Abstract
Organic anion transporter-3 (OAT3) is a member of the organic anion transporter family that mediates the body disposition of a diverse array of clinically important drugs. We previously demonstrated that activation of protein kinase C (PKC) inhibits OAT3 transport activity by accelerating OAT3 internalization from cell surface into intracellular compartments. In the current study, we established that PKC-induced inhibition of OAT3 transport activity in monkey kidney COS-7 cells and in human kidney HEK293 cells occurred through an enhanced OAT3 ubiquitination, a process catalyzed by an E3 ubiquitin-protein ligase Nedd4-2 (neural precursor cell expressed, developmentally down-regulated 4-2). Overexpression of Nedd4-2 enhanced OAT3 ubiquitination, decreased OAT3 expression at the cell surface, and inhibited OAT3 transport activity. In contrast, overexpression of the ubiquitin ligase-dead mutant Nedd4-2/C821A or siRNA knockdown of endogenous Nedd4-2 had opposite effects on OAT3. Furthermore, immunoprecipitation experiments conducted both in culture cells and with rat kidney slices showed that there was a physical interaction between OAT3 and Nedd4-2. In conclusion, our results provided the first evidence that Nedd4-2 is an important regulator for OAT3 ubiquitination, expression and transport activity.
Keywords: Membrane Transporter, Drug Transporter, Regulation, Ubiquitin Ligase, Ubiquitination
Graphical Abstract

INTRODUCTION
The organic anion transporter (OAT) subfamily, which constitutes roughly half of the SLC22 (solute carrier 22) transporter family, plays critical role in body handling of clinical important drugs, including anti-viral therapeutics, anti-cancer drugs, antibiotics, anti-hypertensives, and anti-inflammatories 1–5. Therefore, understanding the regulation of these transporters has profound clinical significance.
OAT isoforms are expressed in distinct tissues and cell membranes. In the kidney, OAT1 and OAT3 are the gatekeepers to move organic anions across the basolateral membrane into the proximal tubule cells for subsequent exit across the apical membrane into the urine for elimination 1–5. OAT1 and OAT3 show overlapping and yet distinct substrate specificities. As a cell membrane transporter, the amount of OAT at the cell surface is critical for its transport activity.
Recently, modification of receptors and channels by ubiquitin conjugation has emerged as the major regulatory mechanism of cell surface internalization, intracellular sorting, and turnover of these membrane proteins 6, 7. Ubiquitin is a highly conserved 76-amino-acid protein that forms an isopeptide bond between its C-terminal glycine and a lysine residue on the target protein. Each ubiquitin moiety harbors seven lysine residues, allowing for the formation of ubiquitin chains linked through its internal lysine residues. Therefore, a substrate can be modified by different types of ubiquitin conjugation: monoubiquitination (conjugation of one single ubiquitin to one single lysine on the substrate), multiubiquitination (conjugation of several monoubiquitin molecules to multiple lysine residues on the substrate), or polyubiquitination (extended polyubiquitin chain). More and more evidence indicates that ubiquitination serves as a major signal in PKC-regulated cellular trafficking of transporters, including dopamine transporter (DAT) 8, cationic amino acid transporter (CAT-1) 9, and glutamate transporter (GLT-1) 10. We previously demonstrated that OAT1 and OAT3 constitutively internalize from and recycle back to the cell surface, and that activation of PKC inhibits the transport activity of these transporters through accelerating their internalization from the cell surface to intracellular compartments 11, 12. As a result, the amount of OAT at the plasma membrane is reduced, and their transport activities are therefore decreased. Our follow-up studies further demonstrated that PKC-enhanced OAT1 ubiquitination is an important step preceding OAT1 internalization 13. However, whether ubiquitination is involved in OAT3 trafficking and function is presently unknown. E3 ubiquitin ligases are the enzymes participating in the last stage of ubiquitination and responsible for the transfer of ubiquitin to the specific substrate.
E3 ubiquitin ligases are classified into two main families, RING (~600 ligases) and HECT (~30 ligases). Nedd4-2 is a member of the HECT family and has been implicated in the ubiquitination of many mammalian transporters and ion channels 8, 10, 14, 15. In this report, we provided evidence that OAT3 ubiquitination is a significant mechanism in the regulation of the transporter. Most importantly, we demonstrated for the first time that ubiquitin ligase Nedd4-2 mediates OAT3 ubiquitination, expression and function.
MATERIALS AND METHODS
Materials
COS-7 cells and HEK293T cells were purchased from American Type Culture Collection (Manassas, VA). [3H]-labeled estrone sulfate (ES), a protypical substrate for OAT3, was purchased from PerkinElmer (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin, streptavidin-agarose beads and protein G-agarose beads were purchased from Pierce (Rockford, IL). cDNA for human Nedd4-2 was generously provided by Dr. Peter M. Snyder of the College of Medicine, University of Iowa (Iowa City, IA). cDNA for human αENaC with FLAG tag was generously provided by Dr. Christie P. Thomas of the College of Medicine, University of Iowa (Iowa City, IA). Mouse anti-myc antibody (9E10) was purchased from Roche (Indianapolis, IN). Mouse anti-ubiquitin antibody P4D1, anti-β-actin, and mouse IgG (sc2025) were purchased from Santa Cruz (Santa Cruz, CA). Rabbit anti-Nedd4-2 antibody, rabbit anti-E-Cadherin (M168) antibody and rabbit anti-OAT3 (ab83789) antibody were purchased from Abcam (Cambridge, MA). Mouse anti-FLAG (M2) antibody was purchased from Sigma-Aldrich (St. Louis, MO). Rabbit IgG (12-370) was purchased from EMD Millipore (Billerica, MA). Nedd4-2 siRNA oligonucleotides (Silencer® Select, identification number S23570) and negative control siRNA (Silencer® Select, catalog number 4390843) were purchased from Ambion (Grand Island, NY). PKC activator phorbol 12-myristate 13-acetate (PMA) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and Transfection
Parental COS-7 cells, parental HEK293T cells or COS-7 cells stably expressing human OAT3-myc were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C in 5% CO2. Transfection with plasmids or siRNA was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Cells were harvested following 48 hours after transfection for further experiments.
Preparation of Rat Kidney Slices—Sprague-Dawley rats (200–250g, male) were euthanized by CO2 inhalation, and the kidneys were immediately placed into freshly oxygenated ice-cold saline. Tissue slices (<0.5 mm; 5–10 mg wet weight) were cut with a Stadie-Riggs microtome and kept in oxygenated ice-cold saline afterwards until homogenized and lysed.
Site-Directed Mutagenesis
Nedd4-2 ligase dead mutant Nedd4-2/C821A was generated using site-directed mutagenesis kit from Agilent Technologies (Santa Clara, CA) following the manufacture’s manual of QuickChange® site-directed mutagenesis kit. The sequences of the mutant were confirmed by the dideoxy chain termination method.
Ubiquitination Assay
To inhibit proteasomal degradation of ubiquitinated OAT3, cells were treated with 100 μM N-acetyl-Leu-Leu-norleucinal (ALLN) for 2 hours before cell harvest. Cells were then lysed with ubiquitination lysis buffer (20 mM Tris/HCl, pH 7.5, 1% Triton X-100, 2 mM EDTA, and 25 mM NaF) freshly added with 1% of proteinase inhibitor cocktail and 20 mM N-ethylmaleimide (NEM). OAT3 was then immunoprecipitated with anti-myc antibody, followed by immunoblotting with anti-ubiquitin antibody P4D1.
Transport Measurements
Cells were plated in 48-well plates. For each well, uptake solution was added. The uptake solution consisted of phosphate-buffered saline (PBS)/Ca2+/Mg2+ (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 0.1 mM CaCl2, and 1 mM MgCl2, pH 7.3) and [3H]ES (100 nM). At the time points indicated, uptake process was stopped by aspirating the uptake solution and rapidly washing the cells with ice-cold PBS solution. The cells were then solubilized in 0.2 N NaOH, neutralized in 0.2 N HCl, and aliquotted for liquid scintillation counting.
Cell Surface Biotinylation
Cell surface expression level of OAT3 was examined using the membrane-impermeable biotinylation reagent, NHS-SS-biotin. Cells were plated in 6-well plates. Each well of cells was incubated with 1 ml of NHS-SS-biotin (0.5 mg/ml in PBS/CM) in two successive 20 min incubations on ice with very gentle shaking. The reagent was freshly prepared for each incubation. After biotinylation, each well was briefly rinsed with 3 ml of PBS/CM containing 100 mM glycine, and then incubated with the same solution for 30 min on ice to ensure complete quenching of the unreacted NHS-SS-biotin. The cells were then lysed on ice for 30 min in 400 μl of lysis buffer (10 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100 with 1/100 protease inhibitor mixture and 20 mM NEM). The cell lysates were cleared by centrifugation at 16,000g at 4 °C. 40 μl of streptavidin-agarose beads was then added to the supernatant to isolate cell membrane proteins. OAT3 was detected in the pool of surface proteins by SDS-PAGE and immunoblotting using an anti-myc antibody 9E10.
Immunoprecipitation
Cells or rat kidney slices were lysed with immunoprecipitation lysis buffer (10 mM Tris/HCl, pH 7.5, 10 mM NaCl, 0.5% Triton X-100, 2 mM EDTA, 10% glycerol), freshly added with 1% of proteinase inhibitor cocktail and 20 mM NEM. Cell lysates were precleared with protein G-agarose beads to reduce nonspecific binding at 4 °C for 1.5 hours. Anti-myc antibody (1:100) was incubated with appropriate volume of protein G-agarose beads at 4 °C for 1.5 hours. The precleared protein sample was then mixed with antibody-bound protein G-agarose beads and underwent end-over-end rotating at 4 °C overnight. Proteins bound to the protein G-agarose beads were eluted with Urea buffer containing β-mercaptoethanol and analyzed by immunoblotting with indicated antibodies.
Electrophoresis and Immunoblotting
Protein samples were resolved on 7.5% SDS-PAGE minigels and electroblotted on to polyvinylidene difluoride membranes. The blots were blocked for 1 hour with 5% nonfat dry milk in PBS-0.05% Tween 20, washed, and incubated overnight at 4 °C with appropriate primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies. The signals were detected by SuperSignal West Dura Extended Duration Substrate kit (Pierce). Nonsaturating, immunoreactive protein bands were quantified by scanning densitometry with the FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA).
Data Analysis
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments. Statistical analysis was performed using Student’s paired t tests. A p-value of <0.05 was considered significant and indicated as “*”.
RESULTS
PKC enhanced OAT3 ubiquitination
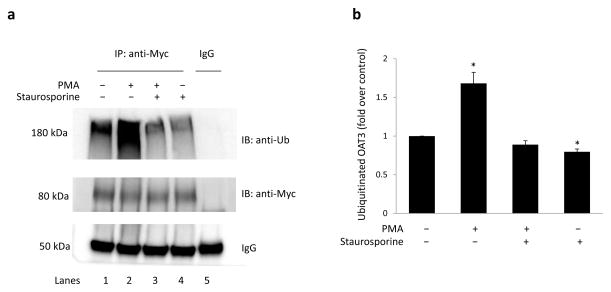
We previously reported that activation of PKC inhibits OAT3 transport activity 12. To examine whether OAT3 ubiquitination was involved in this process, OAT3-expressing COS-7 cells were treated with control vehicle, PKC activator PMA, PMA plus PKC inhibitor staurosporine, or staurosporine alone for 30 minutes. OAT3 was then immunoprecipitated with anti-myc antibody (myc was tagged to OAT3) or with control IgG (as negative control), followed by immunoblotting with anti-ubiquitin antibody. As shown in Fig. 1a, top panel (lane 1), ubiquitin-immunoreactive signal displayed a smeary band starting from 180 kDa, ~100 kDa larger than the size of OAT3 (~80 kDa). Given that each ubiquitin molecule is ~ 8 kDa, OAT3 is most likely to be polyubiquitinated or multiubiquitinated. Treatment of the cells with PMA, a PKC activator, induced a significant increase in OAT3 ubiquitination (lane 2). The PMA-induced OAT3 ubiquitination was reduced in the presence of staurosporine, a general PKC inhibitor (lane 3). The cells treated with staurosporine alone also slightly decreased OAT3 ubiquitination (lane 4), which indicates that inhibition of the endogenous PKC also affected OAT3 ubiquitination. Moreover, the differences in ubiquitination were not due to the differences in the amount of OAT3 immunoprecipitated as evident when the same immunoblot was reprobed with anti-myc antibody. Similar amount of OAT3 was immunoprecipitated in all samples under these conditions (Fig. 1a, middle panel). These data demonstrated the specific involvement of PKC in OAT3 ubiquitination. Similar results were obtained when these experiments were performed in human embryonic kidney cells (HEK293T) (Fig. 2), suggesting that OAT3 ubiquitination is not cell type-specific, but is rather a general feature of this transporter.
Fig. 1. PKC activation induced OAT3 ubiquitination in COS-7 cells.
(a) COS-7 cells, transfected with OAT3, were treated with control vehicle, PKC activator PMA (1 μM), PMA plus PKC inhibitor staurosporine (2 μM), or staurosporine alone (2 μM) for 30 minutes. Treated cells were then lysed, and OAT3 was immunoprecipitated (IP) with anti-myc antibody or with mouse IgG (as negative control), followed by immunoblotting (IB) with anti-ubiquitin antibody (anti-Ub) (top panel). The same immunoblot from (a, top panel) was reprobed with anti-myc antibody (middle panel) to determine the amount of OAT3 immunoprecipitated. IgG bands were shown in bottom panel. (b) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
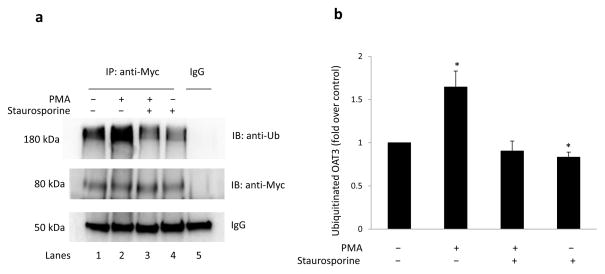
Fig. 2. PKC activation induced OAT3 ubiquitination in HEK293T cells.
(a) HEK293T cells, transiently transfected with OAT3, were treated with control vehicle, PKC activator PMA (1 μM), PMA plus PKC inhibitor staurosporine (2 μM), or staurosporine only (2 μM) for 30 minutes. Treated cells were then lysed, and OAT3 was immunoprecipitated (IP) with anti-myc antibody or with mouse IgG (as negative control), followed by immunoblotting (IB) with anti-ubiquitin antibody (anti-Ub) (top panel). The same immunoblot from (a, top panel) was reprobed by anti-myc antibody (middle panel) to determine the amount of OAT3 immunoprecipitated. IgG bands were shown in bottom panel. (b) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
Effect of Nedd4-2 on OAT3 ubiquitination
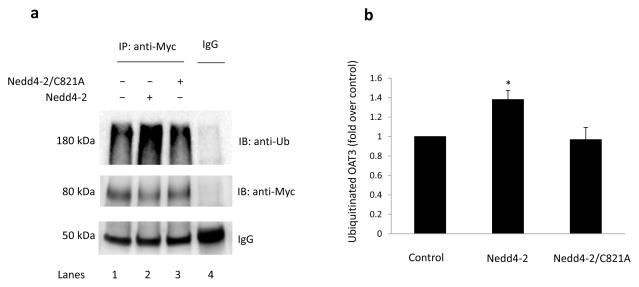
We showed above that OAT3 ubiquitination was significantly enhanced by activation of PKC. Since Nedd4-2 is an ubiquitin ligase that has been reported to promote the ubiquitination of many ion channels and transporters, we therefore examined the role of Nedd4-2 in OAT3 ubiquitination. COS-7 cells were co-transfected with OAT3 and Nedd4-2 wild type or with OAT3 and the ubiquitin ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A). The ligase-dead mutant was unable to transfer ubiquitin to its target protein 16, 17. Transfected cells were lysed, and OAT3 was then immunoprecipitated with anti-myc antibody (myc was tagged to OAT3) or with control IgG (as negative control), followed by immunoblotting with anti-ubiquitin antibody. As shown in Fig. 3a, top panel, OAT3 was ubiquitinated under basal condition (lane 1). The ubiquitination level was augmented in cells transfected with Nedd4-2 wild type (lane 2), whereas the ubiquitin ligase-dead mutant Nedd4-2/C821A was without any significant effects on OAT3 ubiquitination (lane 3). Furthermore, the differences in ubiquitination were not due to the differences in the amount of OAT3 immunoprecipitated as evident when the same immunoblot was reprobed with anti-myc antibody. Similar amount of OAT3 was immunoprecipitated in all samples under these conditions (Fig. 3a, middle panel).
Fig. 3. Effect of Nedd4-2 on OAT3 ubiquitination.
(a) COS-7 cells were co-transfected with OAT3 and Nedd4-2 or with OAT3 and the ubiquitin ligase-dead mutant Nedd4-2/C821A. Transfected cells were then lysed, and OAT3 was immunoprecipitated (IP) with anti-myc antibody or with mouse IgG (as negative control), followed by immunoblotting (IB) with anti-ubiquitin antibody (anti-Ub) (top panel). The same immunoblot from (a, top panel) was reprobed by anti-myc antibody (middle panel) to determine the amount of OAT3 immunoprecipitated. IgG bands were shown in bottom panel. (b) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
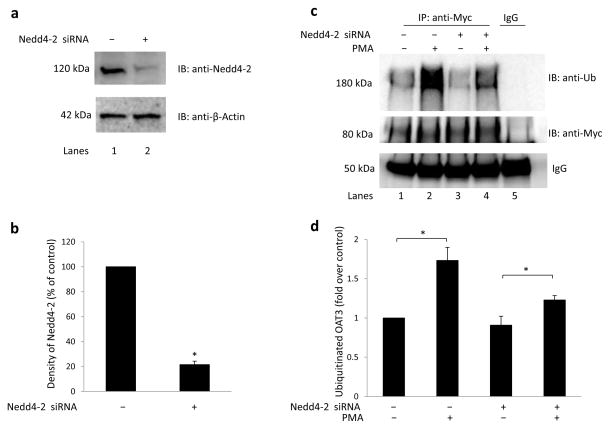
As an independent approach, we used a siRNA strategy to abrogate the endogenous Nedd4-2 and evaluated the role of Nedd4-2 in OAT3 ubiquitination. As shown in Fig. 4a, top panel, the endogenous Nedd4-2 expression was effectively reduced in Nedd4-2 siRNA-transfected cells (lane 2) as compared to that in scrambled siRNA-transfected negative control cells (lane 1). When the same blot was reprobed with anti-β-actin, it was clear that the housekeeping protein β-actin was not affected under these conditions (Fig. 4a, bottom panel). We then proceeded to examine the role of Nedd4-2 in OAT3 ubiquitination. As shown in Fig. 4c, PMA treatment for 30 min significantly enhanced OAT3 ubiquitination in scrambled siRNA-transfected control cells (lanes 1 and 2), whereas in Nedd4-2 knock-down cells (lanes 3 and 4), PMA-induced OAT3 ubiquitination was considerably reduced. Together, our data provided evidence that Nedd4-2 is an important ubiquitin ligase for OAT3 ubiquitination.
Fig. 4. Nedd4-2 siRNA abrogated OAT3 ubiquitination.
(a) COS-7 cells were co-transfected with OAT3 and scrambled control siRNA or with OAT3 and Nedd4-2-specific siRNA. The effectiveness of Nedd4-2-specific siRNA was tested by probing the lysis sample with anti-Nedd4-2 antibody (top panel). The same immunoblot from (a, top panel) was reprobed by anti-β-actin antibody (bottom panel). (b) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05. (c) COS-7 cells, co-transfected with OAT3 and negative control siRNA or with OAT3 and Nedd4-2 siRNA, were treated with either control vehicle or PKC activator PMA (1 μM) for 30 minutes. Treated cells were then lysed, and OAT3 was immunoprecipitated (IP) with anti-myc antibody, or with mouse IgG (as negative control), followed by immunoblotting (IB) with anti-ubiquitin antibody (anti-Ub, top panel). The same immunoblot from (c, top panel) was reprobed by anti-myc antibody (middle panel) to determine the amount of OAT3 immunoprecipitated. IgG bands were shown in bottom panel. (d) Densitometry plot of results from (c, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
Effect of Nedd4-2 on OAT3 expression
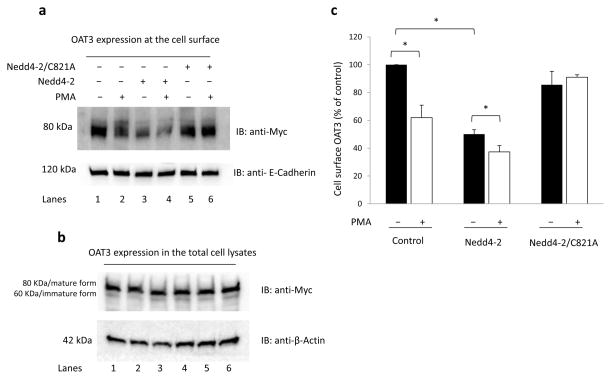
In our previously published work 12, we demonstrated that activation of PKC in OAT3-expressing cells led to an accelerated internalization of the transporter from the cell surface to intracellular compartment. As a result, the amount of OAT3 at the plasma membrane was reduced. In this experiment, we examined the role of Nedd4-2 in this process. The surface expression of OAT3 was evaluated through biotinylation assay as described under “Materials and Methods”. As shown in Fig. 5a, top panel, PMA treatment (30 min) in control cells (lanes 1 and 2) decreased the surface expression of OAT3 to 61.9% ± 9.0%. In cells transfected with Nedd4-2 for 48 hours (lanes 3 and 4), the initial amount of OAT3 at the cell surface (lane 3) was already significantly reduced as compared to that in control cells (lane 1), suggesting that a portion of OAT3 was already ubiquitinated and therefore internalized from the cell surface during the 48-hour period of Nedd4-2 transfection. PMA treatment in these cells further augmented the decrease of OAT3 at the cell surface (lane 4). However, in cells transfected with the ubiquitin ligase-dead mutant Nedd4-2/C821A (lanes 5 and 6), the initial amount of OAT3 at the cell surface (lane 5) was similar to that in control cells (lane 1), and PMA treatment had little effect on OAT3 expression at the cell surface (lane 6). Furthermore, such a change in OAT3 cell surface expression was not due to the general perturbation of cellular proteins as the expression of the cell surface protein marker E-cadherin18, 19 was not affected under these conditions (Fig. 5a, bottom panel). OAT3 total expression (Fig. 5b, top panel, both mature form and immature form 20, 21) and the total expression of the cell protein marker β-actin (Fig. 5b, bottom panel) were not affected under these conditions.
Fig. 5. Effect of Nedd4-2 on OAT3 expression.
(a) OAT3 expression at the cell surface. COS-7 cells, co-transfected with OAT3 and control vector, with OAT3 and wild type Nedd4-2, or with OAT3 and the ubiquitin ligase-dead mutant Nedd4-2/C821A, were treated with either control vehicle or PKC activator PMA (1 μM) for 30 minutes. Cell surface biotinylation was performed. Biotinylated/cell surface proteins were separated with streptavidin beads and analyzed by immunoblotting (IB) with an anti-myc antibody (top panel). The same immunoblot from (a, top panel) was reprobed by anti-E-cadherin antibody (bottom panel) to determine the expression of the cell surface protein marker E-cadherin. (b) The expression of OAT3 and total protein marker β-actin in total cell lysates. COS-7 cells, co-transfected with OAT3 and control vector, with OAT3 and wild type Nedd4-2, or with OAT3 and the ubiquitin ligase-dead mutant Nedd4-2/C821A, were treated with either control vehicle or PKC activator PMA (1 μM) for 30 minutes. Cells were then lysed, followed by immunoblotting (IB) with anti-Myc antibody or anti-β-actin. (c) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05. It is important to note that the OAT3 detected by anti-myc antibody at ~ 80 kDa mainly reflected the unubiquitinated OAT3 as the ubiquitinated OAT3 was weak for visualization due to the fact that the signals were spread out in a wide range (centered ~ 180 kDa).
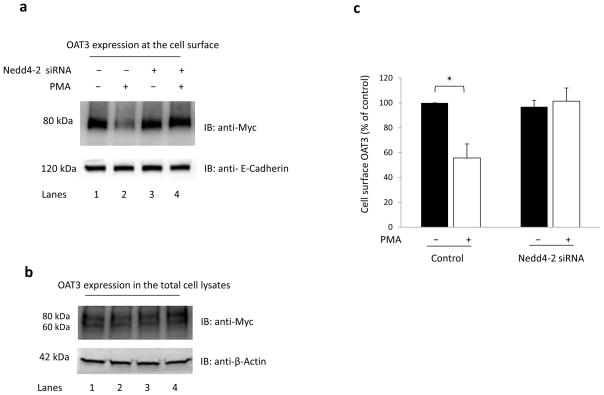
Next, we used a siRNA strategy to knock down the endogenous Nedd4-2 and assessed the role of Nedd4-2 in OAT3 expression. As shown in Fig. 6a, top panel, PMA treatment (30 min) in control cells (lanes 1 and 2) significantly decreased the surface expression of OAT3. However, Nedd4-2 knock-down (lanes 3 and 4) rendered cells significantly less responsive to PMA-induced decrease in OAT3 surface expression. Again, such a change in OAT3 cell surface expression was not due to the general perturbation of cellular proteins as the expression of the cell surface protein marker E-cadherin was not affected under these conditions (Fig. 6a, bottom panel). OAT3 total expression (Fig. 6b, top panel) and the total expression of the cell protein marker β-actin (Fig. 6b, bottom panel) were not affected under these conditions.
Fig. 6. Effect of Nedd4-2 siRNA on OAT3 expression.
(a) OAT3 expression at the cell surface. COS-7 cells, co-transfected with OAT3 and negative control siRNA or with OAT3 and Nedd4-2 siRNA, were treated with either vehicle or PKC activator PMA (1 μM) for 30 minutes. Cell surface biotinylation was performed. Biotinylated (cell surface) proteins were separated with streptavidin beads and analyzed by immunoblotting (IB) with an anti-myc antibody (top panel) or with an anti-E-cadherin antibody (bottom panel). (b) OAT3 expression in total cell lysates. COS-7 cells, co-transfected with OAT3 and negative control siRNA or with OAT3 and Nedd4-2 siRNA, were treated with either vehicle or PKC activator PMA (1 μM) for 30 minutes. Cells were then lysed, followed by immunoblotting (IB) with anti-myc antibody (top panel) or with anti-β-actin antibody (bottom panel). (c) Densitometry plot of results from (a, top panel) as well as from other experiments. The values are mean ± S.E. (n = 3). *P<0.05
Effect of Nedd4-2 on OAT3 transport activity
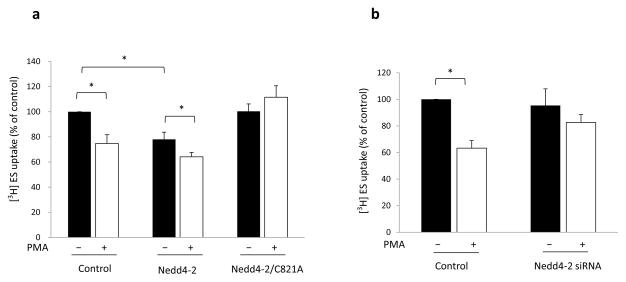
As a cell membrane transporter, the amount of OAT at the cell surface is critical for its transport activity. In our experiments described above, we showed that Nedd4-2 was involved in OAT3 cell surface expression. Therefore, in this experiment, we investigated whether the altered cell surface expression would translate into OAT3 functional change. As shown in Fig. 7a, PMA treatment (30 min) of control cells significantly reduced the uptake function of OAT3 to 74.7% ± 7.0%. In cells transfected with Nedd4-2 for 48 hours, the initial OAT3 uptake function was already considerably decreased as compared to that in control cells, consistent with the much reduced OAT3 expression at the cell surface in these cells (Fig. 5a, top panel, lane 3), and PMA treatment in these cells further amplified such functional decrease. However, in cells transfected with the ubiquitin ligase-dead mutant Nedd4-2/C821A, PMA-induced inhibition of OAT3 uptake function was blocked. Similarly, the PMA-induced inhibition of OAT3 uptake function was blocked when the endogenous Nedd4-2 was knocked-down by Nedd4-2-specific siRNA (Fig. 7b).
Fig. 7. Effect of Nedd4-2 on OAT3 transport activity.
(a) COS-7 cells, co-transfected with OAT3 and control vector, with OAT3 and wild type Nedd4-2, or with OAT3 and the ubiquitin ligase-dead mutant Nedd4-2/C821A, were treated with either control vehicle or PKC activator PMA (1 μM) for 30 minutes. Uptake of [3H]ES was then performed. (b) COS-7 cells, co-transfected with OAT3 and negative control siRNA or with OAT3 and Nedd4-2 siRNA, were treated with either vehicle or PKC activator PMA (1 μM) for 30 minutes. Uptake of [3H] ES was then performed. Uptake activity was expressed as a percentage of the uptake measured in cells without treatment with PMA. The data represent uptake into OAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P<0.05
Nedd4-2 interaction with OAT3
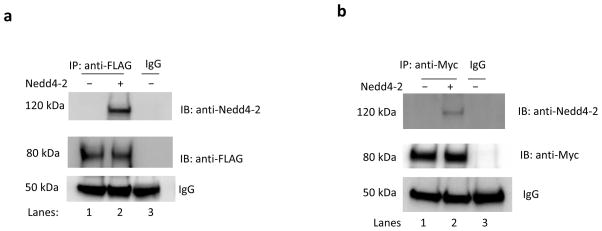
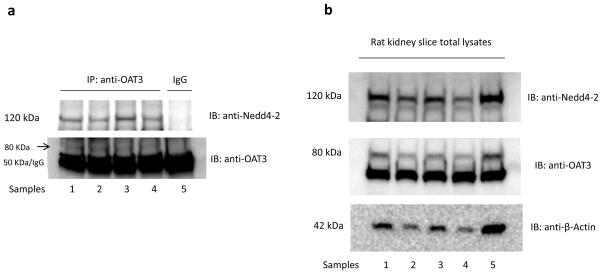
Nedd4-2 is reported to interact either directly or indirectly with its target proteins, and thus, we assessed whether there was an association between Nedd4-2 and OAT3 in COS-7 cells through co-immunoprecipitation assay. We used the interaction between epithelial sodium channel ENaC and Nedd4-2 as a positive control (Fig. 8a) because Nedd4-2 has been shown to regulate ENaC through directly binding to the channel 22. COS-7 cells were co-transfected with α subunit of ENaC (αENaC) and control vector or with αENaC and wild type Nedd4-2. Transfected cells were lysed and αENaC was immunoprecipitated with anti-FLAG antibody (FLAG was tagged to αENaC) or control IgG (as negative control), followed by immunoblotting (IB) with anti-Nedd4-2 antibody. As shown in Fig. 8a, top panel, significant amount of Nedd4-2 was detected in αENaC immunoprecipitates in Nedd4-2-transfected cells (lane 2) as compared to that in control cells (lane 1), confirming the interaction between Nedd4-2 and ENaC. We then proceeded to examine whether there was an interaction between Nedd4-2 and OAT3. COS-7 cells were co-transfected with OAT3 and control vector or with OAT3 and Nedd4-2. Transfected cells were lysed and OAT3 was immunoprecipitated with anti-myc antibody (myc was tagged to OAT3) or with control IgG (as negative control), followed by immunoblotting (IB) with anti-Nedd4-2 antibody. As shown in Fig. 8b, top panel, significant amount of Nedd4-2 was detected in OAT3 immunoprecipitates in Nedd4-2-transfected cells (lane 2) as compared to that in control cells (lane 1), suggesting that there was an association between Nedd4-2 and OAT3. The differences in the amount of Nedd4-2 detected were not due to differences in the amount of OAT3 immunoprecipitated as evident when the same immunoblot was reprobed with anti-myc antibody. Similar amount of OAT3 was immunoprecipitated in both control and Nedd4-2-transfected cells (Fig. 8b, middle panel). A similar experiment was then performed with rat kidney slices where both Nedd4-2 and OAT3 were endogenously expressed. As shown in Fig. 9a, top panel, Nedd4-2 was detected in OAT3 immunoprecipitates in all rats examined. These results provide physiological relevance of our study.
Fig. 8. Interaction between Nedd4-2 and OAT3 in COS-7 cells.
(a) Interaction between ENaC and Nedd4-2. COS-7 cells were co-transfected with αENaC and control vector or with αENaC and wild type Nedd4-2. Transfected cells were lysed and αENaC was immunoprecipitated by anti-FLAG antibody (FLAG was tagged to αENaC) or by mouse IgG (as negative control), followed by immunoblotting (IB) with anti-Nedd4-2 antibody (top panel). The same immunoblot from (a, top panel) was reprobed by anti-FLAG antibody (middle panel) to determine the amount of αENaC immunoprecipitated. IgG bands were shown in bottom panel. (b) Interaction between Nedd4-2 and OAT3. OAT3-expressing COS-7 cells, transfected with control vector or wild type Nedd4-2, were lysed, and OAT3 was then immunoprecipitated (IP) with anti-myc antibody or with mouse IgG (as negative control), followed by immunoblotting (IB) with anti-Nedd4-2 antibody (top panel). The same immunoblot was reprobed by anti-myc antibody (middle panel) to determine the amount of OAT3 immunoprecipitated. IgG bands were shown in bottom panel. The experiments were performed at least three times.
Fig. 9. Interaction between Nedd4-2 and OAT3 in rat kidney slices.
(a) The kidney slices from rats (n=5) were lysed, and OAT3 was then immunoprecipitated (IP) with anti-OAT3 antibody or with rabbit IgG (as negative control), followed by immunoblotting (IB) with anti-Nedd4-2 antibody (top panel). The same immunoblot was reprobed by anti-OAT3 antibody (bottom panel) to determine the amount of OAT3 immunoprecipitated (~ 80 kDa). (b) Total expression of Nedd4-2, OAT3, and β-actin in rat kidney slices. The kidney slices from rats (n=5) were lysed and 30 μg protein was loaded for immunoblot analysis. The expression of Nedd4-2, OAT3 and β-actin was detected by anti-Nedd4-2 antibody (top panel), anti-OAT3 antibody (middle panel), and anti-actin antibody (bottom panel) respectively.
DISCUSSION
The substrates of OAT family members are with a wide range, including endogenous metabolites, like second messengers, hormone derivatives, mono-/di-carboxylates; or clinical drugs, such as β-lactam antibiotics, diuretics, statins, antivirals, antitumor drugs, NSAIDs 5, 23, 24. Hence, to understand the regulation of OAT is an important aspect both in physiology and in clinics. As a cell membrane transporter, the variation of OAT protein on the cell surface leads to the change of overall body elimination of metabolites and clinical drugs, which is in part influenced by the trafficking of OAT to and from the plasma membrane. We previously demonstrated 11, 12 that acute activation of PKC inhibited the transport activities of OAT1 and OAT3 by reducing the cell surface expression of these transporters through accelerating their internalization from the cell surface to EEA1-positive intracellular recycling endosome, yet without directly phosphorylating the transporter itself 12, 25, 26. We further established that PKC-induced OAT1 ubiquitination was a crucial event that preceded OAT1 internalization and trafficking. In the current study, we demonstrated that the activation of PKC also significantly enhanced OAT3 ubiquitination (Fig. 1 and Fig. 2). Our data showed that under basal condition, OAT3 was moderately ubiquitinated. However, acute activation of PKC by PMA induced a significant increase in OAT3 ubiquitination and that PMA-induced ubiquitination was blocked by PKC inhibitor staurosporine, confirming that this PMA effect was indeed PKC-dependent. We then explored the detailed mechanism of OAT3 ubiquitination. E3 ubiquitin ligase Nedd4-2 has been reported to be involved in the PKC-regulated ubiquitination of many transporters, such as dopamine transporter (DAT) 8, cationic amino acid transporter (CAT-1) 9, and glutamate transporter (GLT-1) 10. Thus, we asked whether Nedd4-2 also mediated OAT3 ubiquitination. Our results (Fig. 3) showed that overexpression of Nedd4-2 indeed enhanced OAT3 ubiquitination, whereas overexpression of the ubiquitin ligase-dead mutant Nedd4-2/C821A had opposite effect.
We also took the siRNA strategy to investigate the specific role of Nedd4-2 in OAT3 ubiquitination. We used a siRNA that was designed for knock-down of human Nedd4-2, but proven functional in COS-7 cells 10. This was confirmed in Fig. 4a, top panel: endogenous Nedd4-2 expression is successfully reduced in cells transfected with Nedd4-2-specific siRNA. Knock-down of endogenous Nedd4-2 significantly blocked PKC-induced OAT3 ubiquitination (Fig. 4c, top panel). Furthermore, overexpression of the ubiquitin ligase-dead mutant Nedd4-2/C821A (Fig. 5, top panel) or knock-down of endogenous Nedd4-2 (Fig. 6, top panel) both blocked PKC-induced reduction of OAT3 expression at the cell surface and PKC-induced inhibition of OAT3 transport activity (Fig. 7). These data demonstrated for the first time that Nedd4-2 is an important enzyme involved in OAT3 expression at the cell surface and function.
Our current studies also suggest that the effect of Nedd4-2 on OAT3 occurs through interaction between these two proteins as determined by co-immunoprecipitation experiments (Fig. 8 and Fig. 9). Nedd4-2 has 3–4 WW domains. These WW domains can bind with the proline-rich PY motif (PPxY) of its substrates such as in the case of the epithelial sodium channel ENaC 27, 28. However, it was reported that Nedd4-2 also participates in the regulation of substrates that do not bear PY motifs, such as in the cases of DAT 8 and GLT-110, where the physical association was found for both of them with Nedd4-2. OAT3 does not have that PY structure motif either, and yet we were able to detect its association with Nedd4-2. These data indicate that Nedd4–2 may use an unconventional site on OAT3 in its regulation of the transporter.
Our current studies were carried out in heterologous cell systems – monkey kidney COS-7 cells and human kidney HEK293 cells, and in rat kidney slices. COS-7 cells and HEK293 cells offer several useful advantages for study of the cloned organic anion transporter. (i) These cells were directly derived from the kidney and have been very useful in understanding other renal transport processes and cellular functions, including organic cation transport 29–31. (ii) These cell lines do not express endogenous OATs. Therefore, expression of OAT3 in these cells will allow us to dissect the transport characteristics of OAT3 in relevant mammalian systems without the possibly confounding effects of other organic anion transporters. (iii) They possess endogenous PKC and PKA signaling pathways and provide good experimental model systems for studying the regulatory mechanisms underlying many transport processes 32–35 (iv) The transport characteristics of OATs in these cells were in a good agreement with that observed in other systems 11, 36–38. Our studies in rat kidney slices (Fig. 9) further confirmed the physiological relevance of our work, which will pave the way for the future investigation focusing on determining whether the same mechanisms are operative in vivo.
What would be the physiological implication of our studies? Abnormal OAT3 trafficking may contribute to the impaired drug elimination in bilateral ureteral obstruction (BUO). BUO is a serious and common clinical condition, and an important cause of acute renal failure 39, 40. It is shown 40 that in BUO rats, elimination of drugs was impaired partly due to a redistribution of OAT3 from cell surface to intracellular compartment. In BUO, angiotensin II has elevated level of expression 39, 41, 42. We previously reported that angiotensin II inhibits OAT activity through activation of PKC in cultured cells 43. Therefore, the high level of angiotensin II in BUO rats may inhibit OAT3 activity through PKC-regulated OAT3 ubiquitination and trafficking, in which Nedd4-2 plays a critical role. Future studies using Nedd4-2 knockout mice may address this question.
Taken together, our results revealed, for the first time, that Nedd4-2 is an important ubiquitin enzyme for OAT3 ubiquitination, expression and function (Fig. 10). Ongoing studies in our lab also suggest that Nedd4-2 is an important regulator for other members of OAT family. This investigation provides important insight into the understanding of the molecular and cellular bases of OAT regulation in vivo.
Fig. 10.
The role of Nedd4-2 in OAT3 ubiquitination, trafficking, and function.
Acknowledgments
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
References
- 1.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76(3):481–90. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38(7–8):889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2009;31(1):1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22(6):602–16. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 6.Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol Interv. 2007;7(3):157–67. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- 7.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86(2):669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 8.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26(31):8195–205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286(10):8697–706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287(23):19177–87. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283(47):32570–9. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3(2):242–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2012;83(1):217–24. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem. 2004;279(6):5042–6. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- 15.Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 2010;285(10):7645–56. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007;282(28):20207–12. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- 17.Raikwar NS, Thomas CP. Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel. Am J Physiol Renal Physiol. 2008;294(5):F1157–65. doi: 10.1152/ajprenal.00339.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kock K, Koenen A, Giese B, Fraunholz M, May K, Siegmund W, Hammer E, Volker U, Jedlitschky G, Kroemer HK, Grube M. Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization. J Biol Chem. 2010;285(15):11336–47. doi: 10.1074/jbc.M109.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanoni C, Massari S, Losa M, Carrega P, Perego C, Conforti L, Pietrini G. Increased internalisation and degradation of GLT-1 glial glutamate transporter in a cell model for familial amyotrophic lateral sclerosis (ALS) J Cell Sci. 2004;117(Pt 22):5417–26. doi: 10.1242/jcs.01411. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279(15):14961–6. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67(3):868–76. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 22.Itani OA, Stokes JB, Thomas CP. Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol. 2005;289(2):F334–46. doi: 10.1152/ajprenal.00394.2004. [DOI] [PubMed] [Google Scholar]
- 23.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2010;(201):29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- 24.You G. Towards an understanding of organic anion transporters: structure-function relationships. Med Res Rev. 2004;24(6):762–74. doi: 10.1002/med.20014. [DOI] [PubMed] [Google Scholar]
- 25.You G, Kuze K, Kohanski RA, Amsler K, Henderson S. Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem. 2000;275(14):10278–84. doi: 10.1074/jbc.275.14.10278. [DOI] [PubMed] [Google Scholar]
- 26.Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, Burckhardt G. Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol. 2003;14(8):1959–68. doi: 10.1097/01.asn.0000079040.55124.25. [DOI] [PubMed] [Google Scholar]
- 27.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15(10):2371–80. [PMC free article] [PubMed] [Google Scholar]
- 28.Macias MJ, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, Oschkinat H. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382(6592):646–9. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- 29.Nagai K, Takikawa O, Kawakami N, Fukao M, Soma T, Oda A, Nishiya T, Hayashi M, Lu L, Nakano M, Kajita E, Fujita H, Miwa S. Cloning and functional characterization of a novel up-regulator, cartregulin, of carnitine transporter, OCTN2. Arch Biochem Biophys. 2006;452(1):29–37. doi: 10.1016/j.abb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Evans KK, Wright SH. Molecular cloning of rabbit organic cation transporter rbOCT2 and functional comparisons with rbOCT1. Am J Physiol Renal Physiol. 2002;283(1):F124–33. doi: 10.1152/ajprenal.00367.2001. [DOI] [PubMed] [Google Scholar]
- 31.Kwon M, Choi YA, Choi MK, Song IS. Organic cation transporter-mediated drug-drug interaction potential between berberine and metformin. Arch Pharm Res. 2015;38(5):849–56. doi: 10.1007/s12272-014-0510-6. [DOI] [PubMed] [Google Scholar]
- 32.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2) Am J Physiol Lung Cell Mol Physiol. 2002;282(1):L12–25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- 33.Kazanietz MG, Caloca MJ, Aizman O, Nowicki S. Phosphorylation of the catalytic subunit of rat renal Na+, K+-ATPase by classical PKC isoforms. Arch Biochem Biophys. 2001;388(1):74–80. doi: 10.1006/abbi.2000.2264. [DOI] [PubMed] [Google Scholar]
- 34.Hong M, Hong W, Ni C, Huang J, Zhou C. Protein kinase C affects the internalization and recycling of organic anion transporting polypeptide 1B1. Biochim Biophys Acta. 2015;1848(10 Pt A):2022–30. doi: 10.1016/j.bbamem.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Wong R, Schlichter LC. PKA reduces the rat and human KCa3.1 current, CaM binding, and Ca2+ signaling, which requires Ser332/334 in the CaM-binding C terminus. J Neurosci. 2014;34(40):13371–83. doi: 10.1523/JNEUROSCI.1008-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller DS. Protein kinase C regulation of organic anion transport in renal proximal tubule. Am J Physiol. 1998;274(1 Pt 2):F156–64. doi: 10.1152/ajprenal.1998.274.1.F156. [DOI] [PubMed] [Google Scholar]
- 37.Shuprisha A, Lynch RM, Wright SH, Dantzler WH. PKC regulation of organic anion secretion in perfused S2 segments of rabbit proximal tubules. Am J Physiol Renal Physiol. 2000;278(1):F104–9. doi: 10.1152/ajprenal.2000.278.1.F104. [DOI] [PubMed] [Google Scholar]
- 38.Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, Tanabe K, Nakayama H, Horita S, Kusuhara H, Sugiyama Y. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci. 2014;59:94–103. doi: 10.1016/j.ejps.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Seldin DW, Giebisch G. The Kidney: Physiology & Pathophysiology. 3. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 40.Villar SR, Brandoni A, Anzai N, Endou H, Torres AM. Altered expression of rat renal cortical OAT1 and OAT3 in response to bilateral ureteral obstruction. Kidney Int. 2005;68(6):2704–13. doi: 10.1111/j.1523-1755.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 41.Klahr S. Obstructive nephropathy. Kidney Int. 1998;54(1):286–300. [PubMed] [Google Scholar]
- 42.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283(5):F861–75. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Duan P, You G. Regulation of human organic anion transporter 1 by ANG II: involvement of protein kinase Calpha. Am J Physiol Endocrinol Metab. 2009;296(2):E378–83. doi: 10.1152/ajpendo.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]