Charcot–Marie–Tooth (CMT) disease is a genetically and clinically heterogeneous group of inherited peripheral neuropathies, which affect both motor and sensory nerves. CMT affects 1 in 2500 individuals, making it the most commonly inherited neuromuscular disorder.1 X-linked CMT (CMTX) accounts for 15–20% of cases.2–4 At present, 5 loci have been mapped [CMTX1 (OMIM #302800), CMTX2 (OMIM #302801), CMTX3 (OMIM #302802), CMTX4 Cowchock, OMIM #310490), and CMTX5 (OMIM #311070)].2,5–10 Two genes, gap junction beta 1 GJB1) and phosphoribosyl pyrophosphate synthetase 1 (PRPS1), have been identified for CMTX1 and CMTX5, respectively.7,11 The CMTX3 locus was first described in 1991 in 2 American families. Combined LOD scores of 2.29 (DXS86; h 5 0.0) and 2.32 (DXS105; h 5 0.0) stablished linkage to a 31.2-Mb region on Xq26–28.6 Over a decade later, significant linkage in a large, multigenerational UK and New Zealand family (CMT623) confirmed and refined the CMTX3 locus to a 5.7-Mb interval (Xq26.3–27.1).12 Re-analysis of 1 of the original American CMTX3 families (US-PED2) and a second large Australian family (CMT193-ext) confirmed segregation of a CMTX3 haplotype and prioritized the gene search to the distal region of the 5.7-Mb interval based on a founder haplotype in US-PED2.13 Initial reporting of the CMTX3 locus described USPED2 as an X-linked family, with no male-to-male transmission and asymptomatic carrier females. Both USPED2 and CMT623 show axonal involvement with typical signs of CMT, including distal atrophy, high-arched feet (pes cavus), and loss of deep tendon reflexes. Both families show late onset of the disease, and US-PED2 displayed additional signs of spasticity.6,12 In this study we performed exome sequencing analysis on family members from US-PED2. Several candidate variants were identified; however, a previously reported autosomal mutation for distal hereditary motor neuropathy (dHMN) was discovered in this family.
METHODS
Exome sequencing and variant calling was performed by Axeq Technologies (South Korea). DNA (5 lg) from an affected man, an obligate carrier woman, and an unaffected man. They underwent exon enrichment using the illumina TrueSeq technology, followed by 100-bp paired-end sequencing on HiSeq 2000. Sequence alignment and variant calling was performed against the reference human genome (hg19) using company customized analysis pipelines. Informed consent from family members was obtained according to protocols approved by the Sydney Local Health District Ethics Committee, Concord Hospital (Sydney, Australia). The variant reports were filtered for known single-nucleotide variations, insertions, and deletions using the dbSNP 136 and 1000 genome databases. Tools provided by the Galaxy browser (http://main.-g2.bx.psu.edu/) were used to identify shared variants in the affected man and carrier mother. Further filtering was achieved by eliminating variants present in the unaffected brother and 20 in-house normal exomes when compared with the affected man and obligate carrier mother. Candidate variants were confirmed by Sanger sequencing analysis. All experimentation was in compliance with Australian laws.
RESULTS
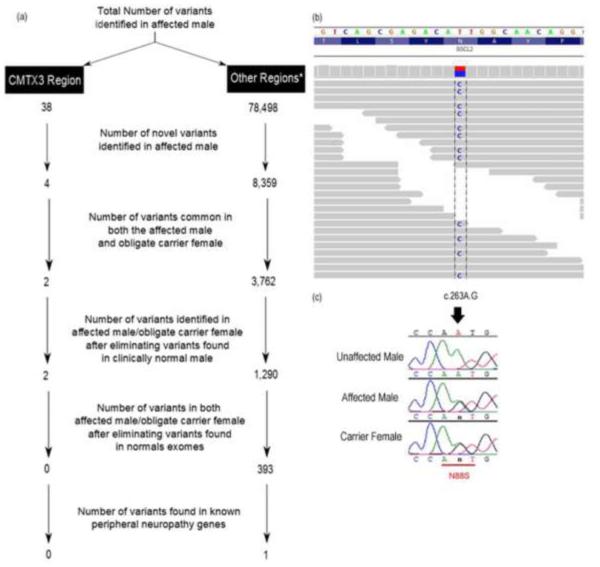
Clinical examination of the proband (III-6)6 indicated onset of weakness of the hands and feet at around 12 years of age. Initial examination at age 14 showed distal weakness and wasting with clawed hands and flat feet. There was a stocking-glove sensory loss to touch and proprioception. Deep tendon reflexes (DTRs) were 21 in arms and 31 in legs with upgoing toes. There was a mild postural tremor. Nerve conduction velocities were normal in sensory nerves but slowed in median and fibular motor nerves (12 and 31 m/s). His course is one of slowly progressive distal weakness and sensory loss. At 36 years of age, he remains ambulatory with the assistance of ankle–foot orthotics (AFO). The carrier mother (II-4)6 denies weakness at age 71 years and is ambulatory without bracing. Osteoarthritic pain in hips and knees limits her activity. A maternal uncle (II-2)6 had onset of weakness around age 20 years. He has not been examined but is reported to have weakness of hands and ankles. Exome sequencing identified a total of 78,536 variants in the affected man. The filtering process used to identify candidate novel variants shared in the affected man and the obligate carrier mother is shown in Figure 1a. A total of 393 shared novel variants were identified. Initially, the CMTX3 region (Xq26.3–27.1) was analyzed for the presence of candidate variants, but none were identified. Having eliminated the CMTX3 locus, a total of 393 novel variants still required validation. To further filter the variants, we analyzed the patient’s exome data for variants in known peripheral neuropathy genes. This filtering strategy identified 1 reported variant on chromosome 11 in the gene Berardinelli–Seip congenital lipodystrophy 2 (BSCL2/seipin). The base change corresponded to a previously identified disease-associated missense mutation, c.263A> G (N88S).14 Coverage analysis of this variant in the BSCL2 gene showed a total of 78 reads, with 48 reads, being the alternate base. Sanger sequencing confirmed the base change, and the variant segregated with the affected man/carrier woman and was absent in the unaffected sibling (Fig. 1b and c).
FIGURE 1.
Identification and validation of a known BSCL2 gene mutation in US-PED2. (a) Filtering strategy to identify shared variants in the affected man and obligate carrier woman. This method enabled the identification of potential pathogenic mutations in the CMTX3 region as well as in known peripheral neuropathy genes. (b) Mapped exome sequence reads in the Integrative Genomics Viewer (IGV)23 for the affected man from US-PED2. The gray bar represents the reference allele (T), and the colored base indicates the alternative allele (C). (c) Sequence traces showing the validation of the change identified by Sanger sequencing. Both the affected and obligate carrier individuals were heterozygous for the change c.263A>G (NM_032667). *Other regions represent the X chromosome, apart from the CMTX3 region, and the autosomes.
DISCUSSION
In this study we performed exome analysis on 1 of the original American families (US-PED2) that contributed to establishing linkage to the CMTX3 locus. In the original study this family was not large enough to demonstrate significant linkage independently.6 We therefore undertook exome analysis for US-PED2 to screen for variants in genes located on both the autosomes and sex chromosomes. We obtained DNA samples from 3 of the 12 family members of the original pedigree,6 and confirmed that coding variants were not present in the CMTX3 region. The family was subsequently assessed for variants in known peripheral neuropathy genes located on the autosomes, and 1 known pathogenic mutation, N88S, in the BSCL2 gene, was identified. This mutation has been reported previously for phenotypes associated with Silver syndrome, dHMN type 5, CMT type 2, and hereditary spastic paraplegia.15 Studies of the N88S mutation suggest a toxic gain of function that causes improper folding of the BSCL2 protein. The incorrectly folded protein accumulates in the endoplasmic reticulum (ER), which leads to ER stress and eventually cellular dysfunction.16–19 Mutations in BSCL2 show a wide spectrum of clinical features, which can affect both motor and sensory neurons.15 One of the more unique clinical symptoms reported in US-PED2 was spastic paraplegia.6 This clinical feature has been reported in families with BSCL2 mutations.15 Interestingly, recent examination of USPED2 (K.M.) revealed that the carrier woman with the BSCL2 mutation was clinically normal, thus reflecting the phenotype for the obligate carriers of an X-linked CMT disease.20 However, variability in penetrance of the phenotype has been reported previously in families with BSCL2 mutations in which individuals carrying the disease mutation appear to be clinically normal.15,21,22 Incomplete penetrance of the N88S mutation is clearly demonstrated in US-PED2.
For over a decade, US-PED2 had been classified as X-linked CMT with evidence indicating linkage to the CMTX3 locus.6 We therefore assumed a novel gene in the CMTX3 locus may lead to the phenotype observed in this family. The reported X-linked inheritance for this family did not make screening genes associated with spasticity a priority. Given the outcome of this study, however, families with signs of spasticity, but that are too small to show significant linkage, may benefit from this approach. In this study, exome sequencing proved to be a fast and cost-efficient method, as it gave us the opportunity to examine both the region of interest and the entire coding exome. In conclusion, we have identified a known BSCL2 mutation in an original CMTX3 family (US-PED2) with the aid of exome sequencing. Despite not finding a pathogenic variant in the CMTX3 region, this family can be revisited once the gene mutation is identified in larger families showing significant linkage to the CMTX3 locus.12,13 This study demonstrates the power of exome analysis to identify gene mutations in the absence of statistically significant linkage in small nuclear families.
Acknowledgments
The authors thank the family members for participating in this investigation. This study was supported by the Australian National Health Medical Research Council and the USA Muscular Dystrophy Association. R.C is a recipient of the Australian Postgraduate Award.
REFERENCES
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Ionasescu VV. Charcot-Marie-Tooth neuropathies: from clinical description to molecular genetics. Muscle Nerve. 1995;18:267–275. doi: 10.1002/mus.880180302. [DOI] [PubMed] [Google Scholar]
- 3.Nelis E, vanBroeckhoven C, deJonghe P, Lofgren A, Vandenberghe A, Latour P, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet. 1996;4:25–33. doi: 10.1159/000472166. [DOI] [PubMed] [Google Scholar]
- 4.Dubourg O, Tardieu S, Birouk N, Gouider R, Leger JM, Maisonobe T, et al. The frequency of 17p11.2 duplication and Connexin 32 mutations in 282 Charcot-Marie-Tooth families in relation to the mode of inheritance and motor nerve conduction velocity. Neuromuscul Disord. 2001;11:458–463. doi: 10.1016/s0960-8966(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 5.Gal A, Mucke J, Theile H, Wieacker PF, Ropers HH, Wienker TF. X-linked dominant Charcot-Marie-Tooth disease: suggestion of linkage with a cloned DNA sequence from the proximal Xq. Hum Genet. 1985;70:38–42. doi: 10.1007/BF00389456. [DOI] [PubMed] [Google Scholar]
- 6.Ionasescu VV, Trofatter J, Haines JL, Summers AM, Ionasescu R, Searby C. Heterogeneity in X-linked recessive Charcot-Marie-Tooth neuropathy. Am J Hum Genet. 1991;48:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- 7.Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, et al. Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- 8.Le Guern E, Ravise N, Gugenheim M, Vignal A, Penet C, Bouche P, et al. Linkage analyses between dominant X-linked Charcot-Marie-Tooth disease, and 15 Xq11–Xq21 microsatellites in a new large family: three new markers are closely linked to the gene. Neuromuscul Disord. 1994;4:463–469. doi: 10.1016/0960-8966(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 9.Priest JM, Fischbeck KH, Nouri N, Keats BJ. A locus for axonal motor-sensory neuropathy with deafness and mental retardation maps to Xq24-q26. Genomics. 1995;29:409–412. doi: 10.1006/geno.1995.9987. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Hong SH, Ki CS, Kim BJ, Shim JS, Cho SH, et al. A novel locus for X-linked recessive CMT with deafness and optic neuropathy maps to Xq21.32-q24. Neurology. 2005;64:1964–1967. doi: 10.1212/01.WNL.0000163768.58168.3A. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, et al. Mutations in PRPS1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5) Am J Hum Genet. 2007;81:552–558. doi: 10.1086/519529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huttner IG, Kennerson ML, Reddel SW, Radovanovic D, Nicholson GA. Proof of genetic heterogeneity in X-linked Charcot-Marie-Tooth disease. Neurology. 2006;67:2016–2021. doi: 10.1212/01.wnl.0000247271.40782.b7. [DOI] [PubMed] [Google Scholar]
- 13.Brewer M, Changi F, Antonellis A, Fischbeck K, Polly P, Nicholson G, et al. Evidence of a founder haplotype refines the X-linked Charcot-Marie-Tooth (CMTX3) locus to a 2.5 Mb region. Neurogenetics. 2008;9:191–195. doi: 10.1007/s10048-008-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windpassinger C, Auer-Grumbach M, Irobi J, Patel H, Petek E, Horl G, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nature Genet. 2004;36:271–276. doi: 10.1038/ng1313. [DOI] [PubMed] [Google Scholar]
- 15.Auer-Grumbach M, Schlotter-Weigel B, Lochmuller H, Strobl-Wildemann G, Auer-Grumbach P, Fischer R, et al. Phenotypes of the N88S Berardinelli–Seip congenital lipodystrophy 2 mutation. Ann Neurol. 2005;57:415–424. doi: 10.1002/ana.20410. [DOI] [PubMed] [Google Scholar]
- 16.Ito D, Suzuki N. Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann Neurol. 2007;61:237–250. doi: 10.1002/ana.21070. [DOI] [PubMed] [Google Scholar]
- 17.Ito D, Fujisawa T, Iida H, Suzuki N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol Dis. 2008;31:266–277. doi: 10.1016/j.nbd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Yagi T, Ito D, Nihei Y, Ishihara T, Suzuki N. N88S seipin mutant transgenic mice develop features of seipinopathy/BSCL2-related motor neuron disease via endoplasmic reticulum stress. Hum Mol Genet. 2011;20:3831–3840. doi: 10.1093/hmg/ddr304. [DOI] [PubMed] [Google Scholar]
- 19.Ito D, Yagi T, Ikawa M, Suzuki N. Characterization of inclusion bodies with cytoprotective properties formed by seipinopathy-linked mutant seipin. Hum Mol Genet. 2012;21:635–646. doi: 10.1093/hmg/ddr497. [DOI] [PubMed] [Google Scholar]
- 20.Kleopa KA, Scherer SS. Molecular genetics of X-linked Charcot-Marie-Tooth disease. Neuromol Med. 2006;8:107–122. doi: 10.1385/nmm:8:1-2:107. [DOI] [PubMed] [Google Scholar]
- 21.Patel H, Hart PE, Warner TT, Houlston RS, Patton MA, Jeffery S, et al. The Silver syndrome variant of hereditary spastic paraplegia maps to chromosome 11q12-q14, with evidence for genetic heterogeneity within this subtype. Am J Hum Genet. 2001;69:209–215. doi: 10.1086/321267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irobi J, van denBergh P, Merlini L, Verellen C, vanMaldergem L, Dierick I, et al. The phenotype of motor neuropathies associated with BSCL2 mutations is broader than Silver syndrome and distal HMN type V. Brain. 2004;127:2124–2130. doi: 10.1093/brain/awh232. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]