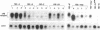Abstract
Erythrocyte development in mammals depends in part upon the interaction of the glycopeptide hormone erythropoietin (EPO) with cell surface receptors on committed erythroid progenitor cells. Both this factor and an EPO receptor polypeptide previously have been cloned, yet little is presently understood concerning molecular mechanisms of receptor activation and signal transduction. To identify cytosolic receptor domains necessary for signaling, we have compared the activities of a series of deletionally mutated EPO receptor constructs by their expression in interleukin 3-dependent, myeloid FDC-P1 cells. EPO-induced growth was transduced efficiently in these cells by the full-length receptor (507 amino acids), and no measurable loss in activity resulted from the deletion of up to 111 carboxyl-terminal residues. In contrast, the deletion of 44 additional residues led to a dramatic loss (86.3% +/- 7.8%; mean +/- SD) in the ability of this receptor to mediate EPO-induced growth, thus indicating that residues between Gly-352 and Met-396 constitute a functionally critical cytosolic subdomain. Interestingly, the expression of full-length EPO receptors in FDC-P1 cells also led to a selective inhibition of normal proliferative responsiveness to the alternative hematopoietic factor granulocyte-macrophage colony-stimulating factor. Moreover, this inhibition was progressively reversed in forms of the EPO receptor in which distal cytosolic residues were sequentially deleted. These results suggest that EPO receptors normally may trans-modulate components in the pathway of granulocyte-macrophage colony-stimulating factor-induced proliferation and that this down-modulation, as exerted by intact EPO receptors, may play a role in promoting erythroid commitment during myeloid blood cell development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Frapier C., Mosthaf L., McKeon C., Elbein S. C., Permutt M. A., Ramos E., Lander E., Ullrich A., Taylor S. I. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989 Sep;8(9):2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussios T., Bertles J. F., Goldwasser E. Erythropoietin. Receptor characteristics during the ontogeny of hamster yolk sac erythroid cells. J Biol Chem. 1989 Sep 25;264(27):16017–16021. [PubMed] [Google Scholar]
- Broudy V. C., Lin N., Egrie J., de Haën C., Weiss T., Papayannopoulou T., Adamson J. W. Identification of the receptor for erythropoietin on human and murine erythroleukemia cells and modulation by phorbol ester and dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6513–6517. doi: 10.1073/pnas.85.17.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
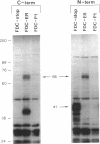
- D'Andrea A. D., Zon L. I. Erythropoietin receptor. Subunit structure and activation. J Clin Invest. 1990 Sep;86(3):681–687. doi: 10.1172/JCI114763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. D., Jarvis J. H., Greenman J. M. A quantitative bioassay for erythropoietin using mouse fetal liver cells. Exp Hematol. 1975 Jan;3(1):65–78. [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Linnekin D., Farrar W. L. Comparative analysis of IL-2 and IL-3 induced tyrosine phosphorylation. Lymphokine Res. 1989 Fall;8(3):215–224. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliniak B. C., Rohrschneider L. R. Expression of the M-CSF receptor is controlled posttranscriptionally by the dominant actions of GM-CSF or multi-CSF. Cell. 1990 Nov 30;63(5):1073–1083. doi: 10.1016/0092-8674(90)90510-l. [DOI] [PubMed] [Google Scholar]
- Harada N., Castle B. E., Gorman D. M., Itoh N., Schreurs J., Barrett R. L., Howard M., Miyajima A. Expression cloning of a cDNA encoding the murine interleukin 4 receptor based on ligand binding. Proc Natl Acad Sci U S A. 1990 Feb;87(3):857–861. doi: 10.1073/pnas.87.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Patil N., Thiel B., Chao M. V. Deletion of cytoplasmic sequences of the nerve growth factor receptor leads to loss of high affinity ligand binding. J Biol Chem. 1990 Jun 15;265(17):9595–9598. [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Jones S. S., D'Andrea A. D., Haines L. L., Wong G. G. Human erythropoietin receptor: cloning, expression, and biologic characterization. Blood. 1990 Jul 1;76(1):31–35. [PubMed] [Google Scholar]
- KRANTZ S. B., GALLIEN-LARTIGUE O., GOLDWASSER E. THE EFFECT OF ERYTHROPOIETIN UPON HEME SYNTHESIS BY MARROW CELLS IN VITRO. J Biol Chem. 1963 Dec;238:4085–4090. [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Accili D., Taylor S. I. Substitution of lysine for asparagine at position 15 in the alpha-subunit of the human insulin receptor. A mutation that impairs transport of receptors to the cell surface and decreases the affinity of insulin binding. J Biol Chem. 1990 Nov 5;265(31):19143–19150. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kreider B. L., Phillips P. D., Prystowsky M. B., Shirsat N., Pierce J. H., Tushinski R., Rovera G. Induction of the granulocyte-macrophage colony-stimulating factor (CSF) receptor by granulocyte CSF increases the differentiative options of a murine hematopoietic progenitor cell. Mol Cell Biol. 1990 Sep;10(9):4846–4853. doi: 10.1128/mcb.10.9.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol. 1983 Aug;11(7):649–660. [PubMed] [Google Scholar]
- Landschulz K. T., Noyes A. N., Rogers O., Boyer S. H. Erythropoietin receptors on murine erythroid colony-forming units: natural history. Blood. 1989 May 1;73(6):1476–1486. [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Farrar W. L. Signal transduction of human interleukin 3 and granulocyte-macrophage colony-stimulating factor through serine and tyrosine phosphorylation. Biochem J. 1990 Oct 15;271(2):317–324. doi: 10.1042/bj2710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- McCaffery P. J., Fraser J. K., Lin F. K., Berridge M. V. Subunit structure of the erythropoietin receptor. J Biol Chem. 1989 Jun 25;264(18):10507–10512. [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Nigon V., Godet J. Genetic and morphogenetic factors in hemoglobin synthesis during higher vertebrate development: an approach to cell differentiation mechanisms. Int Rev Cytol. 1976;46:79–176. doi: 10.1016/s0074-7696(08)60991-2. [DOI] [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Goldwasser E. Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem. 1987 Apr 25;262(12):5554–5562. [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Luna J. Identification of the receptor for erythropoietin by cross-linking to Friend virus-infected erythroid cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3690–3694. doi: 10.1073/pnas.84.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Sawada K. Receptors for erythropoietin in mouse and human erythroid cells and placenta. Blood. 1989 Jul;74(1):103–109. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Walker F., Nicola N. A., Metcalf D., Burgess A. W. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985 Nov;43(1):269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]
- Winkelmann J. C., Penny L. A., Deaven L. L., Forget B. G., Jenkins R. B. The gene for the human erythropoietin receptor: analysis of the coding sequence and assignment to chromosome 19p. Blood. 1990 Jul 1;76(1):24–30. [PubMed] [Google Scholar]
- Wojchowski D. M., Caslake L. Biotinylated recombinant human erythropoietins: bioactivity and utility as receptor ligand. Blood. 1989 Aug 15;74(3):952–958. [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]