Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, is a facultative intracellular pathogen that infects macrophages and other host cells. We show that sonication of M. tuberculosis results in the removal of material from the surface capsule-like layer of the bacteria, resulting in an enhanced propensity of the bacteria to bind to macrophages. This effect is observed with disparate murine and human macrophage populations though, interestingly, not with freshly explanted alveolar macrophages. Enhanced binding to macrophages following sonication is significantly greater within members of the M. tuberculosis family (pathogens) than within the Mycobacterium avium complex (opportunistic pathogens) or for Mycobacterium smegmatis (saprophyte). Sonication does not affect the viability or the surface hydrophobicity of M. tuberculosis but does result in changes in surface charge and in the binding of mannose-specific lectins to the bacterial surface. The increased binding of sonicated M. tuberculosis was not mediated through complement receptor 3. These results provide evidence that the surface capsule on members of the M. tuberculosis family may be an important virulence factor involved in the survival of M. tuberculosis in the mammalian host. They also question the view that M. tuberculosis is readily ingested by any macrophage it encounters and support the contention that M. tuberculosis, like many other microbial pathogens, has an antiphagocytic capsule that limits and controls the interaction of the bacterium with macrophages.
Mycobacterium tuberculosis is the causative agent of tuberculosis and is the single most devastating bacterial pathogen of mankind. Because mycobacteria are known to survive and replicate within macrophages, it is generally assumed that they have evolved strategies that facilitate their association with phagocytes. It is assumed that mycobacteria readily enter any macrophages they encounter and that it is only then that survival mechanisms of bacterial origin come into play. However, recent data have shown that some populations of macrophages are unable to associate efficiently with M. tuberculosis and, surprisingly, this includes resident alveolar macrophages (46, 47).
While much attention has been given to characterizing the macrophage receptors involved in mediating the binding and phagocytosis of M. tuberculosis (19), the mycobacterial ligands that interact with macrophages receptors are less well determined. Isolated mycobacterial cell wall moieties, such as lipoarabinomannan, can bind to macrophages (40), and glucans are able to inhibit the binding of mycobacteria to complement receptor 3 expressed in CHO cells (14). However, very little is known about the cell surface moieties exposed on intact mycobacteria that actually mediate their binding to macrophages.
Intracellular mycobacteria have an outer layer on the cell wall that appears transparent by transmission electron microscopy (53, 16), resulting in its being referred to as the electron transparent zone. Axenic cultures of mycobacteria also possess this electron transparent zone, and by use of ruthenium red staining, it was suggested that this zone consisted of polysaccharides, rich in electronegative groups, that resembled a capsule (36, 20). This layer also contains proteins and lipids but is predominantly composed of glycans, with glucan and arabinomannan being the predominant constituents (15). Monoclonal antibodies directed against capsular glucan and arabinomannan confirm their presence on the surface of M. tuberculosis during growth in vivo (42, 43).
Due to its location, the capsule must be involved in the initial interaction of mycobacteria with host phagocytes such as macrophages and neutrophils. We therefore investigated the role of the capsule in this interaction. We show that removal of capsular material results in greatly increased binding of M. tuberculosis to macrophages that correlates to changes in the surface properties of the bacteria.
MATERIALS AND METHODS
Bacteria.
M. tuberculosis strain Erdman (Trudeau Mycobacterial Collection [TMC] no. 107; American Type Culture Collection [ATCC] no. 35801), M. tuberculosis strain H37Rv (TMC 102, ATCC 27294), M. tuberculosis strain H37Ra (TMC 201, ATCC 25177), M. bovis bacillus Calmette-Guerin strain Pasteur (TMC 1011, ATCC 35734), M. intracellulare strain Dunbar (TMC D673), and M. avium (TMC 724, ATCC 25291) were grown to late log phase in Proskauer and Beck medium supplemented with 0.05% Tween 80. M. smegmatis (ATCC 19420) was grown in Middlebrook 7H9 (Difco). Batch cultures were aliquoted and stored at −70°C. The number of viable mycobacteria in each aliquot was estimated by assessing CFU for two representative tubes following plating serial dilutions on Middlebrook 7H10 agar (Difco, Detroit, Mich.). CFU were quantitated following 2 (M. smegmatis), 10 to 14 (M. intracellulare and M. avium), or 14 to 21 (M. tuberculosis and M. bovis BCG) days of incubation at 37°C. Escherichia coli strain HB101 was grown overnight in L-broth and then passaged into fresh broth and grown to log phase over 4 h.
Isolation of different macrophage populations.
Murine resident peritoneal macrophages, thioglycolate-elicited peritoneal macrophages, freshly explanted alveolar macrophages, and alveolar macrophages maintained in vitro for 4 days were isolated from 6- to 8-week-old BALB/c mice or from CD11b−/− knockout and CD11b+/+ wild-type mice and prepared for use as previously described (9, 46, 47). Human monocyte-derived macrophages and the human acute monocytic leukemia cell line THP-1 were maintained and prepared for use as described (48).
Briefly, peritoneal washes were obtained from untreated mice (resident macrophages) or from mice which had received an intraperitoneal injection of 2 ml of 4% thioglycolate broth in phosphate-buffered saline 3 days prior to collecting the peritoneal washes (elicited macrophages). The exudates were pooled in supplemented RPMI (RPMI 1640 medium plus 10% fetal bovine serum, 10 mM l-glutamine, and 10 mM sodium pyruvate [Gibco, Grand Island, N.Y.]) and allowed to adhere to glass coverslips in 24-well plates (Becton Dickinson, Lincoln Park, N.J.) for 3 h. Nonadherent cells were removed by washing, and adherent macrophages were further incubated overnight before use.
To obtain murine alveolar macrophages, mice were injected with a lethal dose of pentabarbitol, the heart and lung were dissected into cold phosphate-buffered saline, and a 22-gauge catheter (Critikon, Tampa, Fla.) was inserted into the trachea and tied off. The lungs were lavaged, and pooled washes were pelleted and washed with supplemented RPMI. The final pellet was resuspended in supplemented RPMI, and the macrophages were allowed to adhere to glass coverslips for 1 h. They were then used immediately (freshly explanted alveolar macrophages) or maintained in vitro for 4 days before use (4-day alveolar macrophages).
Blood monocytes were obtained by differential centrifugation of heparinized venous blood from healthy adult volunteers. The enriched monocytes were suspended in RPMI plus 15% autologous serum and incubated for 6 days to differentiate. The monocyte-derived macrophages were washed, resuspended in RPMI plus 15% autologous serum, plated on glass coverslips in 24-well tissue culture plates, and allowed to adhere for 1 h before use in binding assays.
THP-1 cells were obtained from the ATCC (Rockville, Md.) and grown in supplemented RPMI. For use in experiments, THP-1 cells were added to glass coverslips in 24-well tissue culture plates containing supplemented RPMI. They were differentiated into adherent, well-spread macrophages by the addition of 100 nM phorbol myristate acetate and maintained for 3 days at 37°C and 5% CO2. At this point the THP-1 cells were used in experiments.
Interaction of mycobacteria with macrophages.
For all cell types, adherent cells were washed twice in binding medium (138 mM NaCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl, 0.6 mM CaCl2, 1 mM MgCl2, and 5.5 mM d-glucose) (45); a 250-μl aliquot of binding medium was then added to each well, the cells were acclimated for 10 min at 37°C and 95% air-5% CO2 and a further 250 μl of binding medium containing the bacteria to be tested was added to the monolayers (46). In some experiments, 10 μg of the monoclonal antibody 5C6 (ATCC; anti-murine CD11b) was added to the macrophages along with the first 250 μl of binding medium. Control experiments demonstrated that 10 μg of 5C6 per ml reduced the macrophage binding of complement-coated erythrocytes prepared as described previously (46) from an average of 12.7 to 2.3 particles per macrophage.
The ratio of bacteria added to macrophages (multiplicity of infection [MOI]) for M. tuberculosis and M. bovis BCG was either 10:1 or 20:1 with peritoneal macrophages (see individual experiments), 20:1 with monocyte-derived macrophages or THP-1, and 500:1 for alveolar macrophages. A higher MOI was used for M. tuberculosis with alveolar macrophages, as they bind and ingest M. tuberculosis significantly less well than do other macrophages (47). Preliminary experiments determined that an MOI of 10:1 was necessary with M. avium and M. intracellulare and an MOI of 2:1 with M. smegmatis in order to obtain comparable levels of binding to peritoneal macrophages as was seen with an MOI of 20:1 with M. tuberculosis and M. bovis BCG. The MOI in individual experiments was confirmed retrospectively by plating out the inocula on 7H10 agar and counting CFU following the appropriate incubation at 37°C (see above).
In one series of experiments, the association of syringed and sonicated M. tuberculosis with peritoneal macrophages was assessed at 4 versus 37°C to differentiate binding from uptake. Macrophage monolayers were prepared as above, and the 10-min preincubation stage was performed on ice at 4 or at 37°C. Bacteria at an MOI of 60:1 were added to the macrophages, which were then incubated at 4 or 37°C. The higher MOI was used to compensate for the reduced association of M. tuberculosis with macrophages at 4°C.
Following the addition of the bacteria, the different macrophage monolayers were incubated for 3 h, gently washed three times with binding medium at 4 or 37°C as appropriate, fixed for 10 min in 10% formaldehyde in ethanol, mounted cell side up on glass slides, stained with Kinyoun's carbol fuschin and malachite green, and enumerated as previously described (46-48).
Phagocytosis refers to the process by which cells bind, ingest, and digest particles. In this study, we were predominantly interested in the binding of mycobacteria to macrophages as a measure of the recognition of mycobacterial ligands by macrophages. As binding must precede ingestion, most of the studies reported here do not differentiate binding from ingestion, and we use the term association to describe the number of bacteria either bound or ingested by macrophages.
Syringing and sonicating mycobacteria to disperse clumps.
Mycobacteria were dispersed by one of two mechanical means. Bacteria were removed from −70°C storage, thawed at room temperature, pelleted by centrifugation at 14,900 × g for 5 min, and gently resuspended in 500 μl of binding medium. The bacteria were then dispersed by either (i) drawing up and expelling the bacterial suspension 10 times through a 25-gauge needle attached to a 1-ml syringe (referred to throughout as syringed) or (ii) three 30-s pulses of bath sonication in cooled water with a VC50T 50-W microcup horn (Sonics & Materials, Danbury, Conn.) tuned to the manufacturer's recommendations (referred to throughout as sonicated). In some experiments, mycobacteria were left suspended in the binding medium and were not dispersed (referred to throughout as untreated).
To assess the effect of these treatments on the viability of M. tuberculosis, bacteria were syringed or sonicated as described above, sonicated in a microcup horn for 5 min continuously in cooled water, or left untreated. The CFU were assessed as described above. To assess the dispersal of clumps following each treatment, an aliquot of the bacterial suspension was also spread on a Reich slide (Belco Glass, Vineland, N.J.), fixed, and stained with auramine O-potassium permanganate as described (11). Preparations were observed with a Zeiss Axioplan 2 fluorescent microscope, and clusters of 1 or 2 bacteria, 3 to 10 bacteria, and more than 10 bacteria were counted for 100 bacterial events on three replicate slides.
Preparation and analysis of capsule protein, carbohydrate, and lipid content.
A 200-ml aliquot of log-phase M. tuberculosis was pelleted by centrifugation at 3,000 × g for 20 min, washed in phosphate-buffered saline with 0.05% Tween 80, and finally resuspended in distilled water at 0.25× the original volume. The bacteria were then dispersed by three 30-s pulses of bath sonication. Bacteria were removed by centrifugation at 3,000 × g for 20 min. The supernatant was filtered through 0.2-μm filters and then lyophilized. The protein content of the capsule was measured with the Bradford assay. Proteins were separated with polyacrylamide gel electrophoresis (PAGE) and either stained with Coomassie blue or blotted to nitrocellulose and probed with a murine polyclonal anti-M. tuberculosis serum and the monoclonal antibodies HYT6 (recognizes 19-kDa glycoprotein) or HYT27 (recognizes antigen 85). Lipids were extracted from the capsule material, dried under nitrogen, weighed, analyzed for polar and apolar lipids by two-dimensional thin-layer chromatography, and stained with molybdophosphoric acid in 70% ethanol followed by charring (total lipid) or naphthol in methanol followed by sulfuric acid (glycolipids), all as described (5). For sugar analysis, lyophilized capsule was dissolved in water, hydrolyzed with 2 M trifluoroacetic acid for 1 h at 125°C, and, following removal of excess acid, glycoses were determined by gas-liquid chromatography-mass spectrometry of the derived alditol acetates (54).
Electron microscopy of syringed and sonicated M. tuberculosis.
Syringed and sonicated M. tuberculosis were processed for transmission electron microscopy essentially as described previously (35) with ruthenium red to stain for anionic polymers (36) in an attempt to visualize the capsule. The bacteria were pelleted at 14,900 × g for 5 min and washed twice in 100 mM HEPES buffer, pH 6.8. The pellet was resuspended in 2.5% (vol/vol) glutaraldehyde in 100 mM HEPES buffer, pH 6.8, containing 500 ppm ruthenium red and left at room temperature for 1 h. Following fixation, the bacteria were washed three times in HEPES buffer containing ruthenium red and then postfixed in 2% (wt/vol) osmium tetroxide for 1 h, enrobed in 2% (wt/vol) Noble agar, cut into blocks (1 by 1 by 4 mm), stained with 2% (wt/vol) uranyl acetate for 1 h, dehydrated through an ethanol series, and embedded in LR White resin. Sections were cut, mounted on 200-mesh carbon- and Formvar-coated copper grids, poststained with uranyl acetate and lead citrate, and viewed in a LEO912AB operating under standard conditions at 100 kV with the anticontaminator in place.
Measurement of isocitrate dehydrogenase.
The presence of isocitrate dehydrogenase (an intracellular enzyme) in bacterial preparations was used as an indication of lysis of bacteria. Approximately 108 M. tuberculosis cells were suspended in 500 μl of binding medium and syringed or sonicated as described above. The bacteria were pelleted by centrifugation, and the supernatants were passed through a 0.22-μm filter and retained for assay. Isocitrate dehydrogenase levels were measured with Sigma kit 153-A (Sigma, St. Louis, Mo.), following the manufacturer's instructions.
Measurement of mycobacterial hydrophobicity.
The adherence of M. tuberculosis to a hydrocarbon in a two-phase aqueous hexadecane system was used as a measure of hydrophobicity (38). Briefly, 40 ml of bacterial culture (M. tuberculosis, M. smegmatis, or E. coli) was spun down and resuspended in PUM buffer (22.2 g of K2HPO4 · 3H2O, 7.26 g of KH2PO4, 1.8 g of urea, 0.2 g of MgSO4 · 7H2O, and distilled water to 1,000 ml; pH 7.1) to an optical density at 600 nm of 0.7. Two M. tuberculosis preparations were set up; one was syringed, and the other was sonicated as described above. Aliquots of 1.5 ml of bacteria were placed in siliconized borosilicate tubes (13 by 100 mm), and 0 to 1,200 μl hexadecane (Aldrich, Milwaukee, Wis.) was added. All tubes were incubated for 8 min at 37°C, vortexed for 8 s to thoroughly mix the aqueous and organic phases, and then left at 22°C for 15 min to facilitate separation of the two phases. The lower aqueous layer was removed with a borosilicate pipette, and its optical density at 400 nm was obtained and expressed as a percentage of that of the bacterial suspension in PUM alone.
Lectin binding to mycobacteria.
A lectin enzyme-linked immunosorbent assay (ELISA) was used to assess the exposure of carbohydrate moieties on the surface of M. tuberculosis. We coated 96-well ELISA plates (Corning, Acton, Mass.) with 107 M. tuberculosis for 2.5 h in 0.25 M carbonate buffer, pH 9.6. Adherent bacteria were washed in 0.05% (vol/vol) Tween 20 in phosphate-buffered saline (wash buffer) and blocked with 0.5% (vol/vol) Tween 20 in phosphate-buffered saline plus 0.1 mg of bovine serum albumin per ml and 77 mM NaN3 (blocking buffer). The blocking buffer was removed, and biotinylated lectins at 40 μg/ml were then added in 50 μl of the appropriate lectin buffer (see below) as recommended by the manufacturer. The wells were washed, and 50 μl of streptavidin-alkaline phosphatase at 50 μg/ml in blocking buffer was added. After washing, color was developed with 1 mg of Sigma 104 substrate (Sigma) per ml in glycine buffer, pH 10.2 (1.5 mg of glycine per ml in 0.2 M NaCl with 2 mM MgCl2 and 2 mM ZnCl2) and read at 405 nm. For each group, controls with no bacteria or with no biotinylated lectin were included to allow for nonspecific binding of the lectin and/or the streptavidin conjugate.
All biotinylated lectins were purchased from EY Laboratories (San Mateo, Calif.) Galanthus nivalis agglutinin (GNA) from Galanthus nivalis (high specificity, recognizes terminal mannose α[1, 3]mannose), Narcissus pseudonarcissus agglutinin (NPA) from Narcissus pseudonarcissus (recognizes terminal and internal mannose residues), peanut agglutinin (PNA) from Arachis hypogea (recognizes β-galactose), wheat germ agglutinin (WGA) from Triticum vulgare (recognizes N-acetylglucosamine and sialic acid), and Ulex europaeus agglutinin (UeA) from Ulex europaeus I (recognizes fucose) were used in 0.01 M phosphate-0.15 M NaCl, pH 7.2 to 7.4. Pisum sativum agglutinin (PSA) from Pisum sativum (low specificity, binding is inhibited by mannose > glucose/N-acetylglucosamine) and Lens culinaris hemagglutinin (LcH) from Lens culinaris (low specificity, binding is inhibited by mannose > glucose > N-acetylglucosamine) were used in 0.05 M Tris-0.15 M NaCl, pH 7.0 to 7.2.
Microelectrophoresis measurements of mycobacteria.
Aliquots of 40 ml of BCG culture were pelleted, resuspended in binding medium, and then syringed, sonicated, or left untreated. The bacteria were pelleted again and resuspended in phosphate-buffered saline at pH 7.2. Larger clumps of bacteria were allowed to settle out, and then samples of bacterial suspension were diluted with 150 mM NaCl (pH 1.98 to 10.88) adjusted with 1 M HCl or 2 M sodium bicarbonate and allowed to equilibrate for 10 min. The electrophoretic mobilities of the bacteria were determined in a Mark I apparatus (Rank, Cambridge, England) with a cylindrical chamber at 25°C (44). Measured velocities were the averages of 10 or more individual readings with reversal of the electrical field. The mobility was calculated from the average velocity, the applied voltage, and the electrical length (44).
Statistical analysis.
Data are expressed as means ± standard error of the mean. When applicable, Student's t test for independent means was used to evaluate the data.
RESULTS
Effect of syringing and sonication on clumping, viability, and capsule appearance of M. tuberculosis.
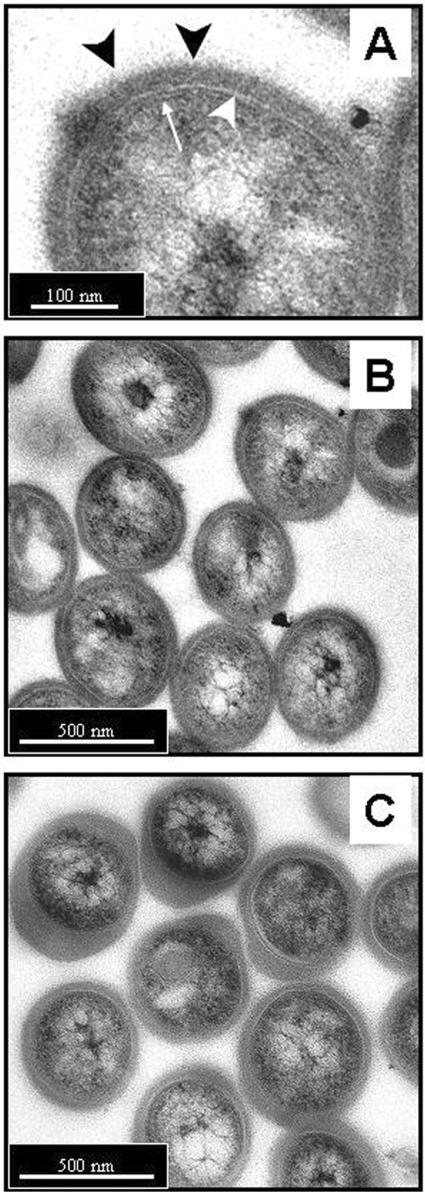
Both syringing and gentle sonication efficiently break up clumps of M. tuberculosis and also result in an apparent increase in viability because clumping results in an underestimation of the true viable count (Table 1). Prolonged sonication (5 min), however, results in a loss of viability (Table 1). Transmission electron microscopy of syringed and sonicated M. tuberculosis stained with ruthenium red demonstrated the presence of a thick capsular outer layer beyond the cell wall (Fig. 1A) and revealed that neither syringing nor sonication noticeably compromised the cellular integrity of the bacterial cells (Fig. 1B and C). However, the appearance of the capsule was dramatically affected by sonication, appearing to cause it to loosen and bulge out around the bacteria, resulting in an increased yet uneven thickness of the material (Fig. 1C). In contrast, the capsule of syringed bacteria remained evenly distributed around the surface of the bacteria, with no evidence of bulging (Fig. 1B).
TABLE 1.
Effect of syringing and sonication on the viable count and clumping of M. tuberculosisa
| Treatment | Batch 1
|
Batch 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| Viable count (CFU/ml) | Clumping (%)
|
Viable count (CFU/ml) | Clumping (%)
|
|||||
| 1 or 2 | 2-10 | >10 | 1 or 2 | 2-10 | >10 | |||
| None | 2.6 × 107 | 30 | 45 | 25 | 4.3 × 107 | 55 | 34 | 11 |
| Syringed | 4.3 × 107 | 73 | 25 | 2 | 8.6 × 107 | 68 | 29 | 3 |
| Sonication | ||||||||
| Three 30-s bursts | 6.0 × 107 | 90 | 9 | 1 | 9.2 × 107 | 85 | 15 | 0 |
| 5 min | 3.9 × 106 | 97 | 2 | 1 | 1.0 × 107 | 79 | 14 | 7 |
Two batches of M. tuberculosis were suspended in binding medium and left untreated, syringed 10 times through a 25 gauge needle, or sonicated. The numbers of viable CFU were assessed for each sample. In addition, smears of each sample were fixed and stained, and clumping was assessed microscopically.
FIG. 1.
Transmission electron microscopy of M. tuberculosis stained with ruthenium red to demonstrate the capsule. The bacteria had been syringed (A and B) or sonicated in three 30-s bursts (C) prior to processing. (A) The cell wall envelope is shown and consists of the plasma membrane and peptidoglycan layers (thin white arrow), an electron-transparent region (large white arrowhead) representing the mycolic acids, glycolipids, and other lipid polymers, and a thick capsule-like outer layer stained strongly by the ruthenium red (blackarrowheads). (B) Syringed bacteria exhibit a capsule of uniform thickness and distribution among the population. (C) Following sonication, the capsule appears to have extended and is unevenly distributed, both around each individual bacteria and between individuals in the population. However, the integrity of the bacterial cells otherwise appears to be comparable to that of the syringed bacteria in B. Bars: 100 nm (A) and 500 nm (B and C).
Effect of syringing and sonication on the association of M. tuberculosis with macrophages in the absence of serum.
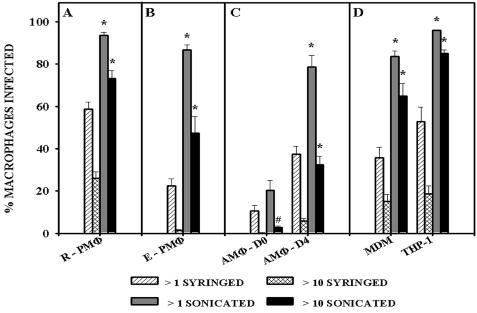
Sonication significantly (P < 0.0001) increased the uptake of M. tuberculosis compared to syringed bacteria, both in the percentage of the macrophages population binding at least one bacterium and, more noticeably, in the percentage of the macrophages population binding more than 10 bacteria (Fig. 2A). We confirmed that elicited murine peritoneal macrophages do not associate with syringed M. tuberculosis as well as do resident peritoneal macrophages (46) (compare Fig. 2A and B); that murine day zero alveolar macrophages do not bind syringed M. tuberculosis efficiently but that following differentiation in vitro for 4 days, they markedly increase their ability to bind these bacteria (47) (Fig. 2C); and that monocyte-derived macrophages and THP-1 bind syringed M. tuberculosis with equal efficiency (48) (Fig. 2D). All the macrophage populations used efficiently bind and ingest control particles such as zymosan or latex beads (46-48).
FIG. 2.
Association of syringed and sonicated (three 30-s bursts) M. tuberculosis, strain Erdman with various macrophage populations was assessed and is expressed as a percentage of the macrophage population that was infected with one or more syringed bacteria (>1 syringed), 10 or more syringed bacteria (>10 syringed), one or more sonicated bacteria (>1 sonicated), and 10 or more sonicated bacteria (>10 sonicated). The mean ± standard error of the mean are shown for (A) murine resident peritoneal macrophages (R-PMΦ), 13 experiments representing a total of 35 replicate coverslips; (B) thioglycolate-elicited peritoneal macrophages (E- PMΦ), four experiments representing a total of eight replicate coverslips; (C) freshly explanted alveolar macrophages at day zero (AMΦ-D0) or in vitro-differentiated alveolar macrophages at day 4 (AMΦ-D4), four experiments representing a total of 12 replicate coverslips; and (D) human monocyte-derived macrophages (MDM) or macrophage-like cells (THP-1), three experiments representing a total of nine replicate coverslips. The MOI was 20 bacteria per macrophage for all macrophage types (A, B, and D) except alveolar macrophages (C), for whichthe MOI was 500:1. *, P < 0.0001, and #, P < 0.05, when comparing sonicated to syringed bacteria.
The percentage of resident peritoneal macrophages, elicited peritoneal macrophages, day 4 alveolar macrophages, monocyte-derived macrophages, and THP-1 binding at least one sonicated bacterium was significantly higher (P < 0.0001) compared to binding of syringed bacteria (Fig. 2). No comparable difference (P > 0.05) was seen with day zero alveolar macrophages (Fig. 2C). There was a dramatic increase in the total number of sonicated bacteria binding to all macrophage populations (as indicated by the percentage of the macrophage population binding more than 10 bacteria), although this was not as significant for day zero alveolar macrophages (P < 0.05) as it was for the other macrophages (P < 0.0001). These results demonstrated that sonication of M. tuberculosis resulted in an increase in the association of the bacteria with macrophages and that this increase was seen for macrophages from different species, from different anatomical sites, and in various states of differentiation.
The possibility that sonication had increased the binding of M. tuberculosis to macrophages without increasing their subsequent ingestion was considered. To isolate binding from ingestion, macrophages were incubated with syringed and sonicated M. tuberculosis at 4 and 37°C (Table 2). At both temperatures, a significantly higher (P < 0.0001) percentage of the macrophage population were able to bind (4°C) or bind and ingest (37°C) sonicated mycobacteria compared to syringed bacteria. This was evident as an ability of macrophages both to bind at least one bacterium and to bind large numbers of bacteria. When measured as the number of bacteria per infected macrophage, binding and ingestion (37°C) were two- to threefold higher than binding alone (4°C) for both syringed and sonicated bacteria. At both 4 and 37°C, the association of sonicated bacteria with macrophages was significantly higher than that of syringed bacteria (P < 0.0001).
TABLE 2.
Effect of syringing and sonicating on binding and ingestion of M. tuberculosis at 4 and 37°Ca
| Treatment | Temp (°C) | Mean % of macrophages ± SEM with:
|
Mean no. of bacteria per infected macrophage ± SEM | |
|---|---|---|---|---|
| ≥1 bacterium | ≥10 bacteria | |||
| Syringed | 4 | 35.0 ± 4.0 | 1.7 ± 0.9 | 4.5 ± 0.2 |
| Sonicated | 4 | 80.7 ± 2.6 | 17.8 ± 1.3 | 12.1 ± 1.0 |
| Syringed | 37 | 79.8 ± 3.2 | 27.3 ± 2.4 | 10.7 ± 1.3 |
| Sonicated | 37 | 98.7 ± 0.3 | 92.2 ± 1.7 | 30.9 ± 2.4 |
Macrophages maintained at 4 or 37°C were incubated with syringed or sonicated M. tuberculosis. The percentage of macrophages binding at least one bacterium or more than 10 bacteria and the number of bacteria associated with each infected macrophage was assessed for two experiments, representing a total of six replicate coverslips.
Because it was not possible to spin and wash the bacteria prior to their addition to macrophages (they would have reaggregated), any material released from the bacteria during syringing or sonication was present in the bacterial suspension. The possibility that the mild sonication used in these studies was releasing material from the bacteria (either from the surface or as a result of rupturing the bacteria) that caused the enhanced binding was therefore considered. The mild sonication used in these studies (three 30-s bursts of bath sonication) did not result in any visible loss of viability or integrity of the bacterial cytoplasm (Fig. 1) and actually resulted in an apparent increase in bacterial viability due to dispersal of clumps (Table 1). However, prolonged sonication (5 min) did decrease bacterial viability (Table 1), indicating that sonication had the potential to damage mycobacteria. We therefore investigated the lysis of the bacteria following sonication by measuring isocitrate dehydrogenase (ICD) release into the supernatant. Compared to water (0 ICD units), untreated bacteria (16 ± 5 ICD units per 108 bacteria), and syringed bacteria (7 ± 7 ICD units per 108 bacteria), mild sonication (three 30-s bursts) did produce a small amount of lysis (79 ± 13 ICD units per 108 bacteria).
In addition to the minimal lysis of M. tuberculosis, we found that sonication releases material from the bacteria into the supernatant, which on analysis was found to consist of 70 to 80% carbohydrate, 10 to 15% protein, and 10 to 15% lipid by weight. Gas-liquid chromatography-mass spectrometry of the derived alditol acetates revealed that the carbohydrate fraction comprised Ara, Man, and Glc in the relative proportions of 1:1.73:8.69, respectively. This probably represents glucan, arabinomannan, and mannan, respectively, as described by Daffé's group (32), though we cannot be certain of this without additional analysis. Polyacrylamide gel electrophoresis and Western blot analysis with polyclonal antiserum and monoclonal antibody recognizing M. tuberculosis revealed numerous proteins in the capsule preparation, including the 19-kDa glycoprotein and antigen 85 (data not shown). Lipid analysis by two-dimensional thin-layer chromatography revealed the presence of numerous polar and apolar lipids, including phosphatidylinositol mannosides, diphosphatidylglycerol, glycolipids, and dimycocerosoates of phthiocerol (data no shown).
When concentrated, soluble, whole-cell lysates or concentrated capsular material prepared from sonicated M. tuberculosis was added to macrophages at concentrations 100 to 1,000-fold higher than was present in the preparations of sonicated bacteria added to macrophages in Fig. 2, both substances actually inhibited the association of M. tuberculosis with macrophages by 60 to 70%. Addition of whole-cell lysate or capsule at concentrations similar to that found in the preparations of sonicated bacteria used in this study had no discernible effect on the ability of macrophages to bind M. tuberculosis. We conclude that sonication of M. tuberculosis did not release a factor that enhanced the binding of the bacteria to macrophages but instead altered the bacterial surface in a way that enhanced its interaction with macrophages.
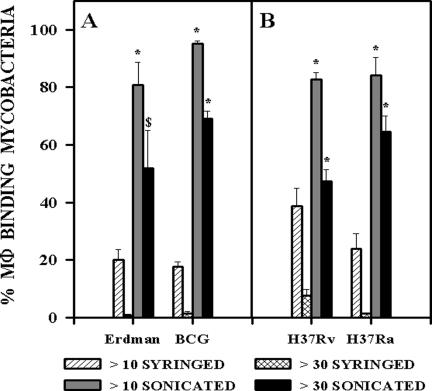
Sonication of members of the M. tuberculosis family but not M. avium complex or M. smegmatis results in greatly increased binding to macrophages in the absence of serum. To investigate whether increased binding of sonicated M. tuberculosis was restricted to virulent strains, we compared the association of syringed and sonicated M. tuberculosis strain Erdman with the vaccine strain M. bovis BCG Pasteur (Fig. 3A) and the mouse-virulent strain M. tuberculosis H37Rv with the mouse-avirulent strain M. tuberculosis H37Ra (Fig. 3B). The binding of all four strains was substantially enhanced by sonication and was significantly higher (P < 0.01 to P < 0.0001) than binding of syringed bacteria of the same strain.
FIG. 3.
Association of syringed and sonicated (three 30-s bursts) members of the M. tuberculosis family with mouse resident peritoneal macrophages was assessed and is expressed as the percentage of macrophages binding more than 10 or more than 30 syringed or sonicated bacteria. Erdman and H37Rv are virulent strains of M. tuberculosis. H37Ra is an avirulent strain of M. tuberculosis. BCG is an avirulent vaccine strain of M. bovis and is closely related to M. tuberculosis. The mean ± standard error of the mean are shown for (A) two experiments representing a total of six replicate coverslips at an MOI of 10:1 and (B) three experiments representing a total of nine replicate coverslips at an MOI of 20:1. *, P < 0.0001, and $, P < 0.01, when comparing sonicated to syringed bacteria in the same group.
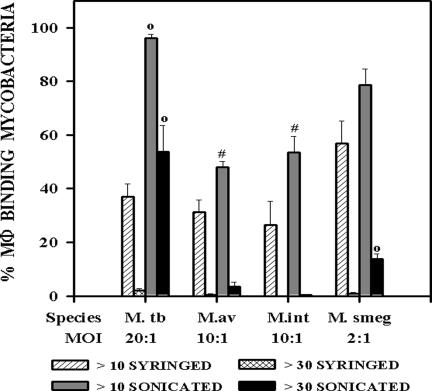
We next investigated whether increased binding of sonicated mycobacteria was restricted to members of the M. tuberculosis family. The association of macrophages with the opportunistic pathogens M. avium and M. intracellulare and the saprophyte M. smegmatis was compared to that of M. tuberculosis following syringing or sonication of the bacteria (Fig. 4). The MOIs of the different strains were adjusted so that about 70% of the macrophage population would bind at least one syringed bacterium. To achieve this for M. tuberculosis required 10 times as many bacteria as it did for M. smegmatis and two times as many as it did for M. avium and M. intracellulare. While a significant increase (P < 0.05) in the binding of sonicated bacteria was seen for every species (Fig. 4), this never reached the levels seen with M. tuberculosis. This was especially noticeable when the percentage of macrophages binding more than 30 bacteria was compared.
FIG. 4.
Association of different species of syringed and sonicated (three 30-s bursts) mycobacteria with mouse resident peritoneal macrophages was assessed and is expressed as the percentage of macrophages binding more than 10 or more than 30 syringed or sonicated bacteria. M. tuberculosis strain Erdman (M. tb), mouse-virulent M. avium (M.av), mouse-virulent M. intracellulare (M. int), and M. smegmatis (M.smeg), a saprophytic mycobacterium, were tested at the MOIs shown. The mean ± standard error of the mean are shown for three experiments representing a total of seven coverslips. ○, P < 0.001, and #, P < 0.05, when comparing sonicated to syringed bacteria in the same group.
Effect of syringing and sonicating mycobacteria on their hydrophobicity.
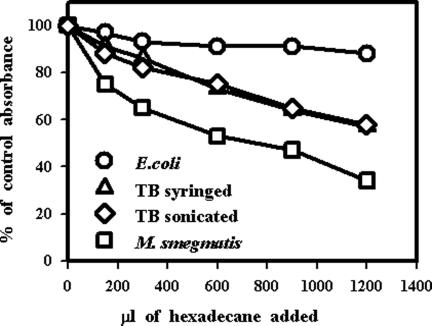
Having demonstrated that sonication of M. tuberculosis resulted in an increased propensity for it to bind to macrophages, we next investigated possible explanations for this phenomenon. We initially considered the possibility that sonication but not syringing resulted in changes to the mycobacterial cell wall hydrophobicity, thereby increasing the interaction with macrophages. Adherence to hexadecane was used as a measure of hydrophobicity (38), which demonstrated that, as expected, E. coli was hydrophilic and mycobacteria were hydrophobic (Fig. 5). M. smegmatis was more hydrophobic than M. tuberculosis, but no difference in hydrophobicity was seen between syringed and sonicated M. tuberculosis.
FIG. 5.
Hydrophobicity of syringed and sonicated (three 30-s bursts) M. tuberculosis strain Erdman compared to that of M. smegmatis and E. coli. Bacteria were suspended in an aqueous buffer and mixed with a range of hexadecane concentrations as indicated. Following separation of the two phases, the optical density at 400 nm (OD400) of the aqueous phase was measured. Results from one experiment are expressed as a percentage of the control absorbance, which was the optical density at 400 nm of bacteria in the aqueous buffer alone.
Effect of syringing and sonicating mycobacteria on their lectin binding properties.
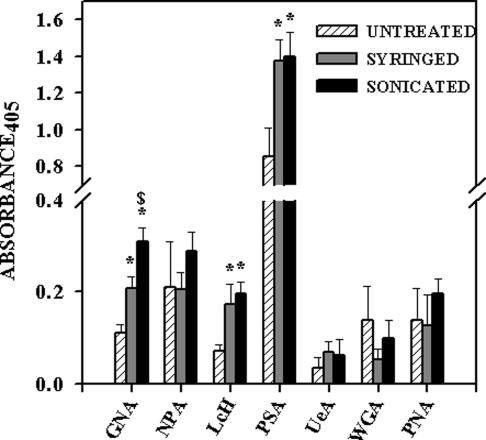
Lectin-like receptors are thought to play a role in the binding of bacteria to mammalian cells through their carbohydrate binding capacity (31). The mycobacterial capsule is predominantly glycan in composition. We therefore considered the possibility that syringing and sonicating M. tuberculosis resulted in variation in the exposure of carbohydrate moieties. A panel of lectins were tested for their binding to untreated, syringed, and sonicated M. tuberculosis (Fig. 6). No significant difference in the binding of NPA, UeA, WGA, or PNA to the three bacterial preparations was seen. The binding of GNA, LcH, and PSA was significantly enhanced following syringing and sonication of the bacteria. In addition, the binding of GNA was significantly higher to sonicated M. tuberculosis than to syringed bacteria.
FIG. 6.
Lectin binding of untreated, syringed, and sonicated (three 30-s bursts) M. tuberculosis strain Erdman was assessed in a lectin enzyme-linked immunosorbent assay. Binding of biotinylated lectins to immobilized M. tuberculosis strain Erdman was quantitated. Each bar is the mean ± standard error of the mean from three separate experiments, each with triplicate readings. Lectin nomenclature and specificity are described in Materials and Methods. *, P < 0.05 when comparing syringed or sonicated bacteria with untreated bacteria. $, P < 0.05 when comparing sonicated with syringed bacteria.
Effect of syringing and sonicating mycobacteria on their surface charge.
Surface charge on a bacterium is a reflection of the moieties exposed on the outer envelope and therefore available for receptor-ligand interactions. An important consequence of the existence of electrical charges on surfaces is that particles will be affected by an applied electric field and will move electrophoretically. The quantity measured in electrophoresis is the electrophoretic mobility, which is directly proportional to the zeta potential, which in turn reflects the overall charge that a particle acquires. The most important factor that affects zeta potential is pH, and thus the electrophoretic mobility of particles in solutions of different pH will provide information about the surface charge on that particle.
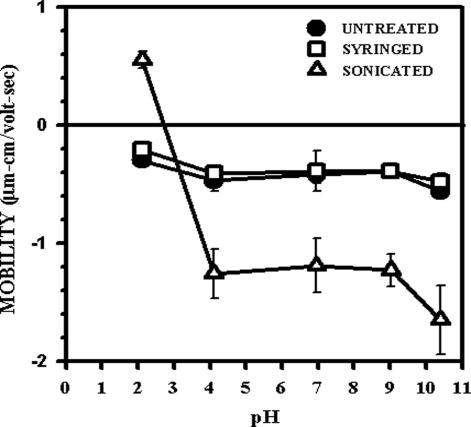
The surface charge on mycobacteria after syringing or sonication was compared with that of untreated cells by measuring the electrophoretic mobility of M. bovis BCG over a pH range of 1.98 to 10.88 (Fig. 7). Due to safety issues, M. bovis BCG not M. tuberculosis was used in these studies. As BCG has been shown to have an antiphagocytic capsule comparable to that of M. tuberculosis (Fig. 3), this was considered an acceptable model organism. Untreated bacteria showed a negative surface charge at all pHs. The surface charge on syringed bacteria paralleled that of untreated bacteria, indicating that no significant changes had resulted from the syringing. Sonicated bacteria, however, exhibited major alterations in their surface charge. They revealed a predominantly negative charge which was significantly more negative than that on untreated and syringed bacteria. On titration, the negative charge was neutralized at around pH 3, below which positive charges became evident. Assuming these were associated with inherent surface structures and not irreversibly adsorbed materials, the results indicate that, compared to the untreated and syringed bacteria, the sonicated bacteria had more negatively charged groups exposed on their surface along with some positive groups not seen on the untreated or syringed bacteria.
FIG. 7.
Microelectrophoresis measurements of the mobility of untreated, syringed, and sonicated (three 30-s bursts) M. bovis, BCG was measured in 150 mM NaCl over a range of pHs. Mobility was calculated from the average velocity, the applied voltage, and the electrical length. Each point is the average of 10 readings ± standard error of the mean.
Involvement of complement receptor 3 (CD11b/CD18) on the binding of syringed and sonicated mycobacteria in the absence of serum.
Complement receptor 3 is a macrophage receptor that mediates approximately 50% of the binding of M. tuberculosis in both the presence and the absence of serum opsonins (29). Besides the epitope for complement components, complement receptor 3 also binds to carbohydrates via a lectin-like epitope (50), including binding to mycobacterial polysaccharides (14). Thus, complement receptor 3 was considered a candidate receptor for mediating the differential binding of syringed and sonicated bacteria.
The binding of syringed or sonicated M. tuberculosis to CD11b+/+ and CD11b−/− peritoneal macrophages in the absence of serum was assessed in five separate experiments. As previously shown (29), CD11b−/− macrophages associated with syringed M. tuberculosis significantly less well than did CD11b+/+ macrophages with 14.6 ± 2.0% and 23.7 ± 3.7% peritoneal macrophages, respectively, binding more than 10 bacteria (P < 0.05). Sonication of the bacteria resulted in a highly significant (P < 0.0001) increase in peritoneal macrophages binding more than 10 bacteria for both CD11b−/− macrophages (to 95.5 ± 1.4%) and CD11b+/+ macrophages (to 96.7 ± 1.2%). However, no difference (P > 0.05) was observed between CD11b+/+ and CD11b−/− peritoneal macrophages in their ability to bind sonicated M. tuberculosis even when comparing the ability to bind more than 30 sonicated bacteria (CD11b−/−, 75.1 ± 7.0%; CD11b+/+, 73.3 ± 6.4%).
In an alternative approach, BALB/c mouse resident peritoneal macrophages were incubated with syringed or sonicated M. tuberculosis in the presence or absence of the CD11b-specific monoclonal antibody 5C6. As expected (46), 5C6 inhibited the binding of syringed M. tuberculosis to peritoneal macrophages (93.5% reduction in peritoneal macrophages binding more than 10 bacteria; P < 0.01) but had no effect on the increased binding of sonicated M. tuberculosis to the same macrophage population (no difference in peritoneal macrophages binding more than 10 bacteria; P > 0.05). These results indicate that the increased binding of sonicated bacteria by macrophages was not mediated by complement receptor 3.
DISCUSSION
Within the mammalian host, M. tuberculosis is commonly found within macrophages. It is therefore generally accepted that any interaction of M. tuberculosis with macrophages will result in the rapid internalization of bacteria via one or more macrophage receptors binding to ligands present on the outer bacterial surface. The association of M. tuberculosis with macrophages can occur in the presence or absence of serum opsonins (41, 46, 49). The serum-independent interaction of M. tuberculosis with macrophages is important in the early stages of pulmonary infection because, under normal physiological conditions, the lung is an environment in which serum opsonins are relatively scarce or absent (30) and resident alveolar macrophages express low levels of receptors for serum opsonins (1, 47). Serum-mediated uptake of M. tuberculosis may be more important at later stages of the infection, when the bacteria will encounter elicited macrophages (46, 47).
Because M. tuberculosis is generally found within macrophages when it is located in the mammalian host, it has long been supposed that M. tuberculosis readily enters any macrophage. Here, we present evidence that members of the M. tuberculosis family have a capsule that, in the absence of serum opsonins, can limit the interaction of the bacteria with macrophages, thereby reducing and/or regulating the uptake of the bacteria by the phagocytes. This observation, in conjunction with the chemical analysis of the capsule (27, 32, 33), supports the idea that the capsule of M. tuberculosis is comparable to the polysaccharide-rich capsules of other pathogens such as Cryptococcus neoformans, Histoplasma capsulatum, and Streptococcus pneumoniae (6, 18, 28).
A recent description of an M. tuberculosis strain mutated in the polyketide synthase gene msl3, in which the lipoglycans diacyltrehalose and polyacyltrehalose were deficient, indicated that the attachment of the capsule of the mutant was compromised. This was postulated to be because the diacyltrehalose and polyacyltrehalose (and possibly other cell envelope lipoglycans with trehalose residues) were involved in attaching the hydrophilic capsule to the hydrophobic lipid cell wall (39). An interesting observation with this mutant was that it bound more efficiently to macrophages, though not as efficiently as did the sonicated M. tuberculosis described in this study, supporting the contention that M. tuberculosis has a capsule that limits the association of the bacteria with macrophages.
Many lines of evidence indicate that pathogenic mycobacteria have mechanisms to limit their association with macrophages: (i) uptake of M. tuberculosis by macrophages is inefficient compared to that of such particles as latex beads or zymosan (46, 47), (ii) saprophytic and opportunistic pathogenic mycobacteria are more readily ingested than are members of the M. tuberculosis family (49) (Fig. 4), (iii) some macrophage phenotypes, most notably alveolar macrophages, do not associate with mycobacteria efficiently yet readily ingest other particles (47) (Fig. 2), and (iv) members of the M. tuberculosis family have an antiphagocytic capsule (Fig. 3).
It is improbable that the increased binding of sonicated bacteria is merely a nonspecific increase in the adhesiveness of the bacteria for several reasons: (i) day zero alveolar macrophages did not associate with the sonicated M. tuberculosis very efficiently (Fig. 2C) even though these macrophages are capable of avidly ingesting other particles such as zymosan and latex (47), (ii) alveolar macrophages can develop the ability to bind sonicated bacteria more efficiently (Fig. 2C), (iii) sonication of M. tuberculosis, M. avium, M. intracellulare, and M. smegmatis did not result in comparable increases in binding (Fig. 4), and (iv) we did not observe nonspecific binding of sonicated M. tuberculosis to glass coverslips. Increased binding of sonicated bacteria was dependent on both the mycobacterial species and the macrophage phenotype, indicating a specific receptor-ligand interaction.
Binding of syringed and sonicated M. tuberculosis was dissociated from binding and ingestion (referred to as association in this study) by incubation of the macrophages at 4 or 37°C, respectively (24). Removal of surface material from M. tuberculosis by sonication significantly increased both binding and association of the bacteria to the same degree, demonstrating that the presence of an intact capsule restricted binding but that, once bound, bacteria are ingested whether the capsule is present or not. This indicates that certain receptor-ligand interactions are inhibited by the presence of the capsule.
If it is not due to a nonspecific increase in binding, what has happened to the capsule of M. tuberculosis following sonication to explain the increased binding to macrophages? Sonication resulted in removal of substantial amounts of material with a composition comparable to that of the capsule described by Daffé's group (27, 32), although we noted that the surface material that was removed by sonication contained more lipid (by percent) than did that of Daffé's group (32, 33). This may be explained by the difference in growth conditions of the bacteria (suspension culture versus pellicle) or the methods used to remove the capsular material (sonication versus agitation with glass beads). Like Daffé's group, we found that the capsule material was predominantly composed of carbohydrate, with glucose being the most prevalent sugar, along with significant amounts of both mannose and arabinose (32, 34). Proteins were also a major constituent, with the 19-kDa lipidated glycoprotein and the antigen 85 complex present in both our study and that of Daffé's group (32).
However, despite the removal of substantial amounts of capsular material from the bacteria, electron microscopy indicated that, following sonication, the capsule was still present. Nevertheless, there were alterations in the appearance and physical properties of the sonicated capsule, with bulging and an overall extension of the capsule (Fig. 1), indicating a loss of integrity. The increase in mannose ligands exposed and the dramatic change to the surface charge following sonication suggest that material masking charged and/or mannosylated moieties has been removed, perhaps neutral glycans such as the α-glucan that makes up a large part of the capsule (32).
Lectin enzyme-linked immunosorbent assay studies showed that M. tuberculosis express little if any fucose, β-galactose, or N-acetylglucosamine. Four different mannose- or glucose-specific lectins bound well to M. tuberculosis, in support of the contention that its surface has numerous mannose residues (32, 34). The increased binding of sonicated bacteria to macrophages did not appear to be mediated by a general increase in the availability of mannose on the bacterial surface, as both syringed and sonicated bacteria showed increased binding of LcH, PSA, and GNA, yet sonicated bacteria bound to macrophages more readily that did syringed bacteria. However, the binding of GNA to sonicated bacteria was significantly higher than to syringed bacteria, perhaps indicating that the epitope for this lectin is involved in the enhanced binding. LcH and PSA are both relatively nonspecific in their binding profiles, interacting with both mannose and glucose groups, whereas GNA specifically binds mannose α-(1-3)mannose groups (51). Interestingly, this does not correspond to the 1-6 and 1-2 linkages of mannose in M. tuberculosis arabinomannan (26), the 1-6 linkages in phosphatidylinositol mannoside (9), or the 1-2 linkages in the mannose caps of lipoarabinomannan (8). If increased binding of sonicated M. tuberculosis is due to increased availability of a GNA binding site, this does not appear to be mediated by three of the dominant mannosylated moieties in the mycobacterial outer envelope.
Studies with Cryptococcus neoformans found that there was a correlation between phagocytosis and a strong negative surface charge on the yeast cell surface (23). We also demonstrated a concomitant association of surface charge and binding of BCG to macrophages; the surface of sonicated bacteria had increased levels of negative charges along with previously unexposed positive charges. As mammalian cell surfaces have a predominantly negative charge, it seems unlikely that the negative charges on the sonicated M. tuberculosis surface would be mediating binding, though it is possible that the positive charges do. The origin of the negative charge in BCG has been attributed to carboxylic acids, phosphoesters, and possibly sulfates but not polysaccharides (25). We have no information on the source of the positively charged groups, but they may be due to amine groups. Considering the high glycan content of the mycobacterial capsule (27, 32), it seems likely that neutral sugars could be masking numerous charged groups which become exposed following sonication. However, it should be remembered that the capsule of M. tuberculosis is not just neutral sugars; surface exposure of proteins, phospholipids, lipoglycans, and peptidoglycolipids may all contribute to the surface charge and represent ligands that can bind to macrophages.
The capsule limits the uptake of M. tuberculosis by macrophages but does not totally prevent it. Of the binding that does occur, approximately 50% is mediated by complement receptor 3, in both the presence and the absence of serum opsonins (29). Mycobacterial capsular polysaccharides, have been reported to mediate binding to complement receptor 3-transfected CHO cells (14), and Cryptococcus neoformans interacts with macrophages via glucan binding to complement receptor 3 as well (12, 13). However, following sonication, the increased binding of M. tuberculosis could not be attributed to binding to complement receptor 3 and must be mediated by some as yet unidentified receptor.
The entry of microbial pathogens via complement receptor 3 is believed to allow them to avoid macrophage killing mechanisms such as reactive oxygen species (4, 17, 52) or other postingestion consequences mediated by the distinct mechanisms of phagocytosis, resulting from binding to different receptors (7, 10). Our data thus present the possibility that the role of the capsule is to ensure that a large percentage of encapsulated M. tuberculosis uptake by macrophages is directed toward complement receptor 3. It will be interesting to determine whether removal of the capsule results in a reduced ability of M. tuberculosis to survive in macrophages and triggers macrophage killing mechanisms. This study will be best achieved with stable acapsular mutants, as we do not yet know the rate of resynthesis of capsule following mechanical removal and so could not be sure of the status of any intracellular bacteria that had been sonicated.
We have shown that members of the M. tuberculosis family but not opportunistic pathogens or saprophytes have antiphagocytic capsules. All species of mycobacteria studied (including M. avium and M. smegmatis) appear to have capsules, though the thickness and composition of them vary (21, 27, 36). Saprophytes like M. smegmatis bind to macrophages more readily than does M. tuberculosis (Fig. 4) and appear to have thin capsules (21) that have high protein and low glycan contents (27), suggesting that thick glycan capsules are a virulence factor aiding the survival of pathogenic mycobacteria within mammalian hosts by, at least in part, limiting macrophage-bacterium interactions. However, we found that avirulent members of the M. tuberculosis family (H37Ra and BCG) also have an antiphagocytic capsule. Strains BCG and H37Ra are laboratory-produced avirulent strains that will still infect and grow within mammalian hosts, though they do not lead to the chronic disease that their virulent counterparts do. Perhaps the capsule is important in the early stages of infection, during initial interactions with macrophages, and may not be so important during later stages of chronic infection. A definitive demonstration of whether an antiphagocytic capsule is a virulence factor of pathogenic mycobacteria awaits the availability of capsular mutants.
The tendency of mycobacteria to clump together interferes with studies investigating their interaction with macrophages. To circumvent this problem, a number of mechanical means for breaking up clumps of mycobacteria have been utilized, including vortexing with glass beads (33, 41), sonication (22, 37), and passage through a syringe needle (46, 49). A practical consideration resulting from the current study is that the practice of dispersing clumps of M. tuberculosis by sonication or agitation with glass beads in order to prepare them for studies with macrophages should be abandoned as it will lead to alterations in the mycobacterial surface that will alter the interaction with macrophages. It is better to disperse the bacteria with repeated syringing, which results in a surface more like that of an untreated bacterium.
The demonstration of an antiphagocytic capsule on the M. tuberculosis family, along with our earlier observations showing that mycobacteria interact with certain macrophage phenotypes inefficiently (46, 47), supports our contention that mycobacteria have evolved mechanisms for controlling or even evading ingestion by certain macrophage phenotypes. Possible explanations for why M. tuberculosis has mechanisms that limit its nonopsonic interaction with macrophages are that (i) this is a strategy to avoid ingestion by macrophages in anatomic sites where serum opsonins are at very low levels, such as the alveolar space and within the granuloma, (ii) the capsule may allow M. tuberculosis to remain outside of a macrophage and enable it to enter lung epithelial or endothelial cells (2, 3), (iii) it is a strategy to control the type of macrophages that are infected and the mechanism by which the bacteria are ingested. As we know that M. tuberculosis can and does enter some macrophages and can survive and multiply therein, it seems more likely that the antiphagocytic capsule is directing the bacteria to specific receptor-ligand interactions that benefit the subsequent survival of intracellular M. tuberculosis. Confirmation of this awaits the availability of capsular mutants, which we are currently isolating.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research grant (MOP- 43924) to R.W.S., a CIHR/CBS Partnership Fund award to D.E.B., and the Network Centres of Excellence, Canadian Bacterial Diseases Network (R.W.S. and T.J.B.). R.W.S. is the recipient of a BC Research Institute for Children's & Women's Health Investigatorship Award. The electron microscopy was done in the NSERC Guelph Regional STEM Facility, which is partially funded by an NSERC-Major Facility Access Grant to T.J.B.
We thank D. Moyles and R. Harris for invaluable assistance with the EM studies, G. Besra, University of Bimingham, United Kingdom, for advice on lipid analysis, and J. Richards of the Institute for Biological Sciences, National Research Council of Canada, for the sugar analysis. Monoclonal antibodies HYT6 and HYT27 were a generous gift from P. Andersen of the Statens Seruminstitut, Copenhagen, Denmark.
Editor: J. D. Clements
REFERENCES
- 1.Berger, M., T. M. Norvell, M. F. Tosi, S. E. Emancipator, M. W. Konstan, and J. R. Schreiber. 1993. Tissue-specific Fcg and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Paediatr. Res. 35:68-77. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berton, G., C. Laudanna, C. Sorio, and F. Rossi. 1992. Generation of signals activating neutrophil functions by leukocyte integrins: LFA-1 and gp150, 95 but not CR3, are able to stimulate the respiratory burst of human neutrophils. J. Cell Biol. 116:1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besra, G. S. 1998. Preparation of cell wall fractions from mycobacteria, p. 91-107. In T. Parish and N. G. Stoker (ed.) Methods in molecular biology: mycobacteria protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 6.Bose, I., A. J. Reese, J. J. Ory, G. Janbon, and T. L. Doering. 2003. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryotic Cell 2:655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee, D., S. W. Hunter, M. Mcneil, and P. J. Brennan. 1992. Lipoarabinomannan - multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 267:6228-6233. [PubMed] [Google Scholar]
- 9.Chatterjee, D., and K. H. Khoo. 1998. Mycobacterial lipoarabinomannan—an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8:113-120. [DOI] [PubMed] [Google Scholar]
- 10.Chimini, G., and P. Chavrier. 2000. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2:E191-E196. [DOI] [PubMed] [Google Scholar]
- 11.Collins, F. M., N. E. Morrison, A. M. Dhople, and S. R. Watson. 1980. Microscopic counts carried out on Mycobacterium leprae and M. tuberculosis suspensions. A comparison of three staining procedures. Int. J. Lepr. 48:402-407. [PubMed] [Google Scholar]
- 12.Cross, C. E., and G. J. Bancroft. 1995. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and β-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, C. E., H. L. Collins, and G. J. Bancroft. 1997. CR3-dependent phagocytosis by murine macrophages—different cytokines regulate ingestion of a defined CR3 ligand and complement-opsonized Cryptococcus neoformans. Immunology 91:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cywes, C., H. C. Hoppe, M. Daffe, and M. R. W. Ehlers. 1997. Nonopsonic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect. Immun. 65:4258-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daffé, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 16.Draper, P., and R. J. W. Rees. 1973. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J. Gen. Microbiol. 77:79-87. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers, M. R. W., and M. Daffé. 1998. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 6:328-335. [DOI] [PubMed] [Google Scholar]
- 18.Eissenberg, L. G., S. Poirier, and W. E. Goldman. 1996. Phenotypic variation and persistence of Histoplasma capsulatum yeasts in host cells. Infect. Immun. 64:5310-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Etr, S. H., and J. D. Cirillo. 2001. Entry mechanisms of mycobacteria. Front. Biosci. 6:D737-D747. [DOI] [PubMed] [Google Scholar]
- 20.Frehel, C., N. Rastogi, J.-C. Benichou, and A. Ryter. 1988. Do test tube grown pathogenic mycobacteria possess a protective capsule? FEMS Microbiol. Lett. 56:225-230. [Google Scholar]
- 21.Frehel, C., A. Ryter, N. Rastogi, and H. David. 1986. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann. Inst. Pasteur Microbiol. 137B:239-257. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, C. S., J. J. Ellner, D. G. Russell, and E. A. Rich. 1994. Complement receptor-mediated uptake and tumor necrosis factor-a-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J. Immunol. 152:743-753. [PubMed] [Google Scholar]
- 23.Kozel, T. R., E. Reiss, R. Cherniak. 1980. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect. Immun. 29:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel, T. R., and R. P. Mastroianni. 1976. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect. Immun. 14:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensen, S., Y. Q. Tian, M. E. Klegerman, and M. J. Groves. 1992. Origins of BCG surface charge - effect of ionic strength and chemical modifications on zeta-potential of Mycobacterium bovis BCG, tice substrain, cells. Microbios 70:185-198. [PubMed] [Google Scholar]
- 26.Lemassu, A., and M. Daffé. 1994. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem. J. 297:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemassu, A., A. Ortalo-Magne, F. Bardou, G. Silve, M. A. Laneelle, and M. Daffé. 1996. Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiology 142:1513-1520. [DOI] [PubMed] [Google Scholar]
- 28.Llull, D., R. Lopez, and E. Garcia. 2001. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr. Mol. Med. 1:475-491. [DOI] [PubMed] [Google Scholar]
- 29.Melo, M. D., I. R. Catchpole, G. Haggar, and R. W. Stokes. 2000. Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell. Immunol. 205:13-23. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell, T. G., and J. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofek, I., J. Goldhar, Y. Keisari, and N. Sharon. 1995. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 49:239-276. [DOI] [PubMed] [Google Scholar]
- 32.Ortalo-Magne, A., M. A. Dupont, A. Lemassu, A. B. Andersen, P. Gounon, and M. Daffé. 1995. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology 141:1609-1620. [DOI] [PubMed] [Google Scholar]
- 33.Ortalo-Magne, A., A. Lemassu, M. A. Laneelle, F. Bardou, G. Silve, P. Gounon, G. Marchal, and M. Daffé. 1996. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J. Bacteriol. 178:456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortalo-Magne, A., A. B. Andersen, and M. Daffé. 1996. The outermost capsular arabinomannans and other mannoconjugates of virulent and avirulent tubercle bacilli. Microbiology 142:927-935. [DOI] [PubMed] [Google Scholar]
- 35.Paul, T. R., and T. J. Beveridge. 1992. Reevaluation of envelope profiles and cytoplasmic ultrastructure of mycobacteria processed by conventional embedding and freeze-substitution protocols. J. Bacteriol. 174:6508-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi, N., C. Fréhel, and H. L. David. 1986. Triple-layered structure of mycobacterial cell wall: evidence for the existence of a polysaccharide rich outer layer in 18 mycobacterial species. Curr. Microbiol. 13:237-242. [Google Scholar]
- 37.Roecklein, J. A., R. P. Swartz, and H. Yeager. 1992. Nonopsonic uptake of Mycobacterium avium complex by human monocytes and alveolar macrophages. J. Lab. Clin. Med. 119:772-781. [PubMed] [Google Scholar]
- 38.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 39.Rousseau, C., O. Neyrolles, Y. Bordat, S. Giroux, T. D. Sirakova, M.-C. Prevost, P. E. Kolattukudy, B. Gicquel, and M. Jackson. 2003. Deficiency in mycolipenate- and mycosanoate-derived acyltrehaloses enhances early interactions of Mycobacterium tuberculosis with host cells. Cell. Microbiol. 5:405-415. [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger, L. S., T. M. Kaufman, S. Iyer, S. R. Hull, and L. K. Marchiando. 1996. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 157:4568-4575. [PubMed] [Google Scholar]
- 41.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 42.Schwebach, J. R., A. Glatman-Freedman, L. Gunther-Cummins, Z. Dai, J. B. Robbins, R. Schneerson, and A. Casadevall. 2002. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect. Immun. 70:2566-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwebach, J. R., A. Casadevall, R. Schneerson, Z. Dai, X. Wang, J. B. Robbins, and A. Glatman-Freedman. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 69:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seaman, G. V. F. 1975. Electrokinetic behaviour of red blood cells, p. 1135-1229. In The red blood cell, 2nd ed., vol. 2. Academic Press; New York, N.Y. [Google Scholar]
- 45.Smith, R. J., and S. S. Iden. 1981. Properties of calcium ionophore-induced generation of superoxide anion by human neutrophils. Inflammation 5:177-192. [DOI] [PubMed] [Google Scholar]
- 46.Stokes, R. W., I. D. Haidl, W. A. Jefferies, and D. P. Speert. 1993. Mycobacteria-macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J. Immunol. 151:7067-7076. [PubMed] [Google Scholar]
- 47.Stokes, R. W., L. M. Thorson, and D. P. Speert. 1998. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J. Immunol. 160:5514-5521. [PubMed] [Google Scholar]
- 48.Stokes, R. W., and D. Doxsee. 1999. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell. Immunol. 197:1-9. [DOI] [PubMed] [Google Scholar]
- 49.Swartz, R. P., D. Naai, C.-W. Vogel, and H. Yeager. 1988. Differences in uptake of mycobacteria by human monocytes: a role for complement. Infect. Immun. 56:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thornton, B. P., V. Vetvicka, M. Pitman, R. C. Goldman, and G. D. Ross. 1996. Analysis of the sugar specificity and molecular location of the b-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156:1235-1246. [PubMed] [Google Scholar]
- 51.Van Damme, E. J. M., W. J. Peumans, A. Pusztai, and S. Bardocz. 1998. Handbook of plant lectins: properties and biomedical applications. John Wiley and Sons, London, England.
- 52.Wright, S. D., and S. C. Silverstein. 1983. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto, T., M. Nishiura, N. Harada, and T. Imaeda. 1958. Electron microscopy of ultra-thin sections of lepra cells and Mycobacterium leprae. Int J. Lepr. 26:1-8. [PubMed] [Google Scholar]
- 54.York, W. S., A. G. Darvill, M. McNeil, and P. Albersheim. 1985. 3-deoxy-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 138:109-126. [Google Scholar]