Abstract
AIM
To examine whether high-flow nasal oxygen (HFNO) availability influences the use of general anesthesia (GA) in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) and associated outcomes.
METHODS
In this retrospective study, patients were stratified into 3 eras between October 1, 2013 and June 30, 2014 based on HFNO availability for deep sedation at the time of their endoscopy. During the first and last 3-mo eras (era 1 and 3), no HFNO was available, whereas it was an option during the second 3-mo era (era 2). The primary outcome was the percent utilization of GA vs deep sedation in each period. Secondary outcomes included oxygen saturation nadir during sedation between periods, as well as procedure duration, and anesthesia-only time between periods and for GA vs sedation cases respectively.
RESULTS
During the study period 238 ERCP or EUS cases were identified for analysis. Statistical testing was employed and a P < 0.050 was significant unless the Bonferroni correction for multiple comparisons was used. General anesthesia use was significantly lower in era 2 compared to era 1 with the same trend between era 2 and 3 (P = 0.012 and 0.045 respectively). The oxygen saturation nadir during sedation was significantly higher in era 2 compared to era 3 (P < 0.001) but not between eras 1 and 2 (P = 0.028) or 1 and 3 (P = 0.069). The procedure time within each era was significantly longer under GA compared to deep sedation (P ≤ 0.007) as was the anesthesia-only time (P ≤ 0.001).
CONCLUSION
High-flow nasal oxygen availability was associated with decreased GA utilization and improved oxygenation for ERCP and EUS during sedation.
Keywords: Endoscopic ultrasound, Endoscopic retrograde cholangiopancreatography, Endoscopy, Sedation, Anesthesia, Oxygenation, High flow nasal oxygen
Core tip: This retrospective study demonstrates a decreased use of GA when HFNO is available in the endoscopy unit for patients undergoing endoscopic retrograde cholangiopancreatography and endoscopic ultrasound under sedation. Provision of HFNO and deep sedation was associated with decreased procedure and anesthesia-only times, and oxygenation was improved compared to sedation without HFNO. These findings justify further prospective trials to fully elucidate the role of HFNO during sedation in gastrointestinal endoscopy. HFNO may have the potential to alter sedation practices in the endoscopy suite.
INTRODUCTION
Sedation and analgesia are a critical component of performing gastrointestinal endoscopy, as patients would otherwise often experience anxiety and pain or discomfort. Successful completion and safety of the examination require the patients’ procedural tolerance, particularly for more advanced procedures such as endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS)[1]. Sedation represents a continuum of altered consciousness, ranging from moderate to deep sedation (DS) and may include general anesthesia (GA)[2]. For decades, intravenous sedation using a combination of a benzodiazepine and an opioid administered by a nurse under the supervision of a gastroenterologist was routine practice. However, national trends reveal that both academic and private groups are now moving towards anesthesiologist-administered sedation[3-5]. Although it depresses the respiratory drive, among anesthesia providers propofol has become the agent of choice for several reasons. Propofol has a fast onset and offset of action, it induces a high patient tolerance for complex procedures in a dose dependent manner, and a study comparing propofol to midazolam during ERCP noted higher technical success in the patients that received propofol[6]. Superior recovery time and improved practice efficiency with propofol use have also been described[7]. Because propofol does not provide analgesia, patients often receive doses that result in DS[8]. Worldwide, sedation practices including general anesthesia for procedures such as ERCP vary greatly and no consensus on the optimal strategy exits[9]. A 2011 survey of members of the Italian Society of Digestive Endoscopy revealed that 42.3% and 35.6% of patients undergoing ERCP and EUS in Italy received propofol respectively. In Greece, sedation is administered for 100% of ERCP and EUS procedures with approximately 2/3 of patients receiving a combination of a benzodiazepine and an opioid[9,10]. The majority of gastroenterologists in the United States prefer deep sedation with propofol for complex endoscopic procedures. Data from developing countries is scarce. With regards to the types oxygen delivery systems used, international practice patterns are not known.
Close monitoring of the airway, respiration, and oxygenation is critical for safe sedation, and spontaneous ventilation should be preserved during DS. Supplemental oxygen is routinely provided to all patients regardless of the level of sedation to prevent hypoxemia[11]. Multiple oxygen delivery systems exist for patients undergoing procedural sedation. Both low-flow, providing up to 15 L/min of oxygen, and high-flow systems can be employed including standard nasal cannulae, simple face masks, Venturi masks, and non-rebreathing masks that deliver varying fractions of inspired oxygen (FiO2)[12]. The choice of the oxygen delivery device depends on the clinical circumstances, patient and provider preference, reliability, and ease of use. Inability to effectively heat and humidify gas limits the utility of low-flow modalities in certain situations[12,13]. Conventional high-flow systems (Venturi masks) are able to provide a flow rate of 60 L/min or greater and are superior at providing supplemental oxygen at precise concentrations[14]. However, some patients may not tolerate these devices due to a sensation of obstruction created by the masks. Furthermore, heating and humidification of the inspired air/oxygen mixture needs improvement. Humidified heated high-flow nasal oxygen (HFNO) delivered through nasal cannulae designed to overcome some of these limitations has been introduced to several acute care settings. HFNO is an option in the care of adult patients with hypoxemic respiratory failure and following cardiac, cardiothoracic, and vascular surgery[15-17]. HFNO administration can also be used to maximize oxygenation prior to intubation and to prevent postextubation failure[18,19]. In addition, HFNO during sedation for flexible bronchoscopy has been shown to be safe in patients with stable respiratory parameters, and when compared to Venturi masks, HFNO provides superior oxygenation[20].
Use of HFNO has not been systematically studied during DS for complex gastrointestinal endoscopic procedures such as ERCP and EUS. Indications for these advanced diagnostic and therapeutic techniques are expanding, and patients undergoing ERCP and EUS may have more comorbidities than those receiving esophagogastroduodenoscopy (EGD) and/or colonoscopy[21]. ERCP and EUS procedures can be prolonged, and ERCP is often performed in the prone position, creating a challenge for airway management and ventilation. Many practitioners therefore resort to GA with endotracheal intubation[21]. While GA is safely performed in most endoscopy suites, it requires invasive airway management, carries its own risks, prolongs overall procedural time, and may increase costs[22]. The availability of HFNO may expand the suitability of DS for many patients who would otherwise undergo GA. At our institution, HFNO became available for a 3-mo period shortly after initiating an anesthesiology service in the endoscopy suite. We hypothesized that the availability of HFNO to the anesthesia team in the endoscopy suite would reduce the use of GA for ERCP and EUS.
MATERIALS AND METHODS
Patients and personnel
The Tufts Medical Center and Tufts University Health Sciences Campus Institutional Review Board approved this retrospective study. A chart review was conducted during a 9-mo period of all consecutive patients undergoing ERCP and EUS procedures. Patients were then stratified into 3 eras based on whether or not HFNO was available at the time of their endoscopy. During the first 3-mo era, no HFNO was available (pre-HFNO, era 1). HFNO became an option for clinical use at the anesthesia team’s discretion during the second 3-mo era (HFNO, era 2). Finally, during the third 3-mo era, HFNO was no longer provided (post-HFNO, era 3). Patient characteristics including age, gender, body mass index (BMI, kg/m2), and American Society of Anesthesiologists (ASA) physical status classification were collected. Data analyzed between eras included procedure type (ERCP or EUS), type of anesthesia used (DS or GA), oxygen saturation (SpO2) nadir during sedation, procedure duration, and anesthesia-only time, defined as anesthesia start to end time in minutes minus the procedure time. This included the induction of DS or GA and the time from the end of the endoscopic procedure to the emergence of the patient from DS or GA and completion of the anesthesia report to a nurse in the recovery area.
The anesthesia team’s use of HFNO in era 2 was not consistently recorded and identifiable for each case. However, 60 HFNO disposables dedicated for utilization in the GI suite were employed for the 73 patients undergoing DS, suggesting that 82.2% of the cohort in era 2 received HFNO. We used an intention-to-treat approach for the era 2 study cohort for the purpose of the analysis.
Clinical care and equipment
Expert endoscopists performed all procedures. Patients were positioned supine for EUS and in the semi-prone position with the right side slightly elevated for ERCP. Anesthesia care was provided by rotating teams that included attending physicians, residents, and certified registered nurse anesthetists. The decision to use DS vs GA was at the discretion of the anesthesia teams following discussion with the endoscopists. GA was achieved by standard anesthesia induction with propofol, endotracheal intubation using a short-acting neuromuscular blocking agent such as atracurium and anesthetic maintenance with sevoflurane in oxygen and air followed by controlled ventilation with an anesthesia machine. Deep sedation with or without local anesthetic topicalization of the pharynx was achieved with a propofol infusion and repeated propofol boluses in a spontaneously breathing patient. In this study, recovery from DS was available in the endoscopy suite, while recovery from GA required admission to the post-anesthesia care unit, located on a different floor. Standard of care for oxygen delivery consisted of the use of a nasal cannula at flow rates between 3 and 6 L/min with end-tidal carbon dioxide (ETCO2) sampling. The HFNO oxygen delivery system available for DS was the AIRVO™ 2 (Fisher and Paykel Healthcare Limited, Panmure, New Zealand, Figure 1). This system is characterized by ease of use, silent operation, and a small footprint. It delivers humidified oxygen via specifically designed high-flow nasal prongs that we modified for sedation care to capture ETCO2 (Figure 2). The HFNO oxygen enriched air flow settings are adjustable between 10 and 60 L/min with a maximal oxygen output of 50% when using standard 15 L/min supplemental O2 to the machine inflow port as in our case.
Figure 1.
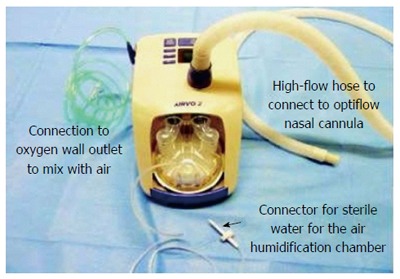
AIRVO™ 2 (Fisher and Paykel Healthcare Limited, Panmure, New Zealand).
Figure 2.
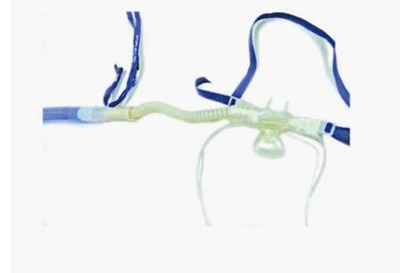
Modified nasal prongs.
Statistical analysis
We employed appropriate testing for comparisons between three different eras as follows: the χ2 test (or Fisher’s exact test, in the case of sparse expected cell counts) was used for nominal outcomes, one-way analysis of variance was used for normally distributed continuous outcomes, and the Kruskal-Wallis test (alongside the Mann-Whitney U test for post-hoc comparisons) was used for ordinal and non-normally distributed continuous outcomes. The Bonferroni correction was used for post hoc analysis of multiple group comparisons as applicable. To determine predictors of DS vs GETA use we performed a multivariate logistic regression including the variables: age, BMI, procedure time and ASA status. Continuous data are reported as mean and standard deviation (SD) or median and interquartile range (IQR); categorical data are reported as counts and percentages. P < 0.050 was considered statistically significant, with the exception of tests in which the Bonferroni correction was used. SPSS Statistics V22.0 (IBM, Armonk, New York) was used in the analysis. The statistical methods of this study were performed and reviewed by Matthew D. Finkelman, PhD, Director of Biostatistics and Experimental Design at Tufts University School of Dental Medicine.
RESULTS
Records of 238 consecutive elective ERCP or EUS cases performed in the endoscopy suite at our institution were analyzed. Of the 238 cases, 63 were performed in era 1, 88 in era 2, and 87 in era 3. There was no statistically significant difference in age, gender, BMI, or ASA class of the patients between the three eras. The difference in the relative proportion of ERCP and EUS procedures between eras was not statistically significant using a 2-sided χ2 test (P = 0.530, Table 1), and neither was the difference between the relative proportions of ERCP and EUS procedures performed under DS (P= 0.700, results not shown).
Table 1.
Patient characteristics and relative proportion of procedures between eras 1-3
| Demographic | Era 1 | Era 2 | Era 3 | P value |
| Age (yr) | 61.1 ± 15.91 | 60.9 ± 15.9 | 62.2 ± 18.2 | 0.850 |
| BMI (kg/m2) | 28.1 ± 8.3 | 26.7 ± 7.4 | 26.3 ± 6.4 | 0.290 |
| Gender (F), n (%) | 28 (44.4) | 45 (51.1) | 53 (60.9) | 0.120 |
| ASA status 1-3 | - | - | - | 0.990 |
| ERCP, n (%) | 33 (52.4) | 38 (43.2) | 40 (46.0) | 0.530 |
| EUS, n (%) | 30 (47.6) | 50 (56.8) | 47 (54.0) | 0.530 |
Data in mean ± standard deviation. BMI: Body mass index; ERCP: Endoscopic retrograde cholangiopancreatography; EUS: Endoscopic ultrasound.
The availability of HFNO was associated with decreased use of GA in our study (Figure 3). Seventeen percent of patients received GA during era 2 compared to 34.9% in era 1 and 29.9% in era 3. The difference was statistically significant following Bonferroni correction (P = 0.012) between eras 1 and 2 but only a trend towards decreased use of GA between eras 2 and 3 (P = 0.045, Table 2) was seen when using the Bonferroni correction. The difference in type of anesthesia between eras 1 and 3 was not significant (P = 0.514).
Figure 3.
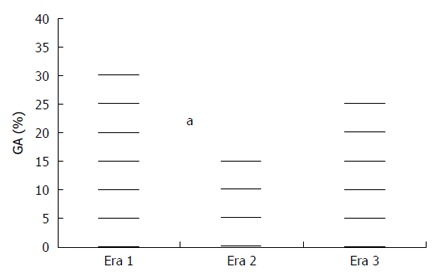
Use of general anesthesia during eras 1, 2 and 3. During era 2 (HFNO available) significantly less cases were performed under GA compared to era 1, although this difference did not reach statistical significance between eras 2 and 3. HFNO: High-flow nasal oxygen; GA: General anesthesia. aP = 0.012 era 1 vs era 2.
Table 2.
Utilization of deep sedation vs general anesthesia and oxygenation during deep sedation between eras
| Anesthesia type | Era 1 | Era 2 | Era 3 | P value |
| DS, n (%) | 41 (65.1) | 73 (83.0) | 61 (70.1) | 0.033 |
| GA, n (%) | 22 (34.9) | 15 (17.0) | 26 (29.9) | 0.033 |
| DS only SpO2 nadir, | 4.5 (98.0) | 3.0 (99.0)a | 4.0 (96.0) | < 0.001 |
There was a significantly lower utilization of GA in era 2 compared to era 1 (P = 0.012) that persisted as a trend only between eras 2 and 3 after Bonferroni correction (P = 0.045). There was a significantly lower median SpO2 nadir in era 3 compared to era 2 (aP < 0.001) that was a trend between eras 1 and 2 after Bonferroni correction (P = 0.028). DS: Deep sedation; GA: General anesthesia.
Improved oxygenation was observed in era 2 compared to eras 1 and 3 with a median oxygen saturation nadir of 99.0%, 98.0%, and 96.0% respectively. Following Bonferroni correction, the difference was statistically significant between eras 2 and 3 (P < 0.001, Table 2) but not between eras 1 and 2 (P = 0.028) or 1 and 3 (P = 0.069).
The median combined procedure time and interquartile ranges for ERCP and EUS in era 1 of 28.0 and 23.0 min improved to 26.0 and 18.3 min and 24.0 and 21.3 min in eras 2 and 3 respectively. The difference in total procedure time for ERCP and EUS, either combined or analyzed separately, was not significantly different between eras. However, the procedure time for complex endoscopies under GA was significantly longer within each era compared to endoscopies under DS (P ≤ 0.007, Table 3).
Table 3.
Procedure times by era
| Procedure | Era 1 | Era 2 | Era 3 | P value |
| Combined ERCP/EUS | 28.0, 23.0 | 26.0, 18.3 | 24.0, 21.3 | 0.340 |
| ERCP only | 30.0, 35.5 | 29.5, 32.0 | 29.5, 28.5 | 0.920 |
| EUS only | 25.0, 20.5 | 24.0, 15.2 | 19.0, 18.2 | 0.170 |
| ERCP/EUS under DS | 25.0, 18.0 | 26.0, 17.0 | 20.0, 18.7 | ≤ 0.007 |
| ERCP/EUS under GA | 40.5, 41.7 | 39.0, 28.0 | 30.5, 24.5 | (time comparison within each era) |
Values are given in medians and interquartile ranges. Time was assessed in minutes from procedure start to finish. There was no significant difference in procedure times between eras, but procedure times were significantly shorter under DS compared to GA within each era. ERCP: Endoscopic retrograde cholangiopancreatography; EUS: Endoscopic ultrasound; DS: Deep sedation; GA: General anesthesia.
Anesthesia-only times are summarized in Table 4. Anesthesia-only time was significantly shorter for DS compared to GA within each era regardless of procedure type (P ≤ 0.001). For complex endoscopies, anesthesia-only time was significantly different between eras (P = 0.006). Anesthesia-only time was significantly shorter by 6.5 min in era 2 compared to era 3 (P = 0.001) but not between eras 1 and 2 or 1 and 3 (P = 0.130 and 0.270, respectively) after Bonferroni correction (results not shown). When analyzed by procedure type, anesthesia-only time was not significantly different between eras for ERCP (P = 0.080), but it was significantly different for EUS (P = 0.005). In post-hoc tests for EUS, the anesthesia-only time was significantly shorter by 5.5 min in era 2 compared to era 3 (P = 0.001). There were no differences in EUS anesthesia-only time between eras 1 and 2 and 1 and 3 (P = 0.500 and 0.060, respectively).
Table 4.
Anesthesia-only times between and within eras
| Anesthesia time | Era 1 | Era 2 | Era 3 | P value |
| ERCP/EUS combined | 26.0, 37.5 | 23.5, 20.51 | 30.0, 28.0 | 0.006 |
| ERCP | 52.5, 48.2 | 31.0, 27.5 | 43.5, 32.0 | 0.080 |
| EUS | 20.0, 12.0 | 17.5, 10.22 | 23.0, 11.2 | 0.005 |
| DS | 21.0, 12.5 | 21.0, 13.0 | 24.0, 13.0 | ≤ 0.001 |
| GA | 68.0, 34.7 | 56.0, 13.0 | 59.0, 24.5 | (time comparison within each era) |
Anesthesia time was significantly shorter in era 2 compared to era 3 (P = 0.001);
Anesthesia time for EUS was significantly shorter in era 2 compared to era 3 (P = 0.001). Values are given in medians and interquartile ranges. Anesthesia-only time was assessed in minutes from anesthesia start to finish after subtraction of the procedure time. ERCP: Endoscopic retrograde cholangiopancreatography; EUS: Endoscopic ultrasound; DS: Deep sedation; GA: General anesthesia.
In the multivariable regression analysis including age, BMI, ASA physical status classification, and procedure time, only ASA status and longer procedure time were significantly associated with GA use (P = 0.009 and < 0.001, respectively). Patients with an ASA status of 4 were significantly more likely to receive GA than ASA 1 - 3 patients (P ≤ 0.014), adjusting for the other variables in the model.
DISCUSSION
In this study, we investigated the association of the availability of humidified heated high-flow nasal oxygen delivery during sedation for ERCP and EUS in the endoscopy suite on the utilization of general anesthesia for these complex endoscopies. Compared to the era before HFNO, general anesthesia was used significantly less frequently during the era with HFNO availability, and a trend towards increased GA re-emerged when HFNO was no longer accessible. There was no significant difference between patients of each era regarding their age, gender, BMI, ASA physical status classification or the proportion of complex endoscopies performed. The lack of a statistically significant difference between GA and DS use between the HFNO (era 2) and post-HFNO (era 3) eras may be explained by the relatively small sample size of this study and that not all DS patients in era 2 received HFNO. It is also possible that the so-called learning contamination bias contributed to this finding[23]. The learning contamination bias would suggest that anesthesia teams became more assured of sedation use during the HFNO period and were more comfortable with sedation even after HFNO was no longer available. However, the consistent observation of a reduced GA use during the availability of HFNO represents a finding that warrants further prospective evaluation in the endoscopy suite as a feasible technique for ventilation support during deep sedation.
The oxygen saturation nadir during the procedures under DS was least during the HFNO era, and significantly so between era 2 and 3. Although the differences may not be clinically significant, HFNO availability was associated with improved oxygenation parameters compared to standard nasal cannula, and did not increase the risk of hypoxemia. This result is consistent with other trials employing HFNO in several different clinical settings[16,17,20,24,25].
As a clinical observation, the decision to use HFNO became strongly biased over time toward oxygen saturation support. That is, the desire to support oxygenation levels became a more frequently expressed reason for using HFNO. At the same time, there was no hesitation to use it due to the presence of conditions such as chronic obstructive pulmonary disease. Again, a prospective evaluation may provide better insight and guidance about the particular circumstances that favor or disfavor the use of HFNO.
HFNO oxygen delivery systems have been in development for decades[12]. Indications for HFNO vary, and this technology was first used to manage respiratory failure in neonates and children[26,27]. The combination of an air/oxygen blender and a heated humidifier allows this system to deliver an air-oxygen mixture via specifically designed nasal cannulae at a rate of up to 60 L/min. Flow rate and FiO2 can be adjusted independently in some HFNO systems. HFNO provides improved access to the airway and gastrointestinal tract compared to oxygen delivery masks, and it is less bulky and wieldy than nasal continuous positive airway pressure devices. In addition, HFNO studies report increased patient comfort, less mucosal desiccation, improved clearance of pulmonary and airway secretions, a reduction in work of breathing, and enhanced oxygen delivery[19,28-32]. Further clinical benefits include improved ventilation efficiency and increased end-expiratory lung volume[16,33].
Studies have shown that up to 60% of adverse cardiopulmonary events following upper gastrointestinal endoscopies are related to administration of sedation and anesthesia[34-36]. In addition, prolonged procedures are a known risk factor for hypoxemia[37]. We also investigated procedure and anesthesia-only times during the study period. Within each era studied, the procedure time under DS was significantly shorter compared to procedure times under GA. This result may be explained by the clinician’s choice of DS for anticipated shorter endoscopies. Although overall procedure times between eras decreased slightly during the study period, the differences were not statistically significant.
Anesthesia-only time was significantly shorter within each era for DS compared to GA. This result is not surprising considering the prolonged time it takes for the induction of general anesthesia and intubation, as well as for emergence and extubation when compared to DS that does not include these steps. The time differences may have been exacerbated in our study by the need to transfer patients to a different floor for recovery following GA. Anesthesia-only time for ERCP and EUS combined was significantly shorter in era 2 compared to era 3 by 6.5 min and for EUS by 5.5 min, observations that are difficult to explain and possibly related to sample size or anesthesia team dynamics that are difficult to control for.
Anesthesia services for endoscopic procedures have increased substantially over the last 15 years and are contributing to rising costs[4]. Shorter anesthesia and procedure times may lead to improved efficiency in the endoscopy suite. In appropriately selected cases, DS may be a less expensive alternative to GA that may reduce the economic burden attributed to anesthesia services and improve throughput.
Interestingly in the multivariate regression analysis with age, BMI, procedure time and ASA physical status classification, only the latter 2 were significant predictors of GA use. This may indicate that anesthesia care providers and GI teams choose GA instead of DS in patients with numerous comorbidities and/or acute illness and for procedures that are anticipated to be long.
Limitations of our study include the retrospective design, and the need to apply an intention-to-treat approach to the era 2 cohort where 82.2% of patients received HFNO. Additional clinically important outcomes could not be retraced from the medical record. For example, the incidence of abdominal distention and pain following the procedure could not be retrieved. Similarly, neither unexpected adverse events nor conversions from HFNC to GA were obvious in the chart review in any era. This finding suggests that the incidence of these events is likely to be low, but prospective targeted confirmation is needed.
The retrospective nature of the study also precluded the use of predetermined guidelines for the choice between HFNO and GA. However, HFNO consistently supported good oxygenation in spontaneously breathing patients receiving propofol as the sole sedative agent. This, in turn, led to a familiar and easily managed recovery profile in the endoscopy recovery area.
Despite these limitations, our conservative analysis indicates that HFNO may be potentially useful during sedation for complex endoscopies, and further study for prospective confirmation is warranted.
In conclusion, our study demonstrates a decreased use of GA when HFNO is available in the endoscopy unit for patients undergoing ERCP and EUS. Provision of HFNO and DS was associated with decreased procedure and anesthesia-only times, and oxygenation was improved compared to sedation without HFNO. Further prospective trials are needed to fully elucidate the role of HFNO during sedation in gastrointestinal endoscopy.
ACKNOWLEDGMENTS
The authors thank the Department of Anesthesiology and the Gastroenterology/Hepatology Division of Tufts Medical Center for providing the clinical care and support required to complete this study.
COMMENTS
Background
Complex endoscopies including endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) may be performed under sedation or general anesthesia. Any anesthetic technique will depress ventilator drive and efficiency, and maintaining adequate ventilation under deep sedation in the spontaneously breathing patient can be challenging and may require a more invasive general anesthetic with endotracheal intubation. This study examines the potential usefulness of high-flow nasal oxygen to improve ventilation in the spontaneously breathing patient under deep sedation for ERCP and EUS. Compared to standard nasal cannula, High-flow nasal oxygen provides an CPAP-like effect and reduces the work of breathing, hence making spontaneous breathing more efficient.
Research frontiers
High-flow nasal oxygen has been employed in neonates and adults with chronic respiratory disease for a long time. In the most recent years, it has emerged as a very useful adjunct in acute care settings, including improvement in oxygenation prior to airway management, and following weaning from the ventilator, expanding its utility. It is now being tested in additional acute care settings.
Innovations and breakthroughs
This study provides a novel approach to maintain adequate ventilation in patients undergoing sedation in the endoscopy suite. Use of this technique has not been extensively reported in this setting and may be beneficial. It may be able to reduce the use of general anesthesia for many patients and expand the feasibility of sedation for this patient population.
Applications
This retrospective study detected a signal that should be prospectively evaluated. It appears that high-flow nasal oxygen (HFNO) is potentially of great clinical value in the endoscopy suite and other environments where procedural deep sedation is required. It may save cost and improve patient safety and comfort. However the equipment still requires modification to improve its ability to monitor end-tidal CO2.
Terminology
High-flow nasal oxygen is a term that describes the delivery of a humidified and heated air/oxygen mixture by a nasal cannula designed for this purpose. The flow rate may go up to 60 L/min with an oxygen content of up to 60% when delivered through the airvo 2 device (Fisher-Paykel, Auckland, NZ).
Peer-review
The study demonstrated the effectiveness of HFNO compared with GA during ERCP and EUS. It is unclear what the standard method of anesthesia and sedation for these procedures is worldwide, but it can be assumed that many institutions still frequently use general anesthesia with endotracheal intubation. HFNO may be able to expand the use of deep sedation when it is available compared to standard nasal cannula use. Because the study is retrospective, it is hypothesis generating and the results need to be confirmed in a prospective trial.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Tufts Medical Center and Tufts University Health Sciences Campus Institutional Review Board.
Informed consent statement: Written consent for both the endoscopic procedures (ERCP and/or EUS) and administration of anesthesia (general anesthesia or deep sedation) was obtained from all patients or their designated legal agent(s). This is a retrospective study on data from clinical care. As such this study was deemed to be minimal risk by the Tufts institutional review board and a waiver of informed consent for this study was granted (see also attached IRB approval document).
Conflict-of-interest statement: None of the authors report a conflict of interest.
Data sharing statement: Technical appendix, statistical code, and the datasets are available from the corresponding author at rschumann@tuftmedicalcenter.org. A waiver of informed consent was obtained from the institutional review board, and data are de-identified with a minimal risk of loss of confidentiality. The benefit of results sharing outweigh this potential risk.
Peer-review started: August 19, 2016
First decision: October 20, 2016
Article in press: November 28, 2016
P- Reviewer: Kawano S, Robles-Medranda S S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Martindale SJ. Anaesthetic considerations during endoscopic retrograde cholangiopancreatography. Anaesth Intensive Care. 2006;34:475–480. doi: 10.1177/0310057X0603400401. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, Aisenberg J. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967–974. doi: 10.1111/j.1572-0241.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003-2009. JAMA. 2012;307:1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 5.Inadomi JM, Gunnarsson CL, Rizzo JA, Fang H. Projected increased growth rate of anesthesia professional-delivered sedation for colonoscopy and EGD in the United States: 2009 to 2015. Gastrointest Endosc. 2010;72:580–586. doi: 10.1016/j.gie.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy. 2000;32:233–238. doi: 10.1055/s-2000-96. [DOI] [PubMed] [Google Scholar]
- 7.Vargo JJ, Bramley T, Meyer K, Nightengale B. Practice efficiency and economics: the case for rapid recovery sedation agents for colonoscopy in a screening population. J Clin Gastroenterol. 2007;41:591–598. doi: 10.1097/01.mcg.0000225634.52780.0e. [DOI] [PubMed] [Google Scholar]
- 8.de Villiers WJ. Anesthesiology and gastroenterology. Anesthesiol Clin. 2009;27:57–70. doi: 10.1016/j.anclin.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Thosani N, Banerjee S. Deep sedation or general anesthesia for ERCP? Dig Dis Sci. 2013;58:3061–3063. doi: 10.1007/s10620-013-2849-9. [DOI] [PubMed] [Google Scholar]
- 10.Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar Risk of Cardiopulmonary Adverse Events Between Propofol and Traditional Anesthesia for Gastrointestinal Endoscopy: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.07.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Bell GD, Quine A, Antrobus JH, Morden A, Burridge SM, Coady TJ, Lee J. Upper gastrointestinal endoscopy: a prospective randomized study comparing continuous supplemental oxygen via the nasal or oral route. Gastrointest Endosc. 1992;38:319–325. doi: 10.1016/s0016-5107(92)70424-6. [DOI] [PubMed] [Google Scholar]
- 12.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest. 2015;148:253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 13.Chanques G, Constantin JM, Sauter M, Jung B, Sebbane M, Verzilli D, Lefrant JY, Jaber S. Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med. 2009;35:996–1003. doi: 10.1007/s00134-009-1456-x. [DOI] [PubMed] [Google Scholar]
- 14.Kallstrom TJ. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility--2002 revision & amp; update. Respir Care. 2002;47:717–720. [PubMed] [Google Scholar]
- 15.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 16.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 17.Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, Cosserant B, Flicoteaux G, Imbert A, Pilorge C, et al. High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA. 2015;313:2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 18.Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, Labbé V, Dufour N, Jean-Baptiste S, Bedet A, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43:574–583. doi: 10.1097/CCM.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 19.Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care. 2010;25:463–468. doi: 10.1016/j.jcrc.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Lucangelo U, Vassallo FG, Marras E, Ferluga M, Beziza E, Comuzzi L, Berlot G, Zin WA. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract. 2012;2012:506382. doi: 10.1155/2012/506382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulson DT, Fragneto RY. Anesthesia for gastrointestinal endoscopic procedures. Anesthesiol Clin. 2009;27:71–85. doi: 10.1016/j.anclin.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Squires RH, Morriss F, Schluterman S, Drews B, Galyen L, Brown KO. Efficacy, safety, and cost of intravenous sedation versus general anesthesia in children undergoing endoscopic procedures. Gastrointest Endosc. 1995;41:99–104. doi: 10.1016/s0016-5107(05)80589-9. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58:635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. 2015;115:629–632. doi: 10.1093/bja/aev262. [DOI] [PubMed] [Google Scholar]
- 26.Manley BJ, Dold SK, Davis PG, Roehr CC. High-flow nasal cannulae for respiratory support of preterm infants: a review of the evidence. Neonatology. 2012;102:300–308. doi: 10.1159/000341754. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39:247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 28.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–413. [PubMed] [Google Scholar]
- 29.Esquinas Rodriguez AM, Scala R, Soroksky A, BaHammam A, de Klerk A, Valipour A, Chiumello D, Martin C, Holland AE. Clinical review: humidifiers during non-invasive ventilation--key topics and practical implications. Crit Care. 2012;16:203. doi: 10.1186/cc10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH. The effects of gas humidification with high-flow nasal cannula on cultured human airway epithelial cells. Pulm Med. 2012;2012:380686. doi: 10.1155/2012/380686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Wagstaff TA, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007;62:492–503. doi: 10.1111/j.1365-2044.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- 33.Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, Stump A, Shaffer TH, Dysart K. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46:67–74. doi: 10.1002/ppul.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA. 1976;235:928–930. doi: 10.1001/jama.235.9.928. [DOI] [PubMed] [Google Scholar]
- 35.Quine MA, Bell GD, McCloy RF, Charlton JE, Devlin HB, Hopkins A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995;36:462–467. doi: 10.1136/gut.36.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Griffin SM, Chung SC, Leung JW, Li AK. Effect of intranasal oxygen on hypoxia and tachycardia during endoscopic cholangiopancreatography. BMJ. 1990;300:83–84. doi: 10.1136/bmj.300.6717.83. [DOI] [PMC free article] [PubMed] [Google Scholar]


