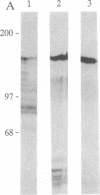
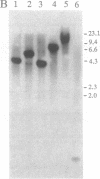
Abstract
Among the variety of specialized intercellular junctions, those of the adherens type have the most obvious association with cytoskeletal elements. This may be with the actin microfilament system as in the zonula adherens or with intermediate filaments as in the macula adherens, or desmosome. In the former case, it is clear that transmembrane glycoproteins of the cadherin family are important adhesive components of the molecular assembly. We now show for desmosomes that a major glycoprotein component (desmosomal glycoprotein DGI) has extensive homology with the cadherins, defining an extended family, but also has unique features in its cytoplasmic domain that are likely to be relevant to the association with intermediate rather than actin filaments. A novel 282-residue extension contains repeats of approximately 29 amino acid residues predicted to have an antiparallel beta-sheet structure, followed by a glycine-rich sequence. As in the cadherins, the extracellular domain contains possible Ca2(+)-binding sequences and a potential protease processing site. The cell adhesion recognition region (His-Ala-Val) of the cadherins is modified to Arg-Ala-Leu.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaschuk O. W., Sullivan R., David S., Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990 May;139(1):227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Green K. J., Parry D. A., Steinert P. M., Virata M. L., Wagner R. M., Angst B. D., Nilles L. A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem. 1990 Feb 15;265(5):2603–2612. [PubMed] [Google Scholar]
- Hirano S., Nose A., Hatta K., Kawakami A., Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987 Dec;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., von dem Borne A. E., Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990 Mar;9(3):765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P. J., Walsh M. J., Schmelz M., Goldschmidt M. D., Zimbelmann R., Franke W. W. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990 Oct;53(1):1–12. [PubMed] [Google Scholar]
- Mansouri A., Spurr N., Goodfellow P. N., Kemler R. Characterization and chromosomal localization of the gene encoding the human cell adhesion molecule uvomorulin. Differentiation. 1988 Jun;38(1):67–71. doi: 10.1111/j.1432-0436.1988.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Mattey D. L., Suhrbier A., Parrish E., Garrod D. R. Recognition, calcium and the control of desmosome formation. Ciba Found Symp. 1987;125:49–65. doi: 10.1002/9780470513408.ch4. [DOI] [PubMed] [Google Scholar]
- Miller K., Mattey D., Measures H., Hopkins C., Garrod D. Localisation of the protein and glycoprotein components of bovine nasal epithelial desmosomes by immunoelectron microscopy. EMBO J. 1987 Apr;6(4):885–889. doi: 10.1002/j.1460-2075.1987.tb04834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Erickson H. P., Bennett V. Desmoplakin I and desmoplakin II. Purification and characterization. J Biol Chem. 1989 May 15;264(14):8310–8318. [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn E. J., Hobson C., Rees D. A., Magee A. I. Structure and assembly of desmosome junctions: biosynthesis, processing, and transport of the major protein and glycoprotein components in cultured epithelial cells. J Cell Biol. 1987 Jul;105(1):57–68. doi: 10.1083/jcb.105.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. J., Jones P., Davison M. D., Patel B., Eperon I. C., Critchley D. R. Isolation and characterization of a vinculin cDNA from chick-embryo fibroblasts. Biochem J. 1987 Jul 15;245(2):595–603. doi: 10.1042/bj2450595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Yoshida T., Terada M., Shimosato Y., Abe O., Hirohashi S. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989 Oct;109(4 Pt 1):1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S., Shida H., Giudice G. J., Shida M., Patel N. H., Blaschuk O. W. On the molecular organization, diversity and functions of desmosomal proteins. Ciba Found Symp. 1987;125:3–25. doi: 10.1002/9780470513408.ch2. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986 Oct;103(4):1441–1450. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Barton C. H., Putt W., Moore S. E., Kelsell D., Spurr N., Goodfellow P. N. N-cadherin gene maps to human chromosome 18 and is not linked to the E-cadherin gene. J Neurochem. 1990 Sep;55(3):805–812. doi: 10.1111/j.1471-4159.1990.tb04563.x. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Parekh R. B., Moore S. E., Dickson G., Barton C. H., Gower H. J., Dwek R. A., Rademacher T. W. Tissue specific O-linked glycosylation of the neural cell adhesion molecule (N-CAM). Development. 1989 Apr;105(4):803–811. doi: 10.1242/dev.105.4.803. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Mattey D. L., Garrod D. R. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol. 1984 Dec;99(6):2211–2215. doi: 10.1083/jcb.99.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Buck C. A., Bechtol K. B., Damsky C. H. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987 Jul;34(3):187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]