antagonists, but none of these compounds made it to the clinic yet. A more recent strategy is the direct inhibition of serine proteases, which shows promising results in a limited number of animal experiments in the field of visceral hypersensitivity. So far, serine protease inhibitors have not been tested in clinical trials for IBS either. However, serine protease inhibitors are well known in the treatment of other diseases, e.g. diabetes and pancreatic cancer. In the domain of gastroenterology, protease inhibitors have already been investigated in animal models, focusing on the effects on intestinal inflammation and permeability. Protease inhibitors were able to ameliorate inflammation as well as permeability, suggesting that proteases may be valuable treatment targets.
The few preclinical studies investigating the effect of protease inhibitors on visceral hypersensitivity show promising results. Therefore it seems necessary that more in-depth research on the therapeutic potential of protease inhibitors in abdominal pain is conducted in the upcoming years. The emphasis should be on the detection and eventually the targeting of specific proteases that might be crucial in visceral hypersensitivity. First of all, as proteases often have overlapping substrate specificities and specific inhibitors are in many cases not available, the methods used to measure the activity of individual proteases should be improved. Furthermore, more and better validated tools are needed to quantify their protein levels. Measuring specific protease activities remains a great challenge. However, investigating the link between certain protease activities and IBS-subtypes would be of great interest and could possibly lead towards the discovery of a new drug target or the development of a new biomarker. Concerning therapeutic options we need to take into account that IBS is not considered to be a life-threatening disease and thus medication needs to be free of substantial side effects. This is certainly an important consideration given the various functions of proteases in the human body, and yet another reason to focus on the specificity of protease inhibitors, thereby not forgetting the potential importance of the equilibrium between proteases and anti-proteases. Direct inhibition of proteases has already been put forward as a possible strategy for the treatment of visceral hypersensitivity in IBS patients. Therefore, we believe that the development of new and more specific protease inhibitors could be of great interest. In the future, not only the safety profile of these compounds but also their route of administration will become a very important subject. Up until now, protease inhibitors are administered via systemic routes, but ideally, the treatment of IBS should focus on the gastrointestinal tract in a further attempt to reduce systemic side-effects. A serine protease inhibitor for local delivery is unfortunately not available at this time and it would be groundbreaking if researchers came up with a solution for this problem. In a recent article from Bermúdez-Humarán, a revolutionary method using recombinant lactic acid bacteria (recLAB) to deliver serine protease inhibitors (Elafin and Secretory Leukocyte Protease Inhibitor- SLPI) at the mucosal level, is described[72]. However, it should be taken into account that in this case the serine protease inhibitors had a protein structure and therefore recombinant bacteria were able to produce and express them. Other serine protease inhibitors such as nafamostat mesilate are synthetic compounds with an organic chemical structure and thus cannot be produced by recombinant bacteria. Therefore, research groups should come up with new strategies to deliver synthetic compounds at the mucosal level. In our opinion, in the following years, research groups should focus on the development of specific serine protease inhibitors, taking into account the potentiality of delivering that compound at the level of the colonic mucosa after correctly measuring the specific protease profile of the individual patient, allowing individually tailored therapy.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: September 22, 2016
First decision: October 20, 2016
Article in press: December 2, 2016
P- Reviewer: Ghannam A, Ksiazek M, Lakatos PL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 5.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 7.De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease - clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther. 2008;27:621–637. doi: 10.1111/j.1365-2036.2008.03624.x. [DOI] [PubMed] [Google Scholar]
- 8.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 9.Spiller R. Clinical update: irritable bowel syndrome. Lancet. 2007;369:1586–1588. doi: 10.1016/S0140-6736(07)60726-0. [DOI] [PubMed] [Google Scholar]
- 10.Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 11.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Anand P, Aziz Q, Willert R, van Oudenhove L. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol Motil. 2007;19:29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen W, De Man JG, Pelckmans PA, De Winter BY. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20:1005–1020. doi: 10.3748/wjg.v20.i4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 15.Van Spaendonk H, Ceuleers H, Witters L, Patteet E, Joossens J, Augustyns K, Lambeir AM, De Meester I, De Man JG, De Winter BY. Gastrointestinal permeability: The role of proteases. World J Gastroenterol. 2016:in Press. doi: 10.3748/wjg.v23.i12.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6:17–26. doi: 10.1097/SPC.0b013e32834f6ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbar A, Walters JR, Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther. 2009;30:423–435. doi: 10.1111/j.1365-2036.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut. 2001;48:125–131. doi: 10.1136/gut.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–G154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 20.de Carvalho Rocha HA, Dantas BP, Rolim TL, Costa BA, de Medeiros AC. Main ion channels and receptors associated with visceral hypersensitivity in irritable bowel syndrome. Ann Gastroenterol. 2014;27:200–206. [PMC free article] [PubMed] [Google Scholar]
- 21.De Winter BY, van den Wijngaard RM, de Jonge WJ. Intestinal mast cells in gut inflammation and motility disturbances. Biochim Biophys Acta. 2012;1822:66–73. doi: 10.1016/j.bbadis.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 23.Deiteren A, De Man JG, Ruyssers NE, Moreels TG, Pelckmans PA, De Winter BY. Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut. 2014;63:1873–1882. doi: 10.1136/gutjnl-2013-305870. [DOI] [PubMed] [Google Scholar]
- 24.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150:875–87.e9. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Song J, Hou X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J Neurogastroenterol Motil. 2016;22:181–192. doi: 10.5056/jnm15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergnolle N. Modulation of visceral pain and inflammation by protease-activated receptors. Br J Pharmacol. 2004;141:1264–1274. doi: 10.1038/sj.bjp.0705750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antalis TM, Shea-Donohue T, Vogel SN, Sears C, Fasano A. Mechanisms of disease: protease functions in intestinal mucosal pathobiology. Nat Clin Pract Gastroenterol Hepatol. 2007;4:393–402. doi: 10.1038/ncpgasthep0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62:597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooper NM. Proteases: a primer. Essays Biochem. 2002;38:1–8. doi: 10.1042/bse0380001. [DOI] [PubMed] [Google Scholar]
- 30.Barrett AJ. The many forms and functions of cellular proteinases. Fed Proc. 1980;39:9–14. [PubMed] [Google Scholar]
- 31.Barrett AJ. Classification of peptidases. Methods Enzymol. 1994;244:1–15. doi: 10.1016/0076-6879(94)44003-4. [DOI] [PubMed] [Google Scholar]
- 32.Powers JC, Odake S, Oleksyszyn J, Hori H, Ueda T, Boduszek B, Kam C. Proteases--structures, mechanism and inhibitors. Agents Actions Suppl. 1993;42:3–18. [PubMed] [Google Scholar]
- 33.Rawlings ND, Barrett AJ, Bateman A. Asparagine peptide lyases: a seventh catalytic type of proteolytic enzymes. J Biol Chem. 2011;286:38321–38328. doi: 10.1074/jbc.M111.260026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 35.Vergnolle N. Protease inhibition as new therapeutic strategy for GI diseases. Gut. 2016;65:1215–1224. doi: 10.1136/gutjnl-2015-309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- 37.Cenac N. Protease-activated receptors as therapeutic targets in visceral pain. Curr Neuropharmacol. 2013;11:598–605. doi: 10.2174/1570159X113119990039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergnolle N. Clinical relevance of proteinase activated receptors (pars) in the gut. Gut. 2005;54:867–874. doi: 10.1136/gut.2004.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 41.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao JH, Dong L, Shi HT, Wang ZY, Shi HY, Ding H. The expression of protease-activated receptor 2 and 4 in the colon of irritable bowel syndrome patients. Dig Dis Sci. 2012;57:58–64. doi: 10.1007/s10620-011-1827-3. [DOI] [PubMed] [Google Scholar]
- 43.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Liang WJ, Zhang G, Luo HS, Liang LX, Huang D, Zhang FC. Tryptase and Protease-Activated Receptor 2 Expression Levels in Irritable Bowel Syndrome. Gut Liver. 2016;10:382–390. doi: 10.5009/gnl14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bian ZX, Li Z, Huang ZX, Zhang M, Chen HL, Xu HX, Sung JJ. Unbalanced expression of protease-activated receptors-1 and -2 in the colon of diarrhea-predominant irritable bowel syndrome patients. J Gastroenterol. 2009;44:666–674. doi: 10.1007/s00535-009-0058-2. [DOI] [PubMed] [Google Scholar]
- 46.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut. 2013;62:985–994. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 47.Lettesjö H, Hansson T, Peterson C, Ung KA, Ringström G, Abrahamsson H, Simrén M. Detection of inflammatory markers in stools from patients with irritable bowel syndrome and collagenous colitis. Scand J Gastroenterol. 2006;41:54–59. doi: 10.1080/00365520510023909. [DOI] [PubMed] [Google Scholar]
- 48.Róka R, Rosztóczy A, Leveque M, Izbéki F, Nagy F, Molnár T, Lonovics J, Garcia-Villar R, Fioramonti J, Wittmann T, et al. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:550–555. doi: 10.1016/j.cgh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Annaházi A, Gecse K, Dabek M, Ait-Belgnaoui A, Rosztóczy A, Róka R, Molnár T, Theodorou V, Wittmann T, Bueno L, et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209–217. doi: 10.1016/j.pain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 51.Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N, et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108.e5. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Tooth D, Garsed K, Singh G, Marciani L, Lam C, Fordham I, Fields A, Banwait R, Lingaya M, Layfield R, et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut. 2014;63:753–760. doi: 10.1136/gutjnl-2012-304042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annaházi A, Ferrier L, Bézirard V, Lévêque M, Eutamène H, Ait-Belgnaoui A, Coëffier M, Ducrotté P, Róka R, Inczefi O, et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am J Gastroenterol. 2013;108:1322–1331. doi: 10.1038/ajg.2013.152. [DOI] [PubMed] [Google Scholar]
- 54.Edgington-Mitchell LE. Pathophysiological roles of proteases in gastrointestinal disease. Am J Physiol Gastrointest Liver Physiol. 2016;310:G234–G239. doi: 10.1152/ajpgi.00393.2015. [DOI] [PubMed] [Google Scholar]
- 55.Kawao N, Ikeda H, Kitano T, Kuroda R, Sekiguchi F, Kataoka K, Kamanaka Y, Kawabata A. Modulation of capsaicin-evoked visceral pain and referred hyperalgesia by protease-activated receptors 1 and 2. J Pharmacol Sci. 2004;94:277–285. doi: 10.1254/jphs.94.277. [DOI] [PubMed] [Google Scholar]
- 56.Kawabata A, Kawao N, Kuroda R, Tanaka A, Shimada C. The PAR-1-activating peptide attenuates carrageenan-induced hyperalgesia in rats. Peptides. 2002;23:1181–1183. doi: 10.1016/s0196-9781(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 57.Augé C, Balz-Hara D, Steinhoff M, Vergnolle N, Cenac N. Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil. 2009;21:1189–e107. doi: 10.1111/j.1365-2982.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 58.Han W, Wang Z, Lu X, Guo C. Protease activated receptor 4 status of mast cells in post infectious irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:113–19, e82. doi: 10.1111/j.1365-2982.2011.01841.x. [DOI] [PubMed] [Google Scholar]
- 59.Kawabata A, Kawao N, Kuroda R, Tanaka A, Itoh H, Nishikawa H. Peripheral PAR-2 triggers thermal hyperalgesia and nociceptive responses in rats. Neuroreport. 2001;12:715–719. doi: 10.1097/00001756-200103260-00020. [DOI] [PubMed] [Google Scholar]
- 60.Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- 61.Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 63.Kawabata A, Kawao N, Kitano T, Matsunami M, Satoh R, Ishiki T, Masuko T, Kanke T, Saito N. Colonic hyperalgesia triggered by proteinase-activated receptor-2 in mice: involvement of endogenous bradykinin. Neurosci Lett. 2006;402:167–172. doi: 10.1016/j.neulet.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 64.Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol. 2013;108:1634–1643. doi: 10.1038/ajg.2013.241. [DOI] [PubMed] [Google Scholar]
- 65.Wang P, Chen FX, Du C, Li CQ, Yu YB, Zuo XL, Li YQ. Increased production of BDNF in colonic epithelial cells induced by fecal supernatants from diarrheic IBS patients. Sci Rep. 2015;5:10121. doi: 10.1038/srep10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceuleers H, Segaert E, Heirbaut J, Hanning N, Francque SM, Joossens J, De Meester I, De Man J, De Winter BY. Su1937 Two Serine Protease Inhibitors, Nafamostat Mesylate and the Newly Developed SPIx, Decrease Post-Inflammatory Visceral Hypersensitivity in Rats. Gastroenterology. 2016;150:S593–S4. [Google Scholar]
- 67.Ceuleers H, De Man JG, Deiteren A, De Schepper H, Joossens J, Francque S. The effect of a potent tryptase inhibitor and a new serine protease inhibitor on visceral pain in a rat model of acute colitis. Friday, 5 June 2015 08: 30-10: 00 Hall B2 FP-01 Sensory mechanisms. Neurogastroenterol Motility. 2015;27:22–25. [Google Scholar]
- 68.Zhao J, Wang J, Dong L, Shi H, Wang Z, Ding H, Shi H, Lu X. A protease inhibitor against acute stress-induced visceral hypersensitivity and paracellular permeability in rats. Eur J Pharmacol. 2011;654:289–294. doi: 10.1016/j.ejphar.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 69.Zhao J, Wang Z, Zou B, Song Y, Dong L. [Camostat mesilate, a protease inhibitor, inhibits visceral sensitivity and spinal c-fos expression in rats with acute restraint stress] Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1546–1550. [PubMed] [Google Scholar]
- 70.Moussa L, Bézirard V, Salvador-Cartier C, Bacquié V, Lencina C, Lévêque M, Braniste V, Ménard S, Théodorou V, Houdeau E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS One. 2012;7:e49547. doi: 10.1371/journal.pone.0049547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moussa L, Bézirard V, Salvador-Cartier C, Bacquié V, Houdeau E, Théodorou V. A new soy germ fermented ingredient displays estrogenic and protease inhibitor activities able to prevent irritable bowel syndrome-like symptoms in stressed female rats. Clin Nutr. 2013;32:51–58. doi: 10.1016/j.clnu.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 72.Bermúdez-Humarán LG, Motta JP, Aubry C, Kharrat P, Rous-Martin L, Sallenave JM, Deraison C, Vergnolle N, Langella P. Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb Cell Fact. 2015;14:26. doi: 10.1186/s12934-015-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mavrakanas TA, Chatzizisis YS. Bivalirudin in stable angina and acute coronary syndromes. Pharmacol Ther. 2015;152:1–10. doi: 10.1016/j.pharmthera.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Reddy P, Giugliano RP. The role of rivaroxaban in atrial fibrillation and acute coronary syndromes. J Cardiovasc Pharmacol Ther. 2014;19:526–532. doi: 10.1177/1074248414525505. [DOI] [PubMed] [Google Scholar]
- 75.McCormack PL. Edoxaban: a review in nonvalvular atrial fibrillation. Am J Cardiovasc Drugs. 2015;15:351–361. doi: 10.1007/s40256-015-0148-x. [DOI] [PubMed] [Google Scholar]
- 76.Fanola CL. Current and emerging strategies in the management of venous thromboembolism: benefit-risk assessment of dabigatran. Vasc Health Risk Manag. 2015;11:271–282. doi: 10.2147/VHRM.S62595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujii K, Kanno Y, Konishi K, Ohgou N. A specific thrombin inhibitor, argatroban, alleviates herpes zoster-associated pain. J Dermatol. 2001;28:200–207. doi: 10.1111/j.1346-8138.2001.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong WB, Taylor TH, Kennedy AR, Melrose RJ, Messadi DV, Gu M, Le AD, Perloff M, Civantos F, Goodwin WJ, et al. Bowman birk inhibitor concentrate and oral leukoplakia: a randomized phase IIb trial. Cancer Prev Res (Phila) 2013;6:410–418. doi: 10.1158/1940-6207.CAPR-13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scully M, Gates C, Neave L. How we manage patients with heparin induced thrombocytopenia. Br J Haematol. 2016;174:9–15. doi: 10.1111/bjh.14102. [DOI] [PubMed] [Google Scholar]
- 80.Heinemann V, Ebert MP, Laubender RP, Bevan P, Mala C, Boeck S. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br J Cancer. 2013;108:766–770. doi: 10.1038/bjc.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uwagawa T, Misawa T, Tsutsui N, Ito R, Gocho T, Hirohara S, Sadaoka S, Yanaga K. Phase II study of gemcitabine in combination with regional arterial infusion of nafamostat mesilate for advanced pancreatic cancer. Am J Clin Oncol. 2013;36:44–48. doi: 10.1097/COC.0b013e31823a53b2. [DOI] [PubMed] [Google Scholar]
- 82.Bao P, Zhao W, Li Y, Liu Y, Zhou Y, Liu C. Protective effect of ulinastatin in patients with non-small cell lung cancer after radiation therapy: a randomized, placebo-controlled study. Med Oncol. 2015;32:405. doi: 10.1007/s12032-014-0405-x. [DOI] [PubMed] [Google Scholar]
- 83.Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, McKee PA, Egleston B, Cohen SJ, Weiner LM, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Wang N, Zhou S, Ye W, Yao Q, Jing G, Zhang M. Preventive effect of ulinastatin on postoperative complications, immunosuppression, and recurrence in esophagectomy patients. World J Surg Oncol. 2013;11:84. doi: 10.1186/1477-7819-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krishna MT, Chauhan A, Little L, Sampson K, Hawksworth R, Mant T, Djukanovic R, Lee T, Holgate S. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. J Allergy Clin Immunol. 2001;107:1039–1045. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- 86.Strange C, Beiko T. Treatment of Alpha-1 Antitrypsin Deficiency. Semin Respir Crit Care Med. 2015;36:470–477. doi: 10.1055/s-0035-1555608. [DOI] [PubMed] [Google Scholar]
- 87.Twigg MS, Brockbank S, Lowry P, FitzGerald SP, Taggart C, Weldon S. The Role of Serine Proteases and Antiproteases in the Cystic Fibrosis Lung. Mediators Inflamm. 2015;2015:293053. doi: 10.1155/2015/293053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas MC, Paldánius PM, Ayyagari R, Ong SH, Groop PH. Systematic Literature Review of DPP-4 Inhibitors in Patients with Type 2 Diabetes Mellitus and Renal Impairment. Diabetes Ther. 2016;7:439–454. doi: 10.1007/s13300-016-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Royston D. The current place of aprotinin in the management of bleeding. Anaesthesia. 2015;70 Suppl 1:46–9, e17. doi: 10.1111/anae.12907. [DOI] [PubMed] [Google Scholar]
- 90.Ono S, Aosasa S, Mochizuki H. Effects of a protease inhibitor on reduction of surgical stress in esophagectomy. Am J Surg. 1999;177:78–82. doi: 10.1016/s0002-9610(98)00300-6. [DOI] [PubMed] [Google Scholar]
- 91.Inagaki H, Nonami T, Kurokawa T, Takeuchi Y, Okuda N, Nakao A, Sakamoto J. Effects of nafamostat mesilate, a synthetic protease inhibitor, on immunity and coagulation after hepatic resection. Hepatogastroenterology. 1999;46:3223–3228. [PubMed] [Google Scholar]
- 92.Ito H, Nakayama H, Yokose T, Yamada K. Prophylaxis for acute exacerbation of interstitial pneumonia after lung resection. Asian Cardiovasc Thorac Ann. 2014;22:948–954. doi: 10.1177/0218492314526187. [DOI] [PubMed] [Google Scholar]
- 93.Joossens J, Ali OM, El-Sayed I, Surpateanu G, Van der Veken P, Lambeir AM, Setyono-Han B, Foekens JA, Schneider A, Schmalix W, et al. Small, potent, and selective diaryl phosphonate inhibitors for urokinase-type plasminogen activator with in vivo antimetastatic properties. J Med Chem. 2007;50:6638–6646. doi: 10.1021/jm700962j. [DOI] [PubMed] [Google Scholar]


