Abstract
The prototype Staphylococcus aureus strain 8325-4 produces high levels of hemolysins and proteases. Recently it has been shown that this property depends on a deficiency of sigma factor B (SigB) activity controlling the activation of regulatory genes such as agr and sarA. SigB deficiency is in turn due to a mutation in the rsbU gene, which is required for posttranslational activation of SigB. The rsbU defect of strain 8325-4 has recently been repaired, and we used this strain (SH1000), along with its isogenic sigB-negative mutant, to investigate the contributions of RsbU and SigB in a murine model of septic arthritis. Intravenous inoculation with the rsbU-repaired isogenic strain SH1000 resulted in significantly more severe arthritis, weight decrease, and mortality compared to those of the parental strain 8325-4 (rsbU-negative) or the isogenic sigB-negative mutant (MJH502). SH1000 also persisted more in kidneys and joints of infected mice. Our data strongly suggest that RsbU and SigB regulate important virulence factors, thereby contributing significantly to the outcome of staphylococcal infection.
Staphylococcus aureus is a very common colonizing microorganism and is one of the major causes of human disease, ranging from mild cutaneous infection to more severe infections such as endocarditis, septic arthritis, osteomyelitis, and sepsis. The ability to cause such a range of diseases has been attributed to the large repertoire of toxins, exoenzymes, and adhesins produced by the staphylococci. These virulence factors are modulated by regulatory genes, of which two of the major determinants are agr (accessory gene regulator) and sar (staphylococcal accessory regulator). Mutation of either locus has been shown to result in attenuation of S. aureus virulence in several models of staphylococcal disease (1, 5, 8, 15). Environmental signals are transduced via agr and sarA to allow appropriate expression of virulence determinants (2). It has been proposed that stress may activate the alternative sigma factor B (SigB or σB), which in turn directly or indirectly controls several virulence genes, e.g., sarA and agr (2, 14). σB controls expression of one of the sarA promoters, and SarA in turn upregulates agr. However, SigB also exerts a negative effect on agr expression independently of SarA (10). SigB activity itself is controlled by a complex interaction of components (7). SigB is activated by RsbU, a positive regulator, which dephosphorylates RsbV and then in turn binds RsbW, an anti-sigma factor, and releases SigB (6, 7, 21, 22).
Recent studies have shown that high production of proteases is associated with low SigB activity. Indeed, S. aureus strains producing large amounts of proteases have been shown to be SigB deficient (A. K. Kanth, K. Tegmark, and S. Arvidson, unpublished data). This is the case with the laboratory strain 8325-4, which produces large amounts of proteases and hemolysins. This strain has been frequently used in assessment of staphylococcal virulence and has now been shown to have a natural defect in σB that is caused by a defect in the rsbU gene (12).
Recent studies using a mouse abscess model indicated that expression of SigB lacks any essential function with respect to virulence and pathogenicity of S. aureus (4, 7). This conclusion is further supported by the results of Nicholas et al., who did not observe any differences between the clinical isolate WCUH29 and its isogenic ΔsigB mutant in the ability to cause infection in three distinct animal models (14).
In this study we wanted to assess the role of SigB expression in a model of septic arthritis, using 8325-4 and its rsbU-repaired mutant, which then acts as a functional RsbU strain, as well as a sigB-negative mutant (MJH502) derived from the RsbU-repaired strain (SH1000). Our results indicate that the rsbU-repaired mutant has significantly increased virulence compared to the 8325-4 parental strain and that sigB mutation attenuates the virulence almost back to 8325-4 level.
MATERIALS AND METHODS
Mice.
Female NMRI mice, 6 to 8 weeks old, were purchased from B&K Universal AB (Sollentuna, Sweden) and maintained in the animal facility of the University of Göteborg.
Bacterial strains.
A functional rsbU strain (SH1000) was constructed from the laboratory strain 8325-4, (10). A sigB-negative mutant (MJH502) was constructed from the SH1000 strain (10). Before the experiments, bacteria were cultured on blood agar plates for 24 h and then reincubated on blood agar for another 24 h. The bacteria were then harvested and kept frozen at −20°C in phosphate-buffered saline (PBS) containing 5% bovine serum albumin and 10% dimethyl sulfoxide. Before administration, the bacterial solutions were thawed, washed in PBS, and adjusted to the appropriate inoculum concentration. The mice were inoculated intravenously in the tail vein with 0.2 ml of the solution. Viable counts were always used to check the numbers of bacteria (CFU) for each inoculation procedure.
Murine infection.
Four independent in vivo experiments were done with declining doses of bacteria (4 × 107, 1 × 107, 6 × 106, and 3 × 106 staphylococci per mouse) of S. aureus strain 8325-4 or its isogenic rsbU-repaired strain, SH1000. Two experiments using an intravenous (i.v.) dose of 3 × 106 to 4 × 106 MJH502 (sigB) staphylococci (n = 10/group) were also done. The mice were monitored for up to 14 days and then sacrificed. Development of arthritis, general appearance, and weight decrease, as well as mortality, were registered during the experiments. All four limbs were analyzed histologically, and the kidneys were assessed for staphylococcal persistence. In addition, sera were obtained for cytokine analyzes.
In one of the experiments with inoculation of strains 8325-4 and SH1000, 5 mice per group were sacrificed at day 3 and the remaining 10 mice per group were sacrificed at day 14 after the bacterial inoculation. In an additional in vivo experiment, the mice were inoculated with a mixture of equal amounts (2 × 106 CFU of each strain per mouse) of the two isogenic strains 8325-4 and SH1000. The mice were sacrificed after 3 and 7 days, respectively. Bacterial cultures were obtained from the kidneys and all four paws (wrist and ankle joints). Another 30 mice were subdivided into three groups and injected intra-articularly in the left knee joint with 2.3 × 104 staphylococci of either of the isogenic strains. Animals were killed 3 days after the injection, and the joints were examined histologically for signs of inflammation.
Clinical and histological evaluation of arthritis.
All of the mice were observed individually by an observer (I.-M.J.) blinded to identity of the groups. Limbs were inspected visually at regular intervals. Arthritis was defined as visible erythema and/or swelling of at least one joint. The intensity of arthritis was evaluated by using a scoring system of 0 to 3 points for each limb (1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema). The arthritic index was constructed by adding the scores from all limbs for each animal. Histopathological evaluation was performed after routine paraformaldehyde fixation, decalcification, and paraffin embedding. Tissue sections were prepared and stained with hematoxylin and eosin. The sections were studied by a blinded observer (I.-M.J.) with regard to synovial hypertrophy, which was defined as a synovial membrane thickness of more than two cell layers (3), and cartilage and bone destruction. Histological scoring was based upon the degree of synovial hypertrophy and degradation of cartilage and/or bone. Scores were 1 for mild, 2 for moderate, and 3 for severe synovial hypertrophy and joint damage. The most affected joint was chosen to represent the score of that particular animal.
Bacteriological examination of infected animals.
The kidneys were cut out aseptically, homogenized, and diluted to appropriate concentrations in PBS. One hundred microliters of the suspension was evenly spread on agar plates and cultured for 24 h, and the numbers of CFU were counted. In the coinoculation experiment, the talocrural and radiocarpal joints also were dissected aseptically, and bacterial samples were obtained with cotton sticks and cultured on agar plates. An isolate was considered positive when ≥15 staphylococcal colonies were found. The identity of isogenic strains was judged visually, since strain SH1000 displays high intensities of yellow pigmentation compared to 8325-4 and MJH502. Throughout the experiments SH1000 was entirely stable as judged by maintenance of yellow coloration.
Analysis of IL-6.
Serum samples were used to analyze circulating interleukin-6 (IL-6) levels. The murine hybridoma cell line B9, which is dependent on IL-6 for growth, was used as an indicator to determine the levels of IL-6 in serum (3, 9)
Statistical analyzes.
Statistical evaluation was done by using the Mann-Whitney U test or the chi-square test with Yates correction. Results are reported as means ± standard errors of the means or as median and interquartile range (IQR).
RESULTS
Clinical course of S. aureus infection.
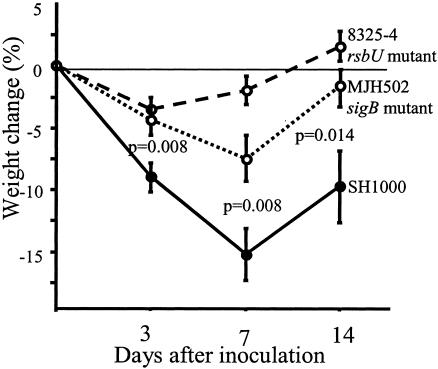
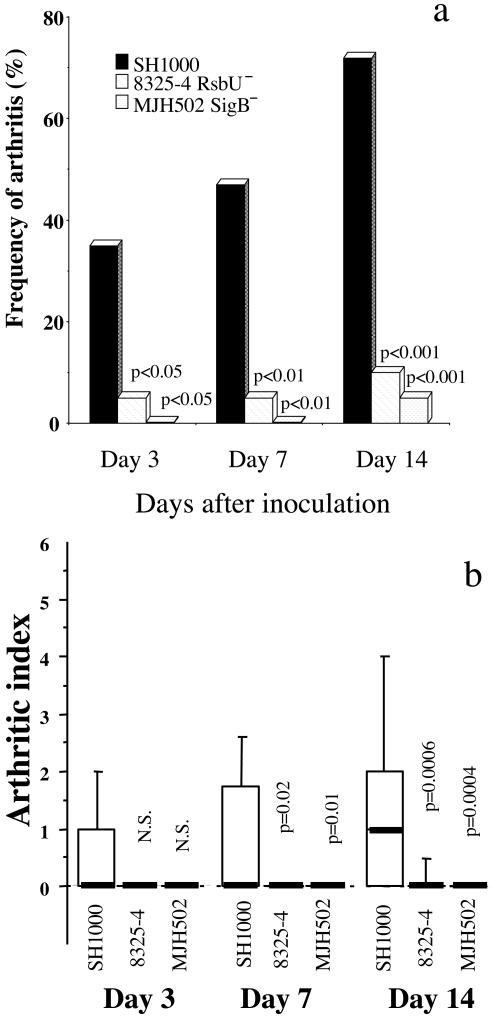
In the first three in vivo experiments with doses of staphylococci ranging from 6 × 106 to 4 × 107 per mouse, the mortality was strikingly increased in mice inoculated with the rsbU+ strain, SH1000, compared to its parental strain, 8325-4. Indeed, an inoculation dose of 107 staphylococci/mouse led to 60% mortality in the SH1000 group, whereas all of the 8425-4 mice survived (P < 0.02) within 10 days following inoculation. Also, inoculation with approximately half of this dose of staphylococci (6 × 106) led to a significantly higher mortality in the SH1000-inoculated group (6 out of 9 mice) than in the 8325-4-inoculated group (1 out of 10) (P < 0.05) within 1 week after bacterial inoculation. Therefore, the inoculation dose was further lowered, to 3 × 106 CFU/mouse, and 15 mice in each group were inoculated i.v. After 3 days, five mice per group were sacrificed, and the remaining were monitored for up to 14 days. With this dose of infection, all animals survived. However, mice inoculated with the SH1000 strain had a significantly more pronounced weight loss than the 8325-4-inoculated mice. At day 7 after inoculation, the SH1000 inoculated mice had lost 9.4% ± 2.7% of their initial weight, whereas mice inoculated with strain 8325-4 had gained weight (0.6% ± 1.6%) (P = 0.005). The frequency of clinical signs of arthritis was low in both groups. Only 3 out of 10 mice inoculated with SH1000 and 1 out of 10 mice inoculated with 8325-4 showed clinical signs of arthritis. Thus, RsbU activity is required for expression of full virulence. To further elucidate the role of SigB as a virulence factor in the staphylococcal arthritis model, mice (20 per group) were inoculated with the sigB knockout mutant MJH502 (3 × 106 to 4 × 106 CFU/mouse), SH1000 (sigB+), and 8325-4 (rsbU mutant). Only one mouse inoculated with MJH502 (sigB) and two animals inoculated with SH1000 died, compared to none inoculated with 8325-4. The weight decrease was again more pronounced in the SH1000-inoculated group than in the 8325-4-inoculated group; the MJH502 (sigB mutant)-inoculated group displayed an intermediate weight decrease (Fig. 1). Fourteen days after bacterial inoculation, the frequency of arthritis reached 72% in the SH1000-inoculated group, compared to 10 and 5%, respectively, in the 8325-4- and MJH502 (sigB)-inoculated groups (P < 0.001) (Fig. 2a). The severity of arthritis was also much more pronounced in the SH1000 inoculated mice than in any of the other two groups (P < 0.02 at day 7 and P < 0.001 at day 14) (Fig. 2b).
FIG. 1.
Changes of body weight in NMRI mice inoculated with 3 × 106 to 4 × 106 CFU of S. aureus 8325-4 (rsbU), SH1000 (rsbU+), or MJH502 (sigB mutant). Data from two experiments are pooled (n = 20 per group). Data shown are means ± standard errors of the means. Comparisons were made by using the Mann-Whitney U test.
FIG. 2.
(a) Frequency of arthritis in mice inoculated with 3 × 106 to 4 × 106 CFU of S. aureus 8325-4 (rsbU mutant), SH1000 (rsbU+), or MJH502 (sigB mutant) (derived from SH1000). Data from two experiments are pooled (n = 20). Statistical comparison between SH1000 and 8325-4 or MJH502 was done by the chi-square test with Yates correction. (b) Severity of arthritis in mice inoculated with 3 × 106 to 4 × 106 CFU of S. aureus 8325-4 (rsbU mutant), SH1000 (rsbU+), or MJH502 (sigB mutant) (derived from SH1000). Data from two experiments are pooled (n = 20). 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema. The scores from all limbs for each animal were added. Data are medians (bold line) and IQRs for each group of mice. Statistical comparison between SH1000 and 8325-4 or MJH502 was done by using the Mann-Whitney U test.
Histopathological analyses of staphylococcal joint infection.
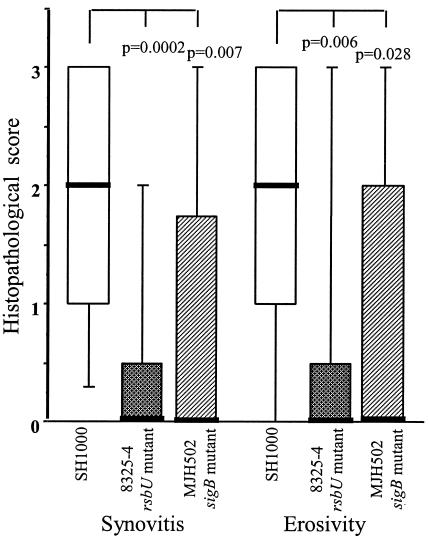
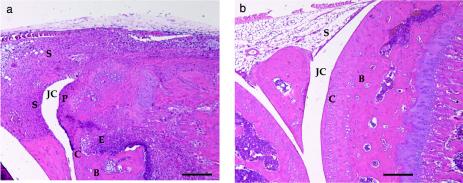
In the experiment with an inoculum of 3 × 106 strain 8325-4 and SH1000 staphylococci per mouse, all four limbs were subjected to histopathological examination. At day 3 after the inoculation, two mice out of five in the SH1000 group and none out of five in the 8325-4 group displayed signs of mild synovitis and erosion of bone and/or cartilage. Fourteen days after the inoculation, the SH1000-inoculated mice had signs of a significantly (P = 0.0046) more severe disease, since all but one mouse (9 of 10) had signs of synovitis and/or erosion. In contrast, only 2 out of 10 mice inoculated with 8325-4 showed signs of synovitis, and one of those also displayed some erosion of cartilage and bone (data not shown). Similar results were obtained in the subsequent experiments in which MJH502 (sigB mutant) was used. Nearly all (16 out of 18) mice inoculated with SH1000 showed signs of severe synovitis and erosion of bone and/or cartilage, and 7 of 19 mice in MJH502 (sigB mutant) group and 5 of 20 in the 8325-4 group displayed histopathological alterations (Fig. 3). Figure 4a shows a micrograph of a knee joint from a mouse inoculated with strain SH1000, displaying signs of severe inflammation of the synovia, pannus formation, and erosion of bone and cartilage. Figure 4b represents a knee joint from a mouse inoculated with MJH502, displaying no signs of inflammation.
FIG. 3.
Histopathological signs of arthritis in mice 14 days after inoculation with 3 × 106 to 4 × 106 CFU of S. aureus wild-type strain 8325-4 (rsbU mutant), SH1000 (rsbU+), or MJH502 (sigB mutant). Data from two experiments are pooled (n = 20, 1,8, and 19, respectively). A scoring system based on the degree of synovial hypertrophy and degradation of cartilage and/or bone was used: 1, mild synovial hypertrophy and joint damage; 2, moderate damage; 3, severe damage. Data are medians (bold line) and IQRs for each group of mice. Comparisons with strain SH1000 were made by using the Mann-Whitney U test.
FIG. 4.
(a) Micrograph showing the knee joint from a NMRI mouse 14 days after inoculation with 4 × 106 CFU of strain SH1000, displaying severe inflammation of synovial tissue and bone and cartilage erosion. (b) Micrograph showing an apparently intact knee joint from a mouse inoculated with the same dose of MJH502 (sigB mutant). JC, joint cavity; C, cartilage; S, synovial tissue; E, erosion of bone and/or cartilage; P, pannus formation. Bar, 200 μm. Hematoxylin and eosin staining was used.
In order to assess whether the decreased arthritogenicity of 8325-4 (rsbU mutant) and MJH502 (sigB mutant) is due to increased elimination of staphylococci before they reach the joint cavity or whether it is caused by the decreased virulence of staphylococci once in situ, the mice received intra-articular rather than i.v. injection of S. aureus, thereby bypassing the requirement of systemic spread of staphylococci. In this case we were unable to find any differences between the strains in their ability to cause inflammation. Indeed, 40 to 50% of injected mice developed inflammation in joints irrespective of the strain inoculated (data not shown).
Staphylococcal persistence in host tissue.
A coinoculation experiment indicated a pronounced ability of SH1000 to persist in kidneys. Indeed, 3 days after the inoculation, SH1000 almost outgrow 8325-4 and showed growth in 9 out of 10 mice, with a median of 6 × 106 CFU (IQR, 5 × 101 to 31 × 106). In contrast, only one of the mice had growth solely of strain 8325-4 and another had both strains in the kidneys (median of 0 CFU; IQR, 0 to 0). At 7 days postinoculation, all mice harbored SH1000 in their kidneys (median CFU of 1 × 108; IQR, 4 × 103 to 6 × 108). Four of the mice had also minor growth of 8325-4 in their kidneys, the median CFU for this strain was 0 (IQR, 0 × 103 to 1 × 103) (data not shown). In the coinoculation experiments, cultures were also obtained from the joints. At 3 days after i.v. inoculation of 2 × 106 CFU of each strain, only one mouse had staphylococci (≥15 colonies) of the SH1000 phenotype in one joint. At day 7, 5 out of 10 mice had SH1000, 1 mouse had 8325-4, and 1 mouse had both strains in equal numbers.
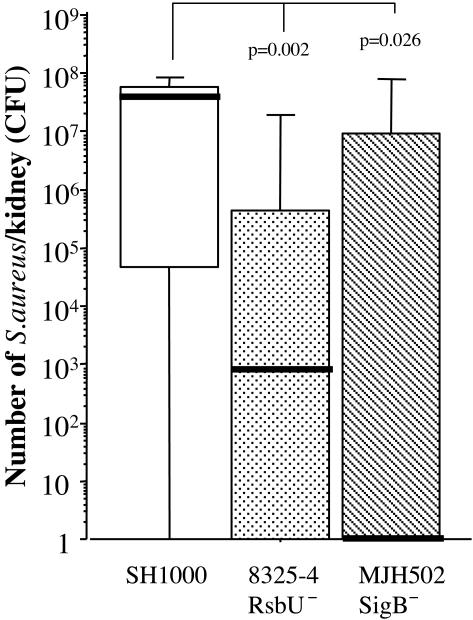
Culture samples were taken from the kidneys on days 3 and 14 after inoculation with 3 × 106 staphylococci per mouse. Mice inoculated with 8325-4 harbored 16.5 × 103 (IQR, 0 × 103 to 376 × 103; n = 5) bacteria in the kidneys, and mice inoculated with SH1000 had a median CFU of 7.8 × 105 (IQR,18 × 103 to 14 × 106; n = 5 [not significant]) 3 days after inoculation. Fourteen days after inoculation, the growth of staphylococci in kidneys was significantly higher in the SH1000-inoculated mice; the median CFU was 3 × 107 (IQR, 4 × 105 to 1.2 × 108; n = 10) in this group, compared with 5 × 102 (IQR, 5 × 101 to 2.6 × 104; n = 10 [P = 0.0126]) in the 8325-4-inoculated mice. Similar results to were also obtained with MJH502 (sigB mutant) (Fig. 5).
FIG. 5.
Persistence of S. aureus in kidneys 14 days following inoculation with 3 × 106 to 4 × 106 CFU of strain 8325-4 (rsbU mutant), SH1000 (rsbU+), or MJH502 (sigB mutant). Data from two experiments are pooled (n = 20, 18, and 19, respectively). The kidneys were homogenized and diluted to appropriate concentrations before being spread on agar plates and cultured for 24 h. The number of CFU was counted. Data are medians (bold line) and IQRs for each group of mice. Comparisons with strain SH1000 were made by using the Mann-Whitney U test.
Inflammatory response in infected animals.
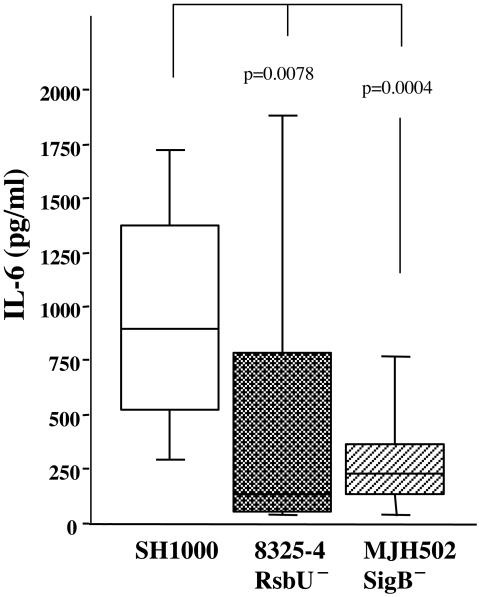
Levels of IL-6, a marker of the inflammatory response, in serum were higher in the mice inoculated with strain SH1000 than in mice inoculated with strain 8325-4. On day 3 after inoculation, the median level of IL-6 was 325 pg/ml (IQR, 272 to 10,262 pg/ml; n = 5) for SH1000-inoculated mice and 170 pg/ml (IQR, 117 to 547 pg/ml; n = 5) for 8325-4-inoculated mice (not significant). At day 14 after bacterial inoculation, the differences in IL-6 levels were even more pronounced; i.e., the SH1000-inoculated mice had a median level of 300 pg/ml (IQR, 245 to 355; n = 10), compared to 130 pg/ml (IQR, 115 to 200; n = 10) for the 8325-4-inoculated mice (P = 0.028). In the two sets of in vivo experiments where the sigB mutant (MJH502) was also inoculated, mice receiving MJH502 displayed significantly lower levels of IL-6 in serum (P = 0.0004) than those inoculated with the parental strain SH1000, 14 days after inoculation (Fig. 6)
FIG. 6.
Levels of IL-6 in serum in mice inoculated with 4 × 106 CFU of strain 8325-4 (rsbU mutant), SH1000 (rsbU+), or MJH502 (sigB mutant). Data from two experiments are pooled (n = 20, 18, and 19, respectively). Data are medians and IQRs for each group of mice. Comparisons with SH1000 were made by using the Mann-Whitney U test.
DISCUSSION
The emergence of antibiotic resistance, even resistance to newly introduced drugs, among S. aureus strains calls for the development of alternative methods for treating staphylococcal diseases. Knowledge about the mechanisms by which pathogenic bacteria cause disease is a prerequisite for finding new molecular targets associated with infection.
The purpose of this study was to analyze the role of SigB as a virulence factor in a murine model of septic arthritis. Previous studies using SigB-negative mutants had failed to prove any role of SigB in the pathogenesis of several staphylococcal diseases (14). Since the laboratory strain 8325-4 is partially SigB deficient due to a natural rsbU mutation, we wanted to compare the virulence of this strain with the virulence of an isogenic strain with a functional rsbU gene (SH1000) (10), using the model of hematogenously mediated septic arthritis and sepsis (3, 20). We report here that mice inoculated with the rsbU+ strain (SH1000) had a significantly more severe arthritis than mice inoculated with rsbU- or sigB-negative strains. The SH1000-inoculated mice also showed signs of a more severe infection, as indicated by increased mortality, a significantly more pronounced weight decrease (Fig. 1), and higher levels of the proinflammatory cytokine IL-6 in blood (Fig. 6).
Earlier in vivo studies have failed to demonstrate any sigB contribution to staphylococcal virulence in murine models of abscess formation and pyelonephritis (4, 10, 14). What are the mechanisms underlying the increased virulence of the rsbU+ strain SH1000 in the mouse arthritis model? SigB has a pleiotropic effect, influencing expression of several secreted toxins and enzymes, cell surface proteins, and biofilm. Production of toxins and enzymes seems to be suppressed by SigB (10, 23), whereas production of cell surface proteins such as clumping factor, fibronectin-binding protein, and coagulase is stimulated by SigB (14, 18). In the septic arthritis and sepsis model, adhesion molecules such as collagen adhesin (17) and clumping factor (11) are crucial virulence factors, and vaccination with either of the molecules markedly reduces severity of the disease (11, 16). Together with the results of the present study, this suggests that RsbU (SigB) acts as a virulence determinant in septic arthritis by increasing expression of bacterial adhesins. The fact that the rsbU-negative strain was even more attenuated then the sigB-negative strain indicates some yet-unknown additional role of RsbU other than activation of SigB. Moreover, direct intra-articular injection of any of the isogenic staphylococcal strains did not show any significant differences in virulence between the strains, suggesting that SigB or RsbU influences the ability of S. aureus to survive in the bloodstream and reach the joints rather that the ability to multiply in the joint and cause inflammation.
SigB is involved in the capacity of staphylococci to be internalized by osteoblasts, which are potentially used by the staphylococci in order to evade host defense and could account for their persistence (13, 19). Interestingly, SH1000 (sigB+) persisted and proliferated significantly more in kidneys than the rsbU and sigB mutant strains, as shown in Fig. 5. This was reflected by the persistence of SH1000 (rsbU+) over and above 8325-4 (rsbU mutant) in the coinoculation study, both in kidneys and in joints.
In summary, mice inoculated with S. aureus with an intact sigB locus displayed a more severe infection, as shown by significantly higher mortality, more severe histologically verified arthritis, weight decrease, and higher levels of the proinflammatory cytokine IL-6. This strain also showed increased persistence in kidneys and joints of inoculated mice. Thus, RsbU and SigB have roles in the regulation of virulence determinant production, which is important in a mouse model of septic arthritis. This alludes to the complexity of the regulatory circuits controlling virulence determinant production in response to specific host signals.
Acknowledgments
We thank Berit Ertman-Ericsson and Margareta Verdrengh for excellent technical assistance.
This work was supported by grants from the Gothenburg Medical Society, the Swedish Association against Rheumatism, the King Gustav V 80 Years Foundation, the Rune and Ulla Amlövs Foundation, the Inflammation Network, the Infection and Vaccination Network, the Nanna Svartz Foundation, the Swedish Research Council, the Börje Dahlin Foundation, the EU, and The University of Gothenburg.
Editor: J. T. Barbieri
REFERENCES
- 1.Abdelnour, A., S. Arvidson, T. Bremell, C. Rydén, and A. Tarkowski. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 61:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidson, S., and K. Tegmark. 2001. Regulation of virulence determinants in Staphylococcus aureus. Int. J. Med. Microbiol. 291:159-170. [DOI] [PubMed] [Google Scholar]
- 3.Bremell, T., A. Abdelnour, and A. Tarkowski. 1992. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 60:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, A. L., M. R. Yeaman, P. M. Sullam, M. D. Witt, and A. S. Bayer. 1994. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect. Immun. 62:1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (Agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helle, M., L. Boeije, and L. Aarden. 1988. Functional discrimination between interleukin 6 and interleukin 1. Eur. J. Immunol. 18:1525-1540. [DOI] [PubMed] [Google Scholar]
- 10.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josefsson, E., O. Hartford, L. O′Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 12.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair, S. P., M. Bischoff, M. M. Senn, and B. Berger-Bächi. 2003. The sigma B regulon influences internalization of Staphylococcus aureus by osteoblasts. Infect. Immun. 71:4167-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas, R. O., T. Li, D. McDevitt, A. Marra, S. Sucoloski, P. L. Demarsh, and D. R. Gentry. 1999. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect. Immun. 67:3667-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson, I.-M., T. Bremell, C. Rydén, A. L. Cheung, and A. Tarkowski. 1996. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect. Immun. 64:4438-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson, I.-M., J. M. Patti, T. Bremell, M. Hook, and A. Tarkowski. 1998. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J. Clin. Investig. 101:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patti, J. M., T. Bremell, D. Krajewska-Pietrasik, A. Abdelnour, A. Tarkowski, C. Rydén, and M. Höök. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor sigma(B) is involved in regulation of biofilm expression in Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheagren, J. N. 1988. Inflammation induced by Staphylococcus aureus, p. 829-840. In J. I. Gallin, I. M. Goldstein, and R. Snyderman (ed.), Inflammation: basic principles and clinical correlates. Raven Press, New York, N.Y.
- 20.Tarkowski, A., L. V. Collins, I. Gjertsson, O. H. Hultgren, I.-M. Jonsson, E. Sakiniene, and M. Verdrengh. 2001. Model systems: modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol. 9:321-326. [DOI] [PubMed] [Google Scholar]
- 21.Voelker, U., A. Dufour, and W. G. Haldenwang. 1995. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J. Bacteriol. 177:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziebandt, A., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and σB. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]