Abstract
Background
Human immunodeficiency virus (HIV) compromises the nutritional status of infected individuals and in turn, malnutrition worsens the effects of the infection itself by weakening the immune system consequently accelerating disease progression and death. However, few studies have examined the association between nutritional status at antiretroviral therapy (ART) initiation and early mortality. Therefore, this study assesses pre-ART nutritional status and other baseline characteristics and mortality among adult patients on ART at Fiche Hospital, Ethiopia.
Methods
A retrospective cohort study was conducted among 489 ART enrolled adult patients between August 01, 2006 and September 30, 2013 in Fiche Hospital. Study participants were selected by using systematic random sampling method. Actuarial table was used to estimate survival of patients after ART initiation and log rank test was used to compare the survival curves. Cox proportional-hazard regression was used to determine independent predictors of time to death.
Results
Most of the study subjects were females 254 (51.9%). A total of 489 patients were included in the analysis, of whom 87 died during a median study follow-up of 22 months. The estimated mortality among malnourished was 21, 28, 33, and 38% at 5, 10, 15, and 25 months respectively with mortality incidence density of 5.63 deaths per 100 person years. The independent predictors of mortality were: BMI <18.5 kg/m2 (AHR = 5.4 95% CI 3.03–9.58), baseline ambulatory functional status (AHR = 3.84; 95% CI 2.19–6.74), bedridden functional status (AHR = 4.78; 95% CI 2.14–10.65), WHO clinical stage III (AHR 2.21; 95% CI 1.16–4.21), WHO clinical stage IV (AHR 4.05; 95% CI 1.50–10.97) and CD4 count less than 200 cells/μl (AHR = 2.95; 95% CI 1.48–5.88), two and more opportunistic infections (AHR 2.30; 95% CI 1.11–4.75).
Conclusions
Undernutrition at the time of ART initiation was associated with increased risk of death, particularly during the first 3 months after ART initiation. Interventions to promote earlier HIV diagnosis and treatment and integrating nutrition counseling at all stages of ART implementation may improve ART outcomes in this vulnerable population.
Keywords: Antiretroviral therapy, Malnutrition, Mortality
Background
Provision of antiretroviral therapy (ART) for HIV-infected individuals is rapidly expanding in sub-Saharan Africa [1]. HIV itself increases resting energy expenditure independently of viral load, further contributing to HIV-associated weight loss. As HIV infection progresses, it can cause a catabolic state that is compounded by a lack of caloric intake, increasing the severity of preexisting undernutrition [2].
Body mass index (BMI) at ART initiation was defined as BMI below 18.5 kg/m2 indicates that a person is underweight, 18.5–24.9 kg/m2 is normal weight, and 25.0–29.9 kg/m2 is overweight and 30.0 kg/m2 or above is obese. HIV compromises the nutritional status of infected individuals and in turn, malnutrition worsens the effects of the infection itself by weakening the immune system consequently accelerating disease progression and death [3].
An improved understanding of the role of nutritional status in HIV disease progression may help in the development of strategies to reduce mortality after ART initiation. To the best of our knowledge, no previous study has examined the effect of pre-ART nutritional status on time to death in the cohort of ART clients enrolled during the specified time in the study area. This study, therefore, intends to examine the associations between nutritional status and its associated mortality among adult patients on ART. Moreover, this study results serve as baseline data for further investigations and provides input for health planners and policy makers.
Methods
Institution based retrospective cohort study was conducted in Fiche Hospital from Jan 01–31/2014. Fiche town has one zonal hospital and two health centers for the catchment area population, which provide ART service for 3937 patients enrolled. Peoples’ living with HIV/AIDS (PLWHA).
Inclusion criteria
HIV positive adults aged 18 years or older who started ART with complete intake form, registers that have been in follow-up from 2006 to 2013.
Exclusion criteria
Diagnosis is made outside of health institution (transfer in).
Loss to follow up, transfer out.
Pregnant and lactating women at the time of ART initiation.
Sample size determination
Sample size was determined using a formula for two population proportions based on the assumption that type I error 5%, power of 90% on exposure (malnourished on pre-ART treatment) and non-exposure (non-malnourished on pre-ART treatment) was taken from previous study [15].
α = level of significance, power = 1 − β = 90%, Zβ = 1.282.
The sample size was 163 for n1 (exposed group) and 326 for n2 (non-exposed group). Using proportional allocation to the malnourished and non-malnourished adult patients, a total of 489 samples taken.
Sampling technique
A cohort of antiretroviral patients who were initiated treatment between August 2006 and September 2013 were included in the study and their profiles were evaluated. After thorough evaluation, the number of HIV clients from the list that fulfills the inclusion criteria were 1228. A total of 489 Study participants were selected by using systematic random sampling method by which one random number in the Patient’s ART unique identification numbers as a starting point. The first HIV client was selected by lottery method among the first sampling intervals from the evaluated profiles (Fig. 1).
Fig. 1.
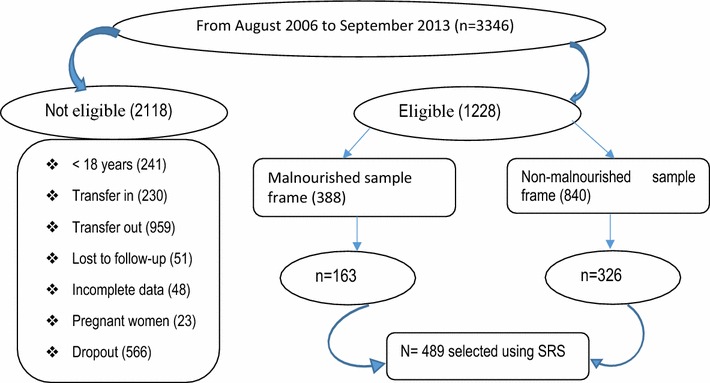
Profile of PLWHA enrolled on ART in Fiche Hospital, North Shoa, Ethiopia
Data collection methods and instruments
A data collection tool was developed from ART entry and follow-up form being used in the ART clinic. The follow-up documents was evaluated thoroughly about its completeness before data collection takes place. The data was collected by reviewing medical record registers, laboratory requests, and follow-up form of ART. The data was collected from Jan 01–31/2014. Data quality was controlled through continuous supervision and random check-up of the data collection. Three days training was given for data collectors and supervisors. Data quality control during data entry was done by double entry to EPI-info Version 3.5.3 computer software and through multivariate analysis.
Ethical approval was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of Harar campus, Haramaya University, College of Medicine and Health Sciences. Following the approval by IHRERC, official letter of ethical clearance was written to the concerned bodies by the School of Public Health. As the study was conducted through review of medical records, the individual patients were not subjected to any harm and personal identifiers were not used on data collection forms and confidentiality was maintained.
Data processing and analysis
The data were entered into Epi-Info Version 3.5.3 and then exported to SPSS Version 16.0 and STATA version 12 statistical packages for data processing and analysis. Actuarial life table analysis was used to estimate cumulative proportion of surviving after initiation of ART. Kaplan–Meier Survival function was used to estimate mortality of HIV patients on ART. The log-rank test was conducted to compare time to death between/among various levels. Before running the Cox regression model, assumption of proportional-hazard was checked by Schoenfeld test and the assumption was not violated. Cox proportional-hazard regression was used to calculate the bivariate and adjusted hazard rate to determine independent determinants (P < 0.05) of time to death.
Results
Socio-demographic characteristics
The study involved a total of 489 adults of people living with HIV/AIDS (PLWHA) on ART; 163 (33.3%) were malnourished (BMI < 18.5 kg/m2) and 326 (66.7%) were non-malnourished adults (BMI ≥ 18.5 kg/m2). Most of the study subjects were females 254 (51.9%) and males 235 (48.1%). The overall mean(±SD) age at ART initiation was 34.36 ± 9.24 years, out of which most of them 201 (41.1%) were in the age range of 18–29 years followed by 149 (30.5%) of 30–39 years (Table 1).
Table 1.
Socio-demographic characteristics of HIV-positive patients at ART initiation in Fiche Hospital, North Shoa, 2006–2013, (N = 489)
| Characteristics | Malnourished (n = 163) | Non malnourished (n = 326) | Total (N = 489) | P value |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Sex | ||||
| Male | 95 (58.3) | 140 (42.9) | 235 (48.1) | 0.002 |
| Female | 68 (41.7) | 186 (57.1) | 254 (51.9) | |
| Age groups (years) | ||||
| 18–29 | 68 (41.7) | 133 (40.8) | 201 (41.1) | 0.786 |
| 30–39 | 53 (32.5) | 96 (29.4) | 149 (30.5) | |
| 40–49 | 31 (19.0) | 69 (21.2) | 100 (20.4) | |
| 50+ | 11 (6.7) | 28 (8.6) | 39 (8.0) | |
| Marital status | ||||
| Single | 24 (14.7) | 37 (11.3) | 61 (12.5) | 0.139 |
| Married | 71 (43.6) | 179 (54.9) | 250 (51.1) | |
| Separated | 21 (12.9) | 43 (13.2) | 64 (13.1) | |
| Divorced | 22 (13.5) | 31 (9.5) | 53 (10.8) | |
| Widowed | 25 (15.3) | 36 (11.0) | 61 (12.5) | |
| Educational status | ||||
| No education | 60 (36.8) | 78 (23.9) | 138 (28.2) | 0.004 |
| Primary | 57 (35.0) | 107 (32.8) | 164 (33.5) | |
| Secondary | 36 (22.1) | 116 (35.6) | 152 (31.1) | |
| Tertiary | 10 (6.1) | 25 (7.7) | 35 (7.2) | |
P value <0.05 = statistically significant difference
Baseline clinical and laboratory information of the cohort
The baseline mean (±SD) values for BMI of the participants was 19.75 ± 2.96. The median weight at ART initiation was 51 kg [interquartile range (IQR 45–57 kg)]. The median CD4 cell count at ART initiation was 145 cells/μl (IQR 80–222). Three hundred thirty five (68.5%) of the patients had CD4 counts <200 cells/μl. The median hemoglobin level was 12.90 g/dl (IQR 10.9–14.6). Most of the study subjects at ART initiation were 220 (45%) in WHO stage III and 197 (40.3%) in WHO stage II. With regard to functional status, 197 (40.3%) participants were ambulatory at baseline and 27 (5.5%) were bedridden. Out of those who participated, 304 (62.2%) had no previous opportunistic infection, 68 (13.9%) had one previous opportunistic infection and 117 (23.9%) had two and more previous opportunistic infections (Table 2).
Table 2.
Baseline clinical characteristics of HIV patients in Fiche Hospital (N = 489)
| Characteristics | Malnourished (n = 163) | Non malnourished (n = 326) | Total (N = 489) | P value |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Functional status | ||||
| Working | 49 (30.1) | 216 (54.2) | 265 (54.2) | 0.0001 |
| Ambulatory | 94 (57.7) | 103 (31.6) | 197 (40.3) | |
| Bedridden | 20 (12.3) | 7 (2.1) | 27 (5.5) | |
| WHO clinical stage | ||||
| Stage I | 7 (4.3) | 41 (12.6) | 48 (9.8) | 0.0001 |
| Stage II | 58 (35.6) | 139 (42.6) | 197 (40.3) | |
| Stage III | 84 (51.5) | 136 (41.7) | 220 (45) | |
| Stage IV | 14 (8.6) | 10 (3.1) | 24 (4.9) | |
| TB history | ||||
| Yes | 75 (46) | 73 (22.4) | 148 (30.3) | 0.0001 |
| No | 88 (54) | 253 (77.6) | 341 (69.7) | |
| Hemoglobin count (g/dl) | ||||
| <10 | 34 (21.5) | 30 (9.7) | 64 (13.7) | 0.0001 |
| ≥10 | 124 (78.5) | 279 (90.3) | 403 (86.3) | |
| Previous OIs | ||||
| None | 86 (52.8) | 218 (66.9) | 304 (62.2) | 0.0001 |
| One | 18 (11.0) | 50 (15.3) | 68 (13.9) | |
| 2+ | 59 (36.2) | 58 (17.8) | 117 (23.9) | |
Baseline demographic and clinical characteristics and associated mortality of patients on ART
From the study subjects, the proportion of mortality is higher among the age group of 50+ years followed by 40–49 years (20.5 vs. 19%). With regard to educational status, 36 (26.1%) of the participants who had no education died after initiation of ART. The proportion of mortality is higher among males than females (19 vs. 17%) (Table 3).
Table 3.
Baseline demographic and clinical characteristics and associated mortality on ART in Fiche Hospital, Ethiopia
| Characteristics | Total | Alive n = 402 | Death n = 87 | P value |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Sex | ||||
| Male | 221 (45.2) | 179 (81) | 42 (19) | 0.604 |
| Female | 268 (54.8) | 223 (83) | 45 (17) | |
| Age groups (years) | ||||
| 18–29 | 201 (41.1) | 167 (83.1) | 34 (16.9) | 0.937 |
| 30–39 | 149 (30.5) | 123 (82.5) | 26 (17.5) | |
| 40–49 | 100 (20.4) | 81 (81) | 19 (19) | |
| 50+ | 39 (8.0) | 31 (79.5) | 8 (20.5) | |
| Nutritional status | ||||
| Malnourished | 163 (33.3) | 122 (74.8) | 41 (25.2) | 0.004 |
| Non-malnourished | 326 (66.7) | 280 (85.9) | 46 (14.1) | |
| Educational status | ||||
| No education | 138 (28.2) | 102 (73.9) | 36 (26.1) | 0.011 |
| Primary | 164 (33.5) | 138 (84.1) | 26 (15.9) | |
| Secondary | 152 (31.1) | 129 (84.9) | 23 (15.1) | |
| Tertiary | 35 (7.2) | 33 (94.3) | 2 (5.7) | |
| Functional status | ||||
| Working | 265 (54.2) | 257 (96.9) | 8 (3.1) | 0.0001 |
| Ambulatory | 197 (40.3) | 128 (64.9) | 69 (35.1) | |
| Bedridden | 27 (5.5) | 17 (62.9) | 10 (37.1) | |
| WHO clinical stage | ||||
| Stage I | 48 (9.8) | 47 (97.9) | 1 (2.1) | 0.0001 |
| Stage II | 197 (40.3) | 192 (97.5) | 5 (2.5) | |
| Stage III | 220 (45) | 149 (67.7) | 71 (32.3) | |
| Stage IV | 24 (4.9) | 14 (58.3) | 10 (41.7) | |
| Previous OIs | ||||
| None | 304 (62.2) | 289 (95.1) | 15 (4.9) | 0.0001 |
| One | 68 (13.9) | 50 (73.5) | 18 (26.5) | |
| 2+ | 117 (23.9) | 63 (53.8) | 54 (46.2) | |
| Initial ART regimen | ||||
| d4t(30)-3TC-NVP | 83 (17) | 72 (86.7) | 11 (13.3) | 0.245 |
| d4t(30)-3TC-EFV | 64 (13.1) | 51 (79.6) | 13 (20.4) | |
| AZT-3TC-NVP | 230 (47) | 193 (83.9) | 37 (16.1) | |
| AZT-3TC-EFV | 112 (22.9) | 86 (76.8) | 26 (23.2) | |
| HIV related counseling | ||||
| Yes | 141 (28.8) | 135 (95.7) | 6 (4.3) | 0.0001 |
| No | 348 (71.2) | 267 (76.7) | 81 (23.7) | |
| TB history | ||||
| Yes | 148 (30.3) | 101 (68.2) | 47 (31.8) | 0.0001 |
| No | 341 (69.7) | 301 (88.2) | 40 (11.8) | |
Survival analysis
A total of 489 HIV infected individuals were enrolled in a retrospective study for a median (IQR) of 22 (14–34) months; 17 (5–34) months among malnourished adults and 23 (17–34) months among non-malnourished adult patients. All the study subjects contributed 1545.4 person year of observation (PYO); 429.35 PYO for those who were malnourished HIV adult patients and 1116.05 PYO for non-malnourished patients. Out of the study subjects, 87 patients died during the study period giving a mortality rate of 5.63 per 100 person-year observations (87 deaths/1545.4 PYO). Of the 87 deaths, 27 (31%) occurred within the first 3 months of ART initiation and 41 (47.1%) died in the first year of follow-up. The overall estimated survival duration after ART initiation was 48 (95% CI 46.32–50.84) months.
Actuarial life table analysis showed that probability of survival time among malnourished adult ART patients was 79, 91, 93, 94, and 98% at 5, 10, 15, 20, and 30 months respectively. The probability of survival time among non-malnourished adults was 97, 99, 99 and 98% at 5, 10, 15 and 35 months respectively (Fig. 2).
Fig. 2.
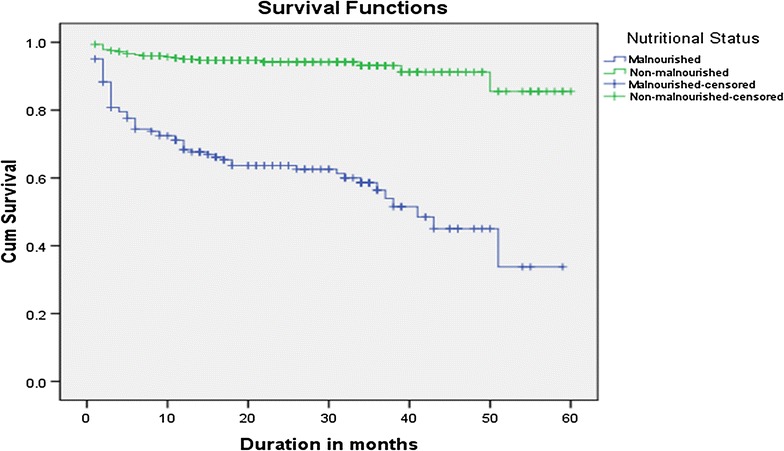
Survival graph of HIV patients at ART initiation by nutritional status in Fiche Hospital, Ethiopia
Survival time of adult HIV-positive patients at ART initiation
Kaplan–Meier analysis of patients at ART by socio-demographic characteristics
The overall estimated survival time after ART initiation using Kaplan–Meier survival analysis was 48 months (95% CI 45.32–50.68). The survival experience after initiation of ART estimated by the Kaplan–Meier survival analysis by educational status showed that there was significant difference in median survival time among the groups, no education 44 months (95% CI 40.18–48.82) and secondary education 53 months (95% CI 49.98–56.02), (log rank test X2 = 14.321, P = 0.002).
Kaplan–Meier analysis of patients at ART by baseline clinical characteristics
There was significant difference in median survival time among working functional status 54 months (95% CI 52.49–57.51), ambulatory status 41 months (95% CI 36.88–44.22) and bedridden 34 months (95% CI 24.86–43.14) (log rank test X2 = 51.976, P = 0.0001).
Predictors of mortality
After adjustment, the following characteristics at the initiation of the ART were the independent significant predictors of mortality: BMI < 18.5 kg/m2, baseline functional status (Ambulatory and Bedridden), WHO stage III and IV, CD4 cell count <200 cells/µl and opportunistic infections with two and more. Patients with a BMI <18 kg/m2 had a 5.4-fold increased risk of mortality (95% CI 3.03–9.58) as compared to those with ≥18.5 kg/m2. The hazard rate for dying is 3.84 times more with baseline functional status of ambulatory compared to patients with functional status of working (AHR = 3.84; 95% CI 2.19–6.74). Similarly, Patients with baseline functional status of bedridden had 4.78 times increased risk of death as compared to patients with baseline functional status of working (AHR = 4.78; 95% CI 2.14, 10.65). HIV-infected patients with baseline WHO clinical stage III had twofold increased risk of death compared to patients with stage I and II (AHR 2.21; 95% CI 1.16–4.21), and the risk of death among WHO clinical stage IV patients was even higher- compared to stage I or II patients (AHR 4.05; 95% CI 1.50, 10.97). Patients starting ART with CD4 count less than 200 cells/μl had threefold higher death hazard (95% CI 1.48–5.88) as compared to those starting ART with more than 200 cells/μl. Patients with two and above opportunistic infections had 2.3 times higher mortality as compared to those who had no starting opportunistic infection (AHR 2.30; 95% CI 1.11, 4.75) (Table 4).
Table 4.
Bivariate and multivariate Cox-regression analysis of socio-demographic and baseline clinical characteristics of the cohort studied in Fiche Hospital, North Shoa during September 2006 to 2013, (N = 489 patients)
| Covariates | Number at risk | Number of deaths | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Educational status | ||||
| No education | 138 | 36 | 1.86 (0.73, 4.76) | 1.35 (0.50, 3.6) |
| Primary | 164 | 26 | 1.60 (0.63, 4.10) | 2.05 (0.78, 5.36) |
| Secondary | 152 | 23 | 0.61 (0.22, 1.71) | 0.92 (0.31, 2.74) |
| Tertiary | 35 | 2 | 1 | |
| Nutritional status | ||||
| Malnourished | 163 | 41 | 7.58 (4.63, 12.39) | 5.40 (3.03, 9.58)** |
| Non-malnourished | 326 | 46 | 1 | |
| Functional status | ||||
| Working | 265 | 8 | 1 | |
| Ambulatory | 197 | 69 | 4.91 (2.91, 8.26) | 3.84 (2.19, 6.74)** |
| Bedridden | 27 | 10 | 6.86 (3.26, 14.44) | 4.78 (2.14, 10.65)** |
| WHO clinical stage | ||||
| Stage I and II | 245 | 6 | 1 | |
| Stage III | 220 | 71 | 3.1 (1.89, 5.07) | 2.21 (1.16, 4.21)* |
| Stage IV | 24 | 10 | 5.93 (2.89, 12.17) | 4.05 (1.50, 10.97)** |
| TB history | ||||
| Yes | 148 | 47 | 1.99 (1.31, 3.05) | 0.95 (0.58, 1.55) |
| No | 341 | 40 | 1 | |
| CD4 count (cells/μl) | ||||
| ≤200 | 335 | 260 | 3.44 (1.83, 6.48) | 2.95 (1.48, 5.88)* |
| >200 | 154 | 142 | 1 | |
| Hemoglobin level (n = 467) | ||||
| <10 g/dl | 64 | 18 | 1.80 (1.06, 3.04) | 0.82(0.45, 1.51) |
| ≥10 g/dl | 403 | 62 | 1 | |
| Previous OIs | ||||
| None | 304 | 15 | 1 | |
| One | 68 | 18 | 1.80 (0.99, 3.52) | 1.31 (0.69, 2.5) |
| 2+ | 117 | 54 | 3.24 (2.05, 5.13) | 2.30 (1.11, 4.75)* |
| HIV related counseling | ||||
| Yes | 141 | 6 | 1 | |
| No | 348 | 81 | 3.85 (2.44, 6.08) | 0.57 (0.26, 1.24) |
1.00 = Reference * P value <0.05, ** P value ≤0.001
Discussion
Mortality in this study was found to be 5.63/100 person years at risk with most of the deaths occurred during the first 3 months following ART initiation. This result is in agreement with the study done in Burkina Faso [4]. However, this finding was low compared to common rates in resources-limited countries [5] and higher compared to 2.03/100 persons-years in the Eastern Ethiopia and 1.89/100 persons-years in Western Ethiopia respectively [6, 7]. The high early mortality observed in our study is in line with other similar studies from resource-limited settings, including Ethiopia [5, 8–12]. This may partly be explained by the fact that the majority of patients that 68.5% patients had advanced disease (CD4 count ≤200 cells/μl) and 45% patients had advanced clinical symptoms (WHO clinical stage III) at the time of treatment initiation and might be due to delayed diagnosis and/or treatment.
This study revealed that a low baseline body mass index (BMI) at the start of ART was an independent predictor of early mortality (i.e., in the first 90 days of therapy). This is in line with studies conducted in several sub-Saharan Africa [5, 11, 13–16]. This might be as a result of the aggregate effects of malnutrition-induced immune system dysfunction, a higher burden of opportunistic infections, metabolic derangement and anthropometric variations. Even after the initiation of ART, the side effects of certain antiretroviral drugs (e.g., nausea, insomnia) may prevent adequate intake [17], and malnutrition and low body weight may potentiate drug toxicity [18].
Patients with advanced clinical diseases (WHO stage III or IV) had higher mortality compared to patients with WHO stage I or II. This finding was supported by several other studies [11, 12, 19–22]. This might be due to the fact that patients died mostly because of their late initiation of ART when they had the worst health conditions. In contrast, a study conducted in Western Ethiopia and South Western Uganda reported that WHO clinical stage was not found to be associated with mortality [7, 23].
In this study, patients with two and above opportunistic infections had 2.3 times higher mortality as compared to those who had no starting opportunistic infection. This study established a similar finding with the studies in sub-Saharan Africa that showed OIs were found to be significant predictors of death among patients under ART [11, 13, 24, 25].
Adult HIV-infected patients who were bedridden at ART initiation had higher risk of mortality compared to the patients with working functional status at treatment initiation. This result is in line with the study done in Eastern Ethiopia and those described elsewhere [6, 21, 23, 26].
Patients starting ART treatment with CD4 cell count ≤200 cells/μl was an independent predictor of mortality in this study. This finding is consistent with studies [4, 6, 12, 20, 25, 27]. Studies have substantiated the fact that low CD4 cell count, a marker of advanced immunodeficiency, was associated with opportunistic infection thereby increasing the likelihood of death [28]. This may partly be explained by the fact that the majority of patients (75.5%) had a CD4 ≤200 cells/μl, which could have made the comparison with higher CD4 counts statistically unstable.
Our study was subjected to several important limitations. Selection bias is possibly introduced due to the fact that patients with incomplete records of variables were excluded. In addition, because we could not ascertain outcomes of patients lost to follow-up, our mortality results might be an underestimation. Anthropometric measurements might not be measured or recorded correctly.
Conclusions
Undernutrition at the time of ART initiation was associated with increased risk of death, particularly during the first 3 months after ART initiation. With regard to nutritional status, there was a significant difference in median survival time between malnourished adults 35 months and non-malnourished adults 52 months. Being malnourished, late WHO stage, having low CD4 cell count, ambulatory and bedridden functional status and two and more opportunistic infections were factors independently associated with death. Interventions to promote earlier HIV diagnosis and treatment and nutrition counseling should be integrated at all stages of ART implementation, such as during adherence counseling, regular follow-up sessions, and meetings of PLWHA support groups may improve ART outcomes in this vulnerable population.
Authors’ contributions
KT conceived and designed the study, data collection, performed statistical analysis and drafted the initial manuscript. NB and KT performed the statistical analysis and revised the manuscript. HK performed the statistical analysis and initial manuscript draft. All of these authors provided critical comments for revision. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge to all staffs of Fiche hospital, particularly Ejigayehu Hailu who helped us during data collection and supervision. We would like to thank the School of Public Health of Haramaya University for the financial support. Our sincere thanks also goes to Delelegn Yilma and Leul Tadesse for their tireless, valuable support and constructive criticism throughout the thesis write up.
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to publish
Ethical approval was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of Harar campus, Haramaya University, College of Medicine and Health Sciences. Following the approval by IHRERC, official letter of ethical clearance was written to the concerned bodies by the School of Public Health. As the study was conducted through review of medical records, the individual patients were not subjected to any harm and personal identifiers were not used on data collection forms and confidentiality was maintained.
Abbreviations
- AHR
adjusted hazard rate
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- BMI
body mass index
- SRS
systematic random sampling
- CHR
crude hazard rate
- CI
confidence interval
- Hgb
hemoglobin
- HIV
human immunodeficiency virus
- HR
hazard rate
- IQR
inter quartile range
- Kg
kilogram
- OI
opportunistic infection
- PLWHA
people living with HIV/AIDS
- PYO
person-year observation
- SD
standard deviation
- TB
tuberculosis
- WHO
World Health Organization
Contributor Information
Kokeb Tesfamariam, Email: kokiadonis@gmail.com.
Negga Baraki, Email: neggabaraki@yahoo.com.
Haji Kedir, Email: hajikedir2007@yahoo.com.
References
- 1.World Health Organization . Epidemic update and health sector progress towards universal access. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836–842. doi: 10.1086/500398. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Nutritional care and support for people living with HIV/AIDS: a training course. Geneva: World Health Organization; 2009. [Google Scholar]
- 4.Poda A, Hema A, Zoungrana J, Kaboré NF. Mortality of HIV-infected patients on antiretroviral therapy in a large public cohort in West Africa, Burkina Faso: frequency and associated factors. Adv Infect Dis. 2013;3:281–289. doi: 10.4236/aid.2013.34043. [DOI] [Google Scholar]
- 5.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS ONE. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9(1):15. doi: 10.1186/1742-6405-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitiku T, Ali A, Dessie Y. Determinants of mortality among HIV positives after initiating antiretroviral therapy in Western Ethiopia: a hospital-based retrospective cohort study. ISRN AIDS. 2013 doi: 10.1155/2013/491601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, Harries AD. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS Res Treat. 2006;20(18):2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and non-death losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43(6):770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, Gundersen SG, Bruun JN. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52–60. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14(1):36–43. doi: 10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am J Clin Nutr. 2010;91(4):1138S–1142S. doi: 10.3945/ajcn.2010.28608D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV infected patients starting antiretroviral therapy. HIV Med. 2006;7(5):323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu E, Spiegelman D, Semu H. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011;204:282–290. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 17.Hardon AP, Akurut D, Comoro C, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. Geneva: World Health Organization; 2003. [Google Scholar]
- 19.Alemu AW, Sebastian MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3:5398. doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mageda K, Leyna GH, Mmbaga EJ. High initial HIV/AIDS-related mortality and-its predictors among patients on antiretroviral therapy in the Kagera Region of Tanzania: a five-year retrospective cohort study. AIDS Res Treat. 2012;2012:843598. doi: 10.1155/2012/843598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatta L, Klouman E, Deuba K, Shrestha R. Survival on antiretroviral treatment among adult HIV-infected patients in Nepal: a retrospective cohort study in far-western Region, 2006–2011. BMC Infect Dis. 2013;13:604. doi: 10.1186/1471-2334-13-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braitstein P, Brinkhof MW, Dabis F, Schechter M. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 23.Tegiste A, Eshetu W. Survival analysis of patients under chronic HIV-care and antiretroviral treatment at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2012;26(1):22–29. [Google Scholar]
- 24.Jean-François E, Ndiaye I, Thierry-Mieg M, Gueye NF. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS Care. 2006;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 25.Ketema K, Wencheko E. Survival analysis of HIV-infected patients under antiretroviral treatment at the Armed Forces General Teaching Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2012;26(3):186–192. [Google Scholar]
- 26.Abebe N, Alemu K, Asfaw T, Abajobir AA. Predictors of mortality among HIV positive adults on antiretroviral therapy in Debremarkos Referral Hospital, Northwest Ethiopia. J AIDS HIV Res. 2014;6(1):19–27. doi: 10.5897/JAHR2013.0275. [DOI] [Google Scholar]
- 27.Eyuel T, Alemayehu W. Assessment of antiretroviral treatment outcome in public hospitals, South Nations, Nationalities and Peoples Region, Ethiopia. Ethiop J Health Dev. 2011;25(2):102–109. [Google Scholar]
- 28.Ghate M, Deshpande S, Tripathy S, Godbole S, Nene M, Thakar M, Risbud A, Bollinger R, Mehendale S. Mortality in HIV infected individuals in Pune, India. Indian J Med Res. 2011;133(4):414–420. [PMC free article] [PubMed] [Google Scholar]


