Abstract
Cytotoxic necrotizing factor type 1 (CNF1) from Escherichia coli activates the small GTP-binding proteins of the Rho family (Rho, Rac, and Cdc42) by catalyzing their deamidation at a specific glutamine residue. Since RhoA, Rac, and Cdc42 play a pivotal role in cell migration during the early phase of wound repair, we investigated whether CNF1 was able to interfere with wound healing in intestinal epithelial monolayers (T84 cells). After mechanical injury, we found that CNF1 blocks epithelial wound repair within 48 h. This effect was characterized by cell elongation and filopodium formation on the leading edge, in association with permanent phosphorylation of the focal adhesion kinase (FAK) via Rho activation. Moreover, inhibition of Rho kinase with Y-27632 decreased CNF1-mediated permanent FAK phosphorylation, leading to complete restitution of wound repair within 24 h. In addition, we found that CNF1 induced upregulation of mitogen-activated protein kinases (MAPK) activation. Moreover, activation of Rac and MAPK by CNF1 increased matrix metalloproteinase 9 expression in wounded T84 monolayers. Taken together, these results provide evidence that CNF1 strongly impairs intestinal epithelial wound healing.
The intestinal and urothelial linings constitute the physical barrier responsible for the partition and integrity of luminal and subepithelial compartments. Trauma, microbes, foreign materials, or high-density neutrophil transepithelial migration can induce intestinal injury by epithelial barrier disruption. Following mucosal injury, rapid barrier restitution occurs as consequence of a complex process that encompasses a number of overlapping phases, including epithelialization, angiogenesis, and matrix deposition and remodeling. Ultimately, these processes are resolved or dampened, which leads to mature wound scar formation.
Epithelial wound closure results from motile epithelial cells that extrude membranous extensions in the direction of motion, which are referred to as lamellipodia or filopodia. The Rho GTPases have been implicated in a wide variety of cellular events, including cytoskeleton organization, cell polarity and adhesion, and control of transcription (18, 34, 41). Most often, Rho has been considered a protein that regulates the formation of contractile actin-myosin filaments in actin stress fiber, whereas Rac and Cdc42 are responsible for the formation of lamellipodia and filopodia, respectively (35, 38, 39). During wound closure, formation and remodeling of focal contacts are dynamic processes that are also regulated by a protein tyrosine kinase, the focal adhesion kinase (FAK) (37). FAK, a 125-kDa cytoplasmic tyrosine kinase localized in focal contacts, has been shown to play an important role in integrin-mediated cell migration (25). FAK turnover is implicated in the regulation of cell migration since it has been shown that FAK-deficient cells migrate poorly in response to chemotactic signals (37). Moreover, previous experience has demonstrated that FAK-deficient cells respond to the specific Rho kinase (ROCK) inhibitor Y-27632, suggesting the possibility that the lack of adhesion complex turnover in FAK-deficient cells may reflect regulation of Rho-regulated contractibility (8, 37).
Tissue injury results in activation of mitogen-activated protein kinase (MAPK) pathways (17) and subsequently in the regulation of the production of different proteins, such as cytokines, growth factors, and matrix metalloproteinases (MMPs) (1, 45, 49). Degradation of fibrillar collagen and other matrix proteins is driven by serine proteases and MMPs under the control of the cytokine network. MMPs not only degrade matrix components but also function as regulatory molecules by driving enzyme cascades and processing cytokines and matrix and adhesion molecules to generate biologically active fragments (45). Although inflammation and repair occur mostly along a proscribed course, the sensitivity of the process is underscored by the consequences of disruption of the balance of regulatory cytokines and MMP activity (45). Specifically, it has been shown that MMP-9 (92- to 82-kDa gelatinase B) plays an essential role during healing of wounded epithelia (40, 46). MMP-9 is stimulated in response to injury, and it is expressed mostly in the migrating epithelial cell sheet (33, 40). A previous study showed that MMP-9 is stimulated by injury in normal human keratinocytes by the mechanical stress due to the injury and not by an epithelial soluble factor produced by wounded cells (48). Moreover, MMP-9 can catalyze the cleavage of type IV collagen and other basement membrane components (48). Taken together, the coordinate events involving these different proteins, epithelial cells, and extracellular matrix remodeling are essential for the initiation, evolution, and resolution of a wound.
Certain Escherichia coli strains that produce a toxin-like protein designated cytotoxic necrotizing factor 1 (CNF1) have been shown to be associated mainly with urinary tract infections or, more rarely, with gastroenteritis (3, 6, 11). CNF1 has been described as a protein that deamidates RhoA GTP-binding protein glutamine 63 and impairs RhoGTPase-activating protein (RhoGAP)-mediated GTP hydrolysis, leading to permanent RhoA activation (16, 42). Consistent with the presence of the DYDRL motif in RhoA, Rac, and Cdc42, CNF1 modified only these proteins in the family of small GTP-binding proteins (5, 15). Moreover, CNF1 potentiates striking actin rearrangements in different human cells (4, 21, 22) and induction of DNA synthesis in quiescent cells, and it impairs cytokinesis (14). By using T84 cells, it was shown previously that CNF1 effaced intestinal cell microvilli and induced a strong decrease in polymorphonuclear leukocyte transepithelial migration in either the luminal-to-basolateral direction or the basolateral-to-luminal direction (21). The attenuated transepithelial migration of polymorphonuclear leukocytes could result in enhanced growth and protection of luminal bacteria. However, the exact signaling mechanisms that link activation of the Rho proteins to these events remain to be determined. Recently, it has been demonstrated that Rho GTPase activation by CNF1 involves the ubiquitin-proteasome complex (9). It was speculated previously that by this means, CNF1 could induce invasive properties in E. coli strains producing this toxin (9). Through its action on Rho, CNF1 induces the tyrosine phosphorylation of focal adhesion complex proteins, leading to the formation of actin stress fibers (28). It is likely that other signaling pathways are also perturbed by activation of the Rho proteins, which leads to increased expression of proinflammatory cytokines (12).
In this study, we investigated whether CNF1 was able to influence epithelial wound healing after mechanical injury. To do this, we used a previously described wound-healing model (49). We obtained the first evidence that CNF1 completely inhibits the closure of wounded T84 monolayers by activating the Rho proteins. By preincubating T84 cells with the specific ROCK inhibitor Y-27632, we showed that the wound repair inhibition induced by CNF1 was impaired by a decrease in FAK phosphorylation due to Rho. In addition, we showed that CNF1 significantly enhanced MAPK (ERK, p38, JNK) activation and MMP-9 expression triggered by wounding.
MATERIALS AND METHODS
Materials and chemicals.
The highly purified CNF1 used throughout this work was kindly provided by Patrice Boquet (INSERM U 452, Faculty of Medicine, University of Nice, Nice, France) and was prepared as described previously (11). The p160/ROCK inhibitor Y-27632 was purchased from Calbiochem (Meudon, France).
Cell culture and wound procedure.
T84 cells (American Type Culture Collection, Rockville, Md.) were grown in a 1:1 mixture of Dulbecco modified Eagle medium and Ham F-12 medium supplemented with 15 mM HEPES buffer (pH 7.4), 14 mM NaHCO3, 40 μg of penicillin per ml, 90 μg of streptomycin per ml, 8 μg of ampicillin per ml, and 5% newborn calf serum (Biowhittaker Europe, Verviers, Belgium) (30). Confluent T84 monolayers grown on 60-mm dishes were incubated for 24 h in serum-deprived medium containing 0.1% bovine serum albumin. Wounding was performed in control monolayers or monolayers incubated with CNF1 (3 h, 4 nM) as previously described (49). Briefly, the apparatus used in this study can generate calibrated injuries since continuous, curvilinear wounds are made throughout the entire surface of the tissue culture dish (49). Thus, when the wounding procedure was performed with a 60-mm-diameter culture dish by using a scalpel blade, the wound length was 7 to 10 m, as previously described (49). This method allowed us to investigate the different molecular events that occurred at various times during epithelial wound closure (49). After wounding, the plates were returned to the incubator (37°C, 5% CO2) for various periods of time (as indicated below) and were analyzed by using a phase-contrast microscope before the cells were lysed and protein extracts were harvested. In some experiments, the p160/ROCK inhibitor Y-27632 (10 μM) was added after wounding. Wound healing in 10 areas that were 200 to 300 μm wide (between the opposite fronts of migration) of six different monolayers were analyzed and photographed at 12, 24, and 48 h for each condition to determine the percentages of wound healing in control and CNF1-treated monolayers incubated with and without Y-27632.
GTPase activation assays.
CNF1 molecules translocated into the cytosol were detected by their ability to upshift the molecular weight of the Rho GTP-binding protein (16, 42). Cell monolayers were incubated for 45 min, washed twice in 3 mM imidazole buffer (pH 7.5) containing 250 mM sucrose, detached from the petri dishes, and suspended in 100 μl of the same solution supplemented with protease inhibitors (Complete EDTA free; Boehringer Mannheim). Cell suspensions were transferred to microcentrifuge tubes and incubated for 10 min at 4°C, and then they were lysed by three cycles of freezing in liquid nitrogen and thawing by incubation at 37°C. Lysates were centrifuged (15, 000 × g, 15 min, 4°C), and the supernatants were collected. As previously described, the in vitro Rho ADP ribosylation reactions were performed by incubating proteins from supernatants (50 μg) for 90 min at 37°C with 15 ng of purified exoenzyme C3 and 106 cpm of [32P]NADP (250 Ci/mmol; ICN Biomedicals, Irvine, Calif.) in 20 mM Tris buffer (pH 7.5) containing 2 mM MgCl2 and 100 mM NaCl (7). Samples were then subjected to sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis (PAGE), and the Rho protein was detected by autoradiography.
The activation status of Rac and Cdc42 was then determined in T84 monolayers following CNF1 treatment. Glutathione S-transferase fusion protein, corresponding to the p21-binding domain (residues 67 to 150) of human p21 activated kinase 1 (PAK1), conjugated to agarose beads was used to pull down activated Rac from control and CNF1-treated monolayers with a Rac activation assay kit (Euromedex, Mundolsheim, France). The positive controls consisted of T84 whole-cell lysates. Activated Rac from the pulldown experiments and total Rac from whole-cell lysates were detected by SDS-PAGE and Western blot analysis by using a monoclonal antibody to Rac. Since PAK1 is also an effector for activated Cdc42, the same nitrocellulose membranes were then stripped in β-mercaptoethanol-based buffer and reblotted with a monoclonal antibody to Cdc42 (Euromedex).
Actin staining and electron microscopy study.
F-actin fluorescence staining of control T84 cells, wounded T84 cells, and T84 cells treated with CNF1 for various times (as indicated below) was performed as follows. Cells were fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4) for 30 min at room temperature and were rinsed in buffer containing 0.2% gelatin and 0.01% Triton X-100. The cells were then incubated for 45 min in the dark with 500 nM rhodamine-phalloidin (Molecular Probes, Junction City, Oreg.), diluted in PBS, and washed in Hanks' balanced salt solution, and slides were mounted in a phenylenediamine-glycerol-PBS medium. The slides were observed and photographed with a laser scanning fluorescence microscope (DMIRBE; Leica, Lyon, France) equipped for epifluorescence. For electron microscopy, control T84 cells, injured T84 cells, and T84 cells treated for various periods of time with CNF1 were fixed in 2% formaldehyde in 0.1 M sodium cacodylate (pH 7.4) for 1 h at 4°C. Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated with a graded alcohol series, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives; thin sections were obtained for multiple areas, mounted on copper mesh grids, and stained with uranyl acetate and lead citrate. Ultrathin sections were examined with a Jeol 1200 EX II electron microscope.
Immunodepletion and Western blotting.
Control, injured, and CNF1-treated T84 cells were washed in Hanks' balanced salt solution (Sigma, Paris, France) and then solubilized at 4°C in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 μM leupeptin, 5 mM benzamidin, 1 μM pepstatin, 25 μM aprotinin, 50 mM sodium β-glycerophosphate, 20 mM sodium pyrophosphate, 0.5 mM dithiothreitol). After sonication with 8-s bursts, lysates were centrifuged at 15,000 × g for 15 min at 4°C.
For immunodepletion, FAK was immunoprecipitated from 1 mg of lysate by incubation at 4°C for 16 h with anti-FAK monoclonal antibody (diluted 1/2,000; Santa Cruz Biotechnologies, Santa Cruz, Calif.) bound to protein A-Sepharose. Immunopellets were then washed twice with lysis buffer and were subjected to SDS-PAGE and subsequently electrophoretically transferred onto nitrocellulose membranes.
For Western blotting, protein lysates (50 μg per sample) were subjected to SDS-PAGE and subsequently electrophoretically transferred onto nitrocellulose membranes. After incubation in blocking buffer, the membranes were probed overnight at 4°C with anti-p42MAPK Erk2 (diluted 1/1,000; New England BioLabs, Beverly, Mass.), anti-JNK1(sc-474) (diluted 1/1,000; Santa Cruz Biotechnologies), anti-p38α (diluted 1/1,000; New England BioLabs), anti-phospho-p42/44MAPK Erk1/Erk2 (diluted 1/2,000; New England BioLabs), anti-phospho-JNK(Thr183/Tyr185) (diluted 1/3,000; New England BioLabs), anti-phospho-P38(Thr180/Tyr182) (diluted 1/1,000; New England BioLabs), anti-phospho-FAK (diluted 1/1,000; Upstate Biotechnologies, Lake Placid, N.Y.), or anti-FAK (diluted 1/1,000; New England BioLabs) antibody. The labeling was visualized by using peroxidase-conjugated secondary antibodies (anti-rabbit immunoglobulin G; diluted 1/2,000; New England BioLabs) and by enhanced chemiluminescence (ECL kit; Amersham International, Little Chalfont, Buckinghamshire, United Kingdom). Phosphorylation protein levels were quantified by densitometric scanning of nonsaturated autoradiographs by using the NIH Image software (version 1.6).
Zymography assay.
Conditioned medium was collected from cells that were cultured in serum-free medium for 24 h after wounding. SDS—9% PAGE gels were copolymerized with 1 mg of gelatin per ml. Samples were resolved under nonreducing conditions. The gels were washed in 2.5% Triton X-100 for 1.5 h and incubated for 48 h at 37°C in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 5 mM CaCl2. MMP-9 protein levels were quantified by densitometric analysis of a negative scan of the zymogram with the NIH Image software (version 1.6).
Data analysis.
An unpaired two-tailed Student t test was used to determine statistical significance for measurements of wound closure. Student's t test for correlated samples was used, and a P value of <0.01 or <0.005 was considered significant.
RESULTS
CNF1 activates RhoA, Rac, and Cdc42 in confluent T84 monolayers.
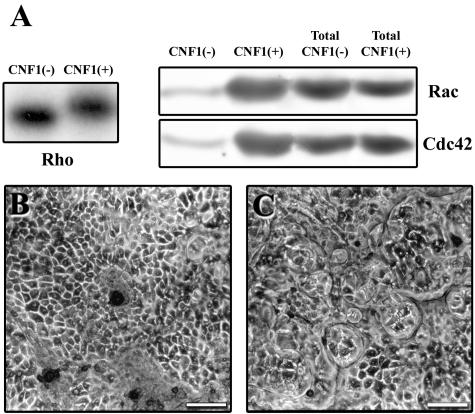
Since it has been shown that CNF1 induces prominent F-actin reorganization in most cultured cells, we first tested the effect of this toxin in confluent T84 monolayers at different times and with different concentrations. The activation of RhoA was analyzed by the ADP ribosylation assay (Fig. 1A). Deamidation of Gln63 to Glu63 by CNF1 results in a significant upshift of the apparent molecular weight of RhoA on SDS-PAGE gels. This shift was demonstrated by using C3 exoenzyme, which specifically added [32P]ADP-ribose to Thr37 of RhoA and therefore radiolabeled RhoA. As indicated in Fig. 1A, GTP-binding proteins belonging to the Rho family were completely modified by 4 nM CNF1 within 3 h of treatment. We observed increased activation of Rac and Cdc42 following incubation of monolayers with CNF1 (Fig. 1A). In parallel, we demonstrated that in T84 cells CNF1 activated Rac and Cdc42 using PAK1 pulldown (Fig. 1A). After incubation with CNF1, the integrity of the monolayer was compared with the integrity of the control preparation by using light microscopy. Like control monolayers, T84 cells treated apically with CNF1 showed complete integrity of the ring structures without intercellular linkage (Fig. 1B and C). However, CNF1-treated cells were larger than control cells and spread over the dishes compared to control cells (Fig. 1B and C).
FIG. 1.
RhoA, Rac, and Cdc42 are activated in CNF1-treated confluent T84 monolayers. (A) Activation of Rho proteins in T84 cells treated or not treated with CNF1 and analyzed by the ADP ribosylation assay. The Rac and Cdc2 activation status was examined by pulldown assays involving binding to the GTPase-binding domains of PAK1 conjugated to agarose beads. The total Rac and Cdc42 protein levels were similar in lysates from control and CNF1-treated monolayers. (B) Phase-contrast image of control T84 monolayers. (C) T84 monolayer apically treated with CNF1 (3 h, 4 nM). Bars = 50 μm.
CNF1 inhibits closure of T84 monolayers after epithelial injury.
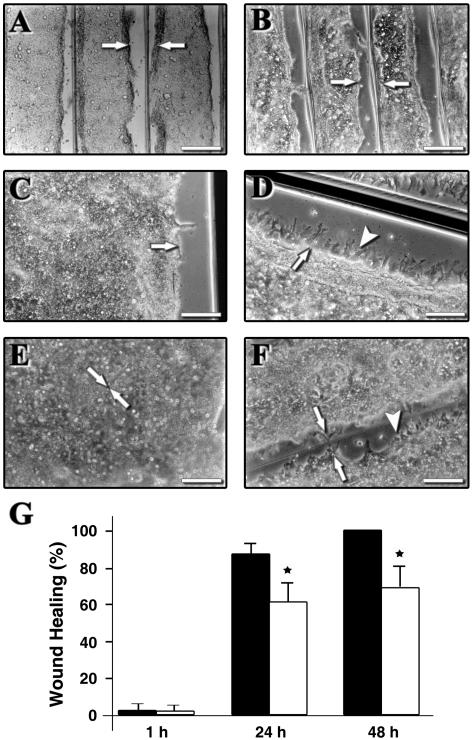
Wound closure was analyzed in control and CNF1-treated T84 monolayers at 1 h (Fig. 2A and B), 24 h (Fig. 2C and D), and 48 h (Fig. 2 E and F) after epithelial injury. There was no difference between control cells and CNF1-treated cells at 1 h (Fig. 2A and B). At 24 h, formation of a few lamellipodia at the wound margin was observed in control cells (Fig. 2C), whereas CNF1-treated cells formed numerous lamellipodia and filopodia (Fig. 2D). Control cells showed complete monolayer restitution at 48 h (Fig. 2E). In contrast to injured control monolayers, the wound size decreased by only approximately 70% after 48 h in CNF1-treated monolayers, indicating that wound healing was partially inhibited by CNF1 treatment (Fig. 2F and G).
FIG. 2.
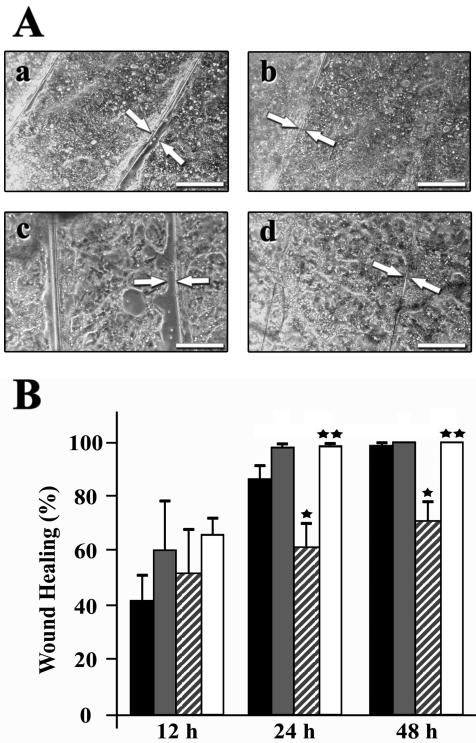
Closure of wounded T84 monolayers is inhibited by CNF1. The effects of CNF1 treatment in wounded epithelial T84 monolayers were analyzed by phase-contrast microscopy at 1 h (A and B), 24 h (C and D), and 48 h (E and F) after injury. (A, C, and E) Control monolayers; (B, D, and F) CNF1-treated monolayers. The arrowheads indicate typical lamellipodia and filopodia induced in CNF1-treated cells; the arrows indicate initial sites of wounds. (A and B) Bars = 40 μm; (C to F) bars = 15 μm. (G) Percentages of wound closure calculated as described in Materials and Methods 1, 24, and 48 h after wounding in control (solid bars) and CNF1-treated (open bars) monolayers. A star indicates that the P value is <0.01.
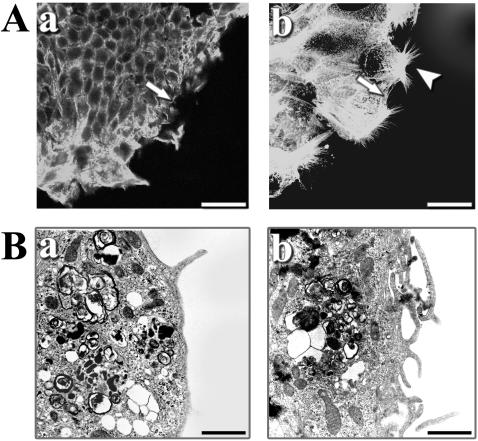
As shown by rhodamine-conjugated phalloidin staining, wounded control cells at 24 h had a few buds at the leading edge (Fig. 3A, panel a), whereas numerous actin-rich filopodium extensions were detected in CNF1-treated wounded cells (Fig. 3A, panel b). As determined by electron microscopy, wounded control cells at 24 h had very few protrusions of the plasma membrane at the leading edge (Fig. 3B, panel a). In contrast, CNF1-treated wounded cells had numerous filopodia at the leading edge (Fig. 3B, panel b).
FIG. 3.
CNF1 induces F-actin reorganization and ultrastructure modifications in wounded T84 monolayers. (A) F-actin stained with rhodamine-phalloidin (500 nM) was observed by confocal microscopy in control (panel a) and CNF1-treated (panel b) T84 cells 24 h after wounding. The wound edges have a few lamellipodia in the control T84 cells, whereas numerous spreading filopodia were observed in the CNF1-treated T84 monolayer (arrowhead). The arrows indicates initial sites of wounding. Bars = 20 μm. (B) Electron microscopy of control (panel a) and CNF1-treated (panel b) T84 monolayers analyzed 24 h after wounding. Increased numbers of filopodia (arrows) were observed at the wound edge in the CNF1-treated monolayer. Bars = 2 μm.
CNF1 maintains FAK activation in T84 epithelial cells.
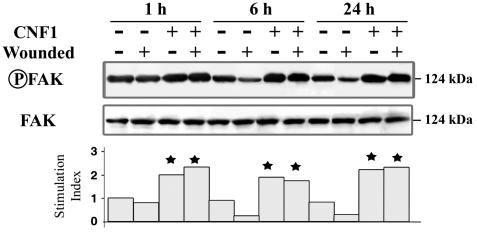
As shown in Fig. 4, FAK phosphorylation was observed in both nonwounded and wounded CNF1-treated T84 monolayers within 24 h, whereas FAK phosphorylation decreased gradually in control wounded T84 cells within 24 h. A high level of FAK phosphorylation was maintained in wounded T84 monolayers treated with CNF1.
FIG. 4.
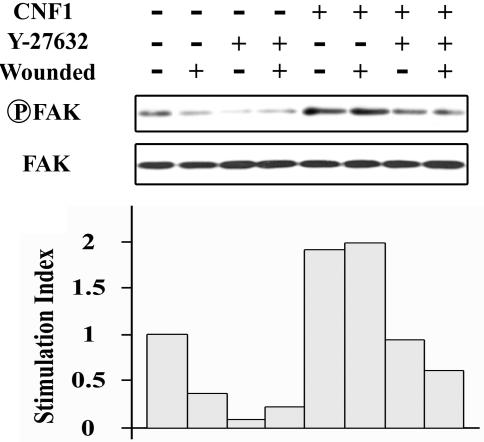
CNF1 induces tyrosine phosphorylation of FAK in T84 monolayers. Quiescent T84 cells that were serum starved for 24 h were incubated in the absence or in the presence of CNF1 (4 nM, 3 h). T84 cells that were incubated or not incubated with CNF1 were lysed after 1, 6, or 24 h of wound healing. Samples were immunodepleted with anti-FAK antibody, resolved by SDS-PAGE, and immunodetected with anti-phospho-FAK and anti-FAK. Densitometric scanning of FAK phosphorylation toward total FAK protein was performed. The results are expressed as an index of stimulation based on the basal level measured in unstimulated T84 cells. The results are representative of the results of three experiments performed with different cell preparations. A star indicates that the P value is <0.01.
Y-27632 restores healing of wounded CNF1-treated monolayers.
Wound closure was accelerated in the presence of Y-27632 in both control and CNF1-treated monolayers (Fig. 5). Compared with control cells (Fig. 5A, panel a), complete restitution of the monolayers was detected at 24 h with Y-27632-treated cells (Fig. 5A, panel b). Treatment of cells with Y-27632 could completely impair the inhibition of wound closure observed in monolayers treated with CNF1 at 24 h (Fig. 5A, panels c and d, and Fig. 5B).
FIG. 5.
Y-27632 inhibitor restores wound closure in CNF1-treated monolayers. The specific p160/ROCK Y-27632 inhibitor restored wound closure in CNF1-treated monolayers and accelerated the closure in wounded control T84 cells. (A) Phase-contrast images of the wound edge 24 h after wounding in control (panel a), Y-27632-treated (panel b), CNF1-treated (panel c), and CNF1- and Y-27632-treated (panel d) T84 monolayers. Rapid wound healing after exposure to the inhibitor was observed in both control and CNF1-treated T84 cells. Bars = 60 μm. (B) Percentages of wound closure, calculated as described in Materials and Methods, 12, 24, and 48 h after wounding. Solid bars, control monolayers; gray bars, Y-27632-treated monolayers; striped bars, CNF1-treated monolayers; open bars, CNF1- and Y-27632-treated monolayers. One star indicates that the P value is < 0.01, and two stars indicate that the P value is <0.005.
The results of an analysis of the effect of Y-27632 on FAK phosphorylation in control wounded monolayers and in CNF1-treated wounded monolayers paralleled the observations for wound closure, since under both conditions the inhibitor Y-27632 induced significant decreases in the levels of phosphorylation (Fig. 6).
FIG. 6.
Y-27632 inhibits CNF1-induced FAK autophosphorylation in T84 monolayers. Quiescent T84 cells that were serum starved for 24 h were incubated with CNF1 (4 nM, 3 h). Nonwounded or wounded cells incubated with or without the Y-27632 inhibitor were lysed 24 h after wounding. (Upper panel) Samples were immunodepleted with anti-FAK antibody, resolved by SDS-PAGE, and immunodetected with anti-phospho-FAK and anti-FAK antibodies. The results are representative of the results of three experiments performed with different cell preparations. (Lower panel) Densitometric scanning of FAK phosphorylation toward FAK. The results are expressed as an index of stimulation based on the basal level measured in unstimulated T84 cells.
MAPK phosphorylation is enhanced in wounded T84 monolayers treated with CNF1.
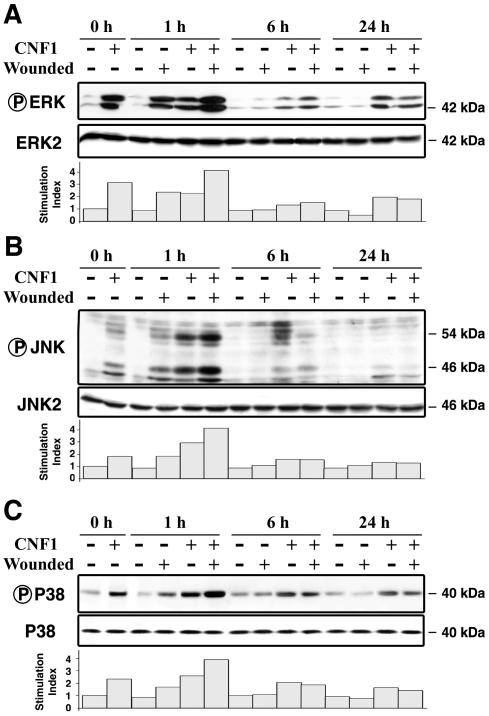
Phosphorylation of ERK1/2, JNK1/2, and p38 MAPK was determined 1, 6, and 24 h after epithelial injury. Before wounding, MAPK was phosphorylated in the CNF1-treated cells. At 1 h after wounding, MAPK was phosphorylated under wounded conditions, and there was an additive effect in T84 cells treated with CNF1 (Fig. 7). The levels of phosphorylation of MAPK at 6 or at 24 h in wounded control cells were similar to the basal levels observed in nonwounded cells, whereas treatment with CNF1 resulted in higher levels of MAPK phosphorylation in wounded cells (Fig. 7).
FIG. 7.
CNF1 enhances MAPK activation in wounded T84 monolayers. Quiescent T84 cells that were serum starved for 24 h were incubated in the presence of CNF1 (4 nM, 3 h). Nonwounded cells and wounded cells were lysed after 1, 6, and 24 h of wound healing. Samples were resolved by SDS-PAGE and immunodetected with anti-phospho-ERK and anti-ERK (A), anti-phospho-JNK and anti-JNK (B), and anti-phospho-p38 and anti-p38 (C) antibodies. The results are representative of the results of three experiments performed with different cell preparations.
MMP-9 activities are increased in wounded T84 monolayers treated with CNF1.
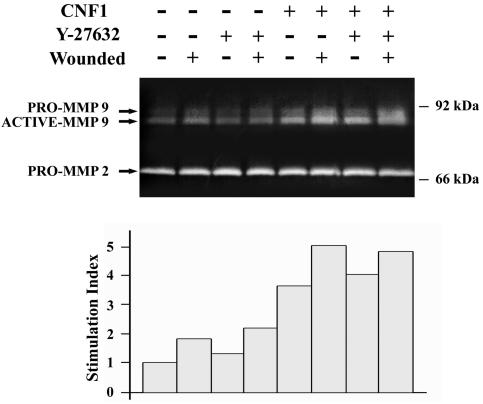
To investigate whether CNF1 could mediate induction and/or activation of MMP, we monitored MMP secretion in culture supernatant by gelatin zymography after 24 h of culture following epithelial injury. In contrast to control monolayers, MMP expression was increased twofold in wounded monolayers, and the major form corresponded to the 82-kDa active protease (Fig. 8). Interestingly, this activity was 5.1-fold greater in CNF1-treated monolayers under both control and wounded conditions (Fig. 8). By contrast, the 72-kDa gelatinolytic activity, probably corresponding to pro-MMP-2, remained stable under the same conditions. As Y-27632 inhibits ROCK and restores wound healing, we analyzed the effect of Y-27632 on MMP-9 activation. Y-27632 did not significantly reverse CNF1-mediated MMP-9 induction, indicating that signaling pathways leading to MMP-9 expression are ROCK independent.
FIG. 8.
CNF1 enhances MMP-9 expression in control and wounded T84 monolayers. (Upper panel) Conditioned media from cultures of nonwounded cells and wounded cells grown in the presence or absence of CNF1 and/or in the presence or absence of Y-27632 were analyzed by gelatin zymography as described in Materials and Methods. (Lower panel) Densitometric negative scanning of MMP-9 toward MMP-2. The results are expressed as an index of stimulation based on the level measured in unstimulated T84 cells.
DISCUSSION
To investigate the effects of CNF1 on wound healing, we used intestinal epithelial cells (T84 cells) wounded over the entire surface area of monolayers. The procedure used in this study resulted in reproducible conditions for mechanical injury with great wound length (49). We showed that CNF1 inhibited monolayer wound closure through irreversible activation of Rho proteins. Since apoptotic or necrotic processes were not detected in T84 cells after 3 h of incubation with CNF1, the hypothesis that impairment of wound healing depended on death of the T84 cells was ruled out. Moreover, in contrast to uroepithelial cells, T84 intestinal epithelial cells did not appear to be susceptible to the CNF1 apoptotic effect (21, 32). As reported for other cell lines, CNF1 induced complete ADP ribosylation of Rho in T84 cells (23). We showed that in wounded T84 monolayers, CNF1 triggered rapid formation of stress fibers and filopodia in epithelial cells at the leading edge. These effects were the consequence of Rho GTPase deamidation by CNF1 and were characterized by the intense actin cytoskeleton polymerization in CNF1-treated cells, which was shown to be indistinguishable from that observed following overexpression of the constitutive form of this protein.
Rho proteins are crucial for cell movement, particularly in wound repair (13, 26, 27, 34, 41, 43). Different RhoA, Rac, or Cdc42 mutants have previously been used to determine the involvement of these proteins in cell movement during the wound-healing process (34, 41). In our study, RhoA, Rac, and Cdc42 were all completely activated by CNF1. Complete healing of control wounded monolayers at 48 h was not observed in CNF1-treated monolayers. In control epithelial cells, the leading edge formed a unit of migrating cells that impelled cells into other cells. Conversely, in CNF1-treated monolayers, epithelial cells at the wound border migrated individually, and then each cell sent filopodia toward the wound. The result was individual cell spreading without migration of the rest of the monolayer. These results demonstrated that CNF1 may delay the early phase of the mucosal healing process through inhibiting the Rho function.
Thus, we investigated the mechanism involved in CNF1-mediated blockage of cell migration by determining the state of FAK phosphorylation in wounded CNF1-treated T84 monolayers. One hallmark of the cell movement is the dynamic turnover of the focal adhesion contacts and one of the key roles played by FAK (37). By using Western blotting, we demonstrated that CNF1 maintained permanent FAK phosphorylation (in both nonwounded and wounded monolayers), whereas FAK activation decreased in wounded control T84 cells. In this regard, a previous study showed that paxillin, a focal adhesion protein, was tyrosine phosphorylated in T84 polarized intestinal epithelial monolayers treated basolaterally with CNF1 (23). We also investigated whether p160/ROCK activation was required for CNF1 to induce phosphorylation of FAK. To do this, quiescent cultures of T84 monolayers were treated with a specific inhibitor of p160/ROCK, Y-27632, which blocks Rho-induced reorganization of the cytoskeleton (31). We showed that Y-27632 strongly attenuated the increase in the CNF1-induced phosphorylation of FAK, indicating that the increase in FAK phosphorylation induced by CNF1 is mediated by protein kinases belonging to the p160/ROCK family. Moreover, the inhibition of closure in wounded CNF1-treated T84 cells was impaired when monolayers were exposed to the p160/ROCK inhibitor. Thus, the inhibition of wound closure in CNF1-treated monolayers was induced by inhibition of FAK turnover, which may dock the monolayer to the extracellular matrix. In parallel, FAK phosphorylation observed at 24 h in wounded CNF1-treated monolayers was not observed when the cells were exposed to the p160/ROCK inhibitor. Moreover, exposure of the cells to Y-27632 strongly inhibited the formation of actin stress fibers in response to CNF1 (data not shown). In this regard, previous study indicated that translocation of FAK to nascent focal adhesions promotes autophosphorylation of the molecule as a result of clustering and/or conformational changes (47).
CNF1 is known to simultaneously activate different members of the Rho family and may thus induce conflicting signaling processes. Consequently, CNF1 not only may activate pathways that are normally regulated by active Rho proteins but also may stimulate pathways in toxin-treated cells or in bacterium-infected cells. A previous report described MAPK activation in epithelial cells upon CNF1 treatment (29). Moreover, several studies showed that a transient increase in MAPK phosphorylation occurred in wounded monolayers (17, 34). Here, we demonstrated that the transient MAPK activation observed in wounded monolayers was strongly enhanced in CNF1-treated cells.
Degradation of the extracellular matrix by MMPs is crucial in the progression of epithelial wound healing (36). Along with other MMPs, MMP-9 plays a key role in physiological processes, not only in development, angiogenesis, tumor invasion, and metastasis but also in inflammation and wound healing (36). MMP-9 expression and/or secretion is mediated or stimulated by a variety of factors, including growth factors, phorbol esters, cytokines, bacterial endotoxins, and MAPK or FAK activation (2, 10, 50, 51). In this regard, we investigated the potential contribution of MAPK phosphorylation induced by CNF1 in MMP-9 expression. Our results demonstrated that MMP-9 was upregulated in both wounded and CNF1-treated T84 monolayers. Although previous studies have shown that both FAK and Rac can modulate both MMP activity and MMP expression (19, 20, 24, 44, 52), Y-27632, which inhibits FAK phosphorylation by targeting ROCK, did not block CNF1-mediated MMP9 induction, suggesting that the constitutive activation of Rac by CNF1 is the major inducer of MMP-9 expression in our model.
Taken together, our results show that CNF1 could be a virulence factor during the wound-healing process by exhibiting three combined activities, (i) inhibition of epithelial cell migration at the leading edge along with ROCK activation and FAK phosphorylation, (ii) maintenance of MAPK phosphorylation, and (iii) increasing extracellular matrix degradation via MMP synthesis.
Acknowledgments
We thank Mireille Mari for her expert technical assistance.
Editor: V. J. DiRita
References
- 1.Birkedal-Hansen, H. 1995. Proteolytic remodeling of extracellular matrix. Curr. Opin. Cell Biol. 7:728-735. [DOI] [PubMed] [Google Scholar]
- 2.Bond, M., R. P. Fabunmi, A. H. Baker, and A. C. Newby. 1998. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 435:29-34. [DOI] [PubMed] [Google Scholar]
- 3.Boquet, P. 1998. Cytotoxic necrotizing factor 1 from Escherichia coli: a toxin with a new intracellular activity for eukaryotic cells. Folia Microbiol. 43:285-289. [DOI] [PubMed] [Google Scholar]
- 4.Brest, P., B. Mograbi, V. Hofman, A. Loubat, B. Rossi, P. Auberger, and P. Hofman. 2003. Rho GTPase is activated by cytotoxic necrotizing factor 1 in peripheral blood T lymphocytes: potential cytotoxicity for intestinal epithelial cells. Infect. Immun. 71:1161-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buetow, L., G. Flatau, K. Chiu, P. Boquet, and P. Ghosh. 2002. Strategies for the structural determination of the catalytic domain of Escherichia coli cytotoxic necrotizing factor 1. Acta Crystallogr. Sect. D Biol. Crystallogr. 58:366-369. [DOI] [PubMed] [Google Scholar]
- 6.Caprioli, A., V. Falbo, L. G. Roda, F. M. Ruggeri, and C. Zona. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chardin, P., P. Boquet, P. Madaule, M. R. Popoff, E. J. Rubin, and D. M. Gill. 1989. The mammalian G protein RhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 8:1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, B. H., J. T. Tzen, A. R. Bresnick, and H. C. Chen. 2002. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 277:33857-33863. [DOI] [PubMed] [Google Scholar]
- 9.Doye, A., A. Mettouchi, G. Bossis, R. Clement, C. Buisson-Touati, G. Flatau, L. Gagnoux, M. Piechaczyk, P. Boquet, and E. Lemichez. 2002. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111:553-564. [DOI] [PubMed] [Google Scholar]
- 10.Eberhardt, W., A. Huwiler, K. F. Beck, S. Walpen, and J. Pfeilschifter. 2000. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J. Immunol. 165:5788-5797. [DOI] [PubMed] [Google Scholar]
- 11.Falzano, L., C. Fiorentini, G. Donelli, E. Michel, C. Kocks, P. Cossart, L. Cabanie, E. Oswald, and P. Boquet. 1993. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9:1247-1254. [DOI] [PubMed] [Google Scholar]
- 12.Falzano, L., M. G. Quaranta, S. Travaglione, P. Filippini, A. Fabbri, M. Viora, G. Donelli, and C. Fiorentini. 2003. Cytotoxic necrotizing factor 1 enhances reactive oxygen species-dependent transcription and secretion of proinflammatory cytokines in human uroepithelial cells. Infect. Immun. 71:4178-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenteany, G., P. A. Janmey, and T. P. Stossel. 2000. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 10:831-838. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentini, C., P. Matarrese, E. Straface, L. Falzano, G. Donelli, P. Boquet, and W. Malorni. 1998. Rho-dependent cell spreading activated by E. coli cytotoxic necrotizing factor 1 hinders apoptosis in epithelial cells. Cell Death Differ. 5:921-929. [DOI] [PubMed] [Google Scholar]
- 15.Flatau, G., L. Landraud, P. Boquet, M. Bruzzone, and P. Munro. 2000. Deamidation of RhoA glutamine 63 by the Escherichia coli CNF1 toxin requires a short sequence of the GTPase switch 2 domain. Biochem. Biophys. Res. Commun. 267:-592. [DOI] [PubMed] [Google Scholar]
- 16.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 17.Goke, M., M. Kanai, K. Lynch-Devaney, and D. K. Podolsky. 1998. Rapid mitogen-activated protein kinase activation by transforming growth factor alpha in wounded rat intestinal epithelial cells. Gastroenterology 114:697-705. [DOI] [PubMed] [Google Scholar]
- 18.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 19.Hauck, C. R., D. A. Hsia, X. S. Puente, D. A. Cheresh, and D. D. Schlaepfer. 2002. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 21:6289-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauck, C. R., D. J. Sieg, D. A. Hsia, J. C. Loftus, W. A. Gaarde, B. P. Monia, and D. D. Schlaepfer. 2001. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 61:7079-7090. [PubMed] [Google Scholar]
- 21.Hofman, P., G. Flatau, E. Selva, M. Gauthier, G. Le Negrate, C. Fiorentini, B. Rossi, and P. Boquet. 1998. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect. Immun. 66:2494-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofman, P., G. Le Negrate, B. Mograbi, V. Hofman, P. Brest, A. Alliana-Schmid, G. Flatau, P. Boquet, and B. Rossi. 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68:522-528. [PubMed] [Google Scholar]
- 23.Hopkins, A. M., S. V. Walsh, P. Verkade, P. Boquet, and A. Nusrat. 2003. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116:725-742. [DOI] [PubMed] [Google Scholar]
- 24.Hsia, D. A., S. K. Mitra, C. R. Hauck, D. N. Streblow, J. A. Nelson, D. Ilic, S. Huang, E. Li, G. R. Nemerow, J. Leng, K. S. Spencer, D. A. Cheresh, and D. D. Schlaepfer. 2003. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160:753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 26.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 27.Keely, P. J., J. K. Westwick, I. P. Whitehead, C. J. Der, and L. V. Parise. 1997. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390:632-636. [DOI] [PubMed] [Google Scholar]
- 28.Lacerda, H. M., G. D. Pullinger, A. J. Lax, and E. Rozengurt. 1997. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21(rho)-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J. Biol. Chem. 272:9587-9596. [DOI] [PubMed] [Google Scholar]
- 29.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madara, J. L., S. Colgan, A. Nusrat, C. Delp, and C. Parkos. 1992. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial monolayers transmigration. J. Tissue Cult. Methods 14:209-216. [Google Scholar]
- 31.Maekawa, M., T. Ishizaki, S. Boku, N. Watanabe, A. Fujita, A. Iwamatsu, T. Obinata, K. Ohashi, K. Mizuno, and S. Narumiya. 1999. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285:895-898. [DOI] [PubMed] [Google Scholar]
- 32.Mills, M., K. C. Meysick, and A. D. O'Brien. 2000. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68:5869-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munaut, C., T. Salonurmi, S. Kontusaari, P. Reponen, T. Morita, J. M. Foidart, and K. Tryggvason. 1999. Murine matrix metalloproteinase 9 gene 5′-upstream region contains cis-acting elements for expression in osteoclasts and migrating keratinocytes in transgenic mice. J. Biol. Chem. 274:5588-5596. [DOI] [PubMed] [Google Scholar]
- 34.Nobes, C. D., and A. Hall. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144:1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobes, C. D., and A. Hall. 1995. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 36.Parks, W. C. 1999. Matrix metalloproteinases in repair. Wound Repair Regen. 7:423-432. [DOI] [PubMed] [Google Scholar]
- 37.Parsons, J. T., K. H. Martin, J. K. Slack, J. M. Taylor, and S. A. Weed. 2000. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19:5606-5613. [DOI] [PubMed] [Google Scholar]
- 38.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389-399. [DOI] [PubMed] [Google Scholar]
- 39.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell 70:401-410. [DOI] [PubMed] [Google Scholar]
- 40.Salo, T., M. Makela, M. Kylmaniemi, H. Autio-Harmainen, and H. Larjava. 1994. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab. Investig. 70:176-182. [PubMed] [Google Scholar]
- 41.Santos, M. F., S. A. McCormack, Z. Guo, J. Okolicany, Y. Zheng, L. R. Johnson, and G. Tigyi. 1997. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J. Clin. Investig. 100:216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz, A. A., E. E. Govek, B. Bottner, and L. Van Aelst. 2000. Rho GTPases: signaling, migration, and invasion. Exp. Cell Res. 261:1-12. [DOI] [PubMed] [Google Scholar]
- 44.Shibata, K., F. Kikkawa, A. Nawa, A. A. Thant, K. Naruse, S. Mizutani, and M. Hamaguchi. 1998. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 58:900-903. [PubMed] [Google Scholar]
- 45.Singer, A. J., and R. A. Clark. 1999. Cutaneous wound healing. N. Engl. J. Med. 341:738-746. [DOI] [PubMed] [Google Scholar]
- 46.Sternlicht, M. D., and Z. Werb. 2001. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17:463-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas, W., G. D. Pullinger, A. J. Lax, and E. Rozengurt. 2001. Escherichia coli cytotoxic necrotizing factor and Pasteurella multocida toxin induce focal adhesion kinase autophosphorylation and Src association. Infect.Immun. 69:1-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turchi, L., A. A. Chassot, I. Bourget, C. Baldescchi, J. P. Ortonne, G. Meneguzzi, E. Lemichez, and G. Ponzio. 2003. Cross-talk between Rho GTPases and stress activated kinases for matrix metalloproteinase-9 induction in response to keratinocytes injury. J. Investig. Dermatol. 121:1291-1300. [DOI] [PubMed] [Google Scholar]
- 49.Turchi, L., A. A. Chassot, R. Rezzonico, K. Yeow, A. Loubat, B. Ferrua, G. Lenegrate, J. P. Ortonne, and G. Ponzio. 2002. Dynamic characterization of the molecular events during in vitro epidermal wound healing. J. Investig. Dermatol. 119:56-63. [DOI] [PubMed] [Google Scholar]
- 50.Underwood, D. C., R. R. Osborn, S. Bochnowicz, E. F. Webb, D. J. Rieman, J. C. Lee, A. M. Romanic, J. L. Adams, D. W. Hay, and D. E. Griswold. 2000. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L895-L902. [DOI] [PubMed] [Google Scholar]
- 51.Yao, J., S. Xiong, K. Klos, N. Nguyen, R. Grijalva, P. Li, and D. Yu. 2001. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene 20:8066-8074. [DOI] [PubMed] [Google Scholar]
- 52.Zhuge, Y., and J. Xu. 2001. Rac1 mediates type I collagen-dependent MMP-2 activation. Role in cell invasion across collagen barrier. J. Biol. Chem. 276:16248-16256. [DOI] [PubMed] [Google Scholar]