Abstract
Cathepsin G (CatG) is a serine protease found in the azurophilic granules of monocytes that is known to have antimicrobial properties, but its role in Mycobacterium tuberculosis infection is unknown. We found that M. tuberculosis infection of human THP-1 monocytic cells induced the down-regulation of CatG mRNA expression, as demonstrated by gene array analysis and reverse transcription-PCR. This was associated with a concomitant decrease in CatG protein and enzymatic activity. In contrast, the expression of lysosomal cathepsins B and D was up-regulated in infected cells. This effect was also observed when THP-1 cells were induced to differentiate into adherent macrophages by exposure to bacterial lipopolysaccharide (LPS). In agreement with this, CatG expression was null in adherent macrophages isolated from bronchoalveolar lavages and normal blood. We wanted to determine if the down-regulation of CatG would be relevant to M. tuberculosis infection. First, we found that addition of CatG to THP-1 cells prior to infection resulted in decreased bacillary viability, presumably due to extracellular killing of bacilli. However, pretreatment of cells with LPS, which decreases intracellular CatG expression, resulted in increased bacillary viability. Second, we found that CatG cationic peptides killed M. tuberculosis bacilli and were five- to sevenfold more bactericidal than full-length CatG. These observations suggest that M. tuberculosis infection of human monocytic cells results in a “cathepsin switch” with down-regulation of CatG rendering M. tuberculosis bacilli more viable. Therefore, the down-regulation of CatG in macrophages is advantageous to M. tuberculosis bacilli and possibly is an important mechanism by which M. tuberculosis is able to evade the host immune defenses.
Tuberculosis (TB) is the leading cause of death worldwide due to an infectious disease, with an estimated 3 million deaths per year (4, 12). The causative agent of TB, Mycobacterium tuberculosis, is an intracellular pathogen that is highly adapted to infect and persist within mammalian tissues. Primary infection occurs when aerosol-droplet nuclei containing a small number of bacilli are deposited in the alveoli of the lung and subsequently phagocytosed by alveolar macrophages. During these early stages of infection, a key determinant of virulence is the ability of the tubercle bacillus to enter and replicate within the phagosome of phagocytic cells, thereby evading the natural host defense mechanisms (24). In this contest for survival between host and pathogen, complex cell-mediated immune responses are elicited that can lead to the formation of caseating granulomas followed by tissue destruction with liquefaction and cavity formation (9).
Early studies have indicated that cellular hypersensitivity responses are responsible for the massive caseous tissue necrosis and liquefaction observed during M. tuberculosis infection (8). Proteolytic damage by macrophage-secreted proteases has been implicated in the pathophysiology of disease, since macrophages are the predominant cell in TB granulomas and contain a large number of proteases capable of degrading the extracellular matrix (ECM) (8, 19). These studies suggest that upon entering the lung, M. tuberculosis elicits a host response characterized by the exaggerated expression and secretion of proteolytic enzymes capable of tissue destruction. Our work has been directed to the identification of matrix-degrading enzymes that could play significant roles in lung tissue remodeling responses during pulmonary TB, the processes involved in extracellular matrix (ECM) expression, deposition, and degradation.
In an effort to gain further insight into the mechanisms of tissue remodeling responses during tuberculosis infection, we conducted gene array analysis to identify host genes differentially regulated in macrophages after M. tuberculosis infection. We found that M. tuberculosis infection of THP-1 monocytes results in the differential expression of genes coding for several lysosomal proteases, integrins, matrix, and cell adhesion molecules (CAMs). Among these genes, we found that lysosomal cathepsins are differentially regulated during infection. In particular, the expression and activity of CatG, a neutral serine protease with known antimicrobial activity (34), was decreased in THP-1 monocytes after M. tuberculosis infection and exposure to lipopolysaccharide (LPS) endotoxin, while the expression of acidic cathepsins B and D was increased. We demonstrate that the down-regulation or inhibition of CatG expression in THP-1 cells coincided with increased M. tuberculosis replication and intracellular survival. In addition, exposure of bacilli to purified CatG and derived synthetic peptides results in decreased survival. Based on these observations, we speculate that CatG down-regulation in macrophages is advantageous to M. tuberculosis replication and an important mechanism for survival of M. tuberculosis bacilli in human macrophages.
MATERIALS AND METHODS
Bacterial cultures and growth conditions.
The M. tuberculosis laboratory strains H37Ra (ATCC 25177), H37Rv (ATCC 27294), and Erdman (ATCC 35801) were obtained from the American Type Culture Collection (ATCC, Manassas, Va.). Mycobacteria were grown in Middlebrook 7H9 broth supplemented with ADC enrichment (Difco Laboratories, Detroit, Mich.) and 2% (wt/vol) glucose and containing 1% (vol/vol) glycerol. Growth occurred at 37°C with slow shaking to mid-log growth phase (7 to 10 days) or to stationary phase (21 days). Bacteria were passaged in broth cultures once and quantified with McFarland equivalence turbidity standards (Remel, Lenexa, Kans.). Cultures of 106 to 108 bacilli/ml were used immediately or stored at −70°C in 7H9 broth containing 20% (vol/vol) glycerol. Bacterial counts in CFU per milliliter were determined by serial titration of cultures 1:100 and 1:1,000 in 7H9 media and plating on 7H11 agar plates for 3 weeks at 37°C.
THP-1 cell culture, treatments, and M. tuberculosis infection.
The THP-1 human macrophage cell line (ATCC TIB-202) was grown at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) and 1% (vol/vol) antibiotic-antimycotic (100-U/ml penicillin G sodium, 100-U/ml streptomycin sulfate, 0.25-g/ml amphotericin B). Before infection, cells were washed in serum-free medium and seeded for 24 h in 75-ml T-flasks or six-well Costar plates containing RPMI 1640 plus 10% (vol/vol) FBS without antibiotics. In several experiments, THP-1 cells were induced to differentiate into adherent cells by 24 h of exposure to LPS at 10 μg/ml. Experiments to determine the effects of CatG were done in serum-free medium (Cellgro, Mediatech, Inc., Herndon, Va.). Cells in suspension, or adherent after LPS induction, were treated with purified CatG at 5 μg/ml (Sigma, St. Louis, Mo.) for 1 h prior to infection with M. tuberculosis Erdman bacilli. Cells were infected with M. tuberculosis bacilli at a multiplicity of infection (MOI) of 1 to 5. Cells and M. tuberculosis bacilli were incubated for 24 h at 37°C in 5% CO2 before being harvested for RNA, protein, CatG activity assays, and/or CFU determinations.
BAL macrophages and PBMCs.
Bronchoalveolar lavage (BAL) macrophages were obtained from discarded individual BAL fluids (three total) collected routinely for diagnostic purposes during bronchoscopy from patients or normal volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of three normal donors by centrifugation in Histopaque 1077 (Sigma) as described before (10). All procedures involving human samples were done in compliance with federal and institutional guidelines. BAL cells and PBMCs were washed twice in serum-free medium, resuspended in RPMI 1640 plus 10% fetal calf serum (FCS), and plated on six-well plates for 2 h at 37°C in 5% CO2. Nonadherent cells were washed off with serum-free medium, and the adherent cells, comprising mostly monocytic cells, were incubated in RPMI 1640 plus 10% FBS without antibiotics for 24 h prior to infection with M. tuberculosis bacilli.
Gene array analysis.
Gene arrays were done for human extracellular matrix and adhesion molecules using the GEArray Q series blots and protocols from SuperArray, Inc., Bethesda, Md. Arrays consisted of nylon membranes with 112 cDNA gene fragments printed in a tetra-spot format, with 96 genes including 43 CAMs, 15 ECMs, 32 proteases, 6 protease inhibitors, and 16 spots with vector and housekeeping gene controls. A list containing the identities and accession numbers of these genes can be found at www.superarray.com. Total RNA was isolated from normal and M. tuberculosis-infected THP-1 cells by treatment with RNA-Bee (Tel-Test, Friendswood, Tex.), treated with DNase I (200 U/ml) to remove any contaminating genomic DNA, and used as template for the synthesis of 32P-labeled cDNA probes. Annealing was done by mixing 1.0 μg of RNA (1 μl) with GEA primer mix (3 μl; SuperArray, Inc.) in 10 μl followed by incubation at 70°C for 3 min and at 42°C for 2 min. Synthesis of the 32P-labeled cDNA probes was done by mixing 10 μl of the annealed mixture and 10 μl of labeling mix (4 μl of 5× GEA buffer, 3 μl of [α-32P]dCTP at 10 μCi/μl, 1 μl of 50 U of reverse transcriptase, 1 μl of RNase inhibitor, 1 μl of RNase-free H2O), and incubation at 42°C for 25 min. The heat-denatured probe mixture (20 μl) was added to prehybridized membranes (60°C for 2 h) in the presence of heat-denatured salmon sperm DNA (100 μg/ml) and hybridizations were done at 60°C for 24 h with continuous mixing. Array membranes were washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% sodium dodecyl sulfate (SDS) and 0.1× SSC-0.5% SDS at 60°C for 10 min before exposure to X-ray film. Analysis was done by densitometry scanning of X-ray films and the use of ImageQuant software from Molecular Dynamics.
RT-PCR.
Analysis of gene expression levels was done by reverse transcription-PCR (RT-PCR) with Promega reagents and protocols (Promega, Madison, Wis.). RNA was isolated from THP-1 cells before and after M. tuberculosis infection and reverse transcribed with gene-specific reverse primers for human cathepsins B, D, G, and a β-actin control. Primers were designed to amplify a 300-bp fragment based on GenBank published sequences and were as follows: CatG (F, AGAAGAGTCAGACGGAATCGA; and R, CCCTGACGACTTTCCATAGGA), CatB (F, GCCAACTCCTGGAACACTGA; and R, CTGTCTGCACTGTAACCACA), CatD (F, CTACACTGTGTTTGACCGTGA; and R, GAAACAGATCTGTGCTCTGGA), and β-actin (F, TGGAGAAAATCTGGCACC; and R, TGAGGTAGTCAGTCAGGT). The first-strand cDNA synthesis reaction was carried out at 42°C for 60 min in a 25-μl reaction mixture consisting of 1.0 μg of RNA, 1 μl of 20 μM reverse primer, 5 μl of 5× Moloney murine leukemia virus (MMLV) buffer, 5 μl of deoxynucleoside triphosphate (dNTP), 10 mM mix, 1 μl of 200 U of MMLV reverse transcriptase, 25 U of rRNasin RNase inhibitor, and nuclease-free H2O. After cDNA synthesis, 5 μl of cDNA was added to 44 μl of PCR master mix (3 μl of 25 mM MgCl2, 5 μl of 10× PCR buffer, 0.2 μl of 1.0 U of Taq DNA polymerase, 4 μl of 10 mM dNTP mix, and diethyl pyrocarbonate-treated water) and 1.0 μl of forward and reverse primers (20 μM) was added to each reaction tube. The thermal cycling parameters consisted of 94°C for 5 min, 20 or 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s; and a final extension of 72°C for 10 min. The RT-PCR products were separated on 1% (wt/vol) Tris-borate-EDTA (TBE) agarose gels and visualized by staining with ethidium bromide, and the intensity of bands was quantified by densitometry scanning.
CatG enzymatic assays.
THP-1 monocytes in suspension were infected with M. tuberculosis Erdman bacilli (at an MOI of 1 to 5) for 24 h. After infection, the cells were centrifuged at 200 × g for 5 min in a Marathon 8K centrifuge, and the cell pellet was washed (gently) in ice-cold phosphate-buffered saline (PBS; pH 7.2) by centrifugation. The cell pellet was resuspended in 500 μl of assay buffer (50 mM HEPES buffer, pH 7.4, 50 mM NaCl) plus 0.1% (vol/vol) Triton X-100 and sonicated in ice (3 to 4 pulses for 20 s). Cell lysates were centrifuged to remove cell debris and filtered through a 22-μm-pore-diameter spin filter. Total protein was determined by the Bradford method (5), and aliquots were stored frozen at −70°C until analyzed for CatG enzyme activity. In other experiments, THP-1 monocytes in suspension or differentiated into adherent cells after treatment with LPS (10 μg/ml), were exposed to M. tuberculosis bacilli (H37Ra, H37Rv, and Erdman) that were killed by fixation in 2% paraformaldehyde as described previously (28). The fixed, killed bacilli were washed three times in Dulbecco's PBS pH 7.2 before added to cells. After exposure for 24 h, the THP-1 cells were harvested as described above and cell lysates were tested for CatG activity.
Enzyme assays for CatG were performed according to Barrett (3). A standard curve for CatG activity was prepared with purified CatG (Sigma) at concentrations of 1, 10, 50, and 100 ng. Cell lysates were diluted to 50, 100, and 200 μg in 450 μl of assay buffer (50 mM HEPES buffer, pH 7.4, 50 mM NaCl). To each sample, 50 μl of CatG substrate (Suc-Ala-Ala-Pro-Phe-pNA; 20 mM in dimethyl sulfoxide) was added, and the reaction mixtures were incubated at 37°C for 1 h. The reactions were stopped by addition of 500 μl of soybean trypsin inhibitor (200 μg/ml), and absorbance was measured at 410 nm. Results are expressed as specific activity (A410 per microgram of protein), and the enzyme concentration in cell lysates was determined based on the standard curve.
Western blot analysis.
Cell lysates were prepared from THP-1 cells after infection or LPS exposure by sonication of cell pellets in lysis buffer (20 mM Tris, pH 7.4, 2.5 mM EDTA, 1% [wt/vol] Triton X-100, 1% [vol/vol] deoxycholic acid, 0.1% [wt/vol] SDS, 100 mM NaCl, 10 mM NaF, 1 mM Na3VO4.). Lysates were centrifuged to remove cell debris, filtered through a 22-μm-pore-diameter spin filter, and the protein concentration was determined. Lysate protein samples of 10 μg were loaded in duplicate; controls consisted of 1 μg of CatG from human neutrophils (ICN Biochemicals) or from human sputum (Sigma) and 10-μg Kaleidoscope size markers (Bio-Rad). Electrophoresis was done in a 12% NuPage Novex Bis-Tris gel (Invitrogen) for 80 min at 200 V in running buffer (50 mM MOPS [morpholinepropanesulfonic acid], 50 mM Tris, 0.5% SDS, 1 mM EDTA). NuPage antioxidant (Invitrogen) was added to the upper chamber buffer. Proteins were transferred to polyvinylidene difluoride membrane for 90 min at 15 V with a Bio-Rad Semi-Dry transfer chamber with NuPage transfer buffer (25 mM Bicine, 25 mM Bis-Tris, 1 mM EDTA, 0.05 mM chlorobutanol, 10% methanol). Membranes were blocked in 5% (wt/vol) nonfat dried milk in TBST (Tris-buffered saline, 0.05% Tween 20, pH 7.5) at room temperature for 2 h. Primary antibody consisted of anti-CatG (from neutrophils) rabbit polyclonal antibody (courtesy of Jan Potempa, University of Georgia, Athens) diluted 1:1,000 and was incubated at 4°C overnight. The blots were washed with shaking three times for 10 min each in TBST. Secondary antibody consisted of goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate at 1:20,000 and was incubated for 90 min at room temperature. The blots were washed three times in TBST for 10 min and rinsed in deionized water. SuperSignal West Pico chemiluminescent substrate (Pierce) was added for detection of CatG bands, and the blots were exposed to X-ray film.
Bactericidal activity assays.
M. tuberculosis Erdman cultures were grown in 7H9/ADC broth to the log phase, and aliquots of 1 ml containing 1 × 106 to 2 × 106 bacilli were incubated for 24 h at 37°C with purified full-length CatG (Sigma), CatG synthetic peptides CG117-136 and C12-CG117-136, full-length CatB (Sigma), and negative control peptide LL1-17, at concentrations of 10, 50, and 100 μg/ml. Peptides CG117-136 and C12-CG117-136 have been described previously (30) and represent residues 117 to 137 (117-RPGTLCTVAGWGRVSMRRGT-136) of the full-length CatG protein (27). The negative control peptide LL1-17 corresponds to amino acids 1 to 17 (1-LLGDFFRKSKEKIGKEF-17) of human cathelicidin peptide hCAP18/LL-37 (13). Peptides were synthesized at the Microchemical Facility of Emory University as previously described (18). After incubation, serial dilutions of 1:100 and 1:1,000 were prepared in Dulbecco's PBS and the number of CFU per milliliter was determined by plating 100 μl on 7H11 agar plates for 3 weeks at 37°C. Average numbers of CFU per milliliter were determined from triplicate experiments, and bactericidal activity was calculated as percent decrease in CFU per milliliter relative to no-treatment controls.
RESULTS
Differential expression of cathepsins and other matrix and cell adhesion genes in THP-1 human macrophages after infection with M. tuberculosis.
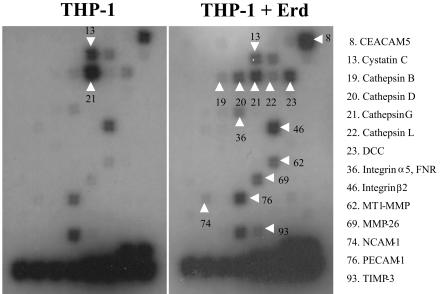
Gene array analyses were conducted to identify differentially expressed genes encoding for ECM, CAM, and proteases in THP-1 human macrophages after a 24-h infection with M. tuberculosis Erdman strain. Arrays contained 96 genes including: 43 CAMs, 15 ECMs, 32 proteases, 6 protease inhibitors, and 13 spots with housekeeping gene and vector controls. Figure 1, a representative array from triplicates, shows that infection of THP-1 cells with M. tuberculosis resulted in differential expression of 14 genes, while the expression of control genes (coding for glyceraldehyde-3-phosphate dehydrogenase [GAPDH], cyclophilin A, ribosomal protein L13a, and β-actin) was not changed. As shown in Table 1, densitometry scanning of the arrays showed 12 genes with a 1- to 2.5-fold increase in expression in THP-1 cells after infection. Among their products were six cell adhesion molecules (CEACAM5, DCC, integrin α5, integrin β2, NCAM-1, and PECAM-1) and six proteases (cathepsins B, D, and L; MT1-MMP; MMP-26; and TIMP-3). Most interestingly is the finding that two genes, coding for CatG and cystatin C (a cathepsin inhibitor), were consistently down-regulated (over onefold decrease) in THP-1 cells after M. tuberculosis infection. These results, showing that gene expression of certain cathepsins B, D, and L (CatB, CatD, and CatL) is up-regulated after infection, while expression of others, like CatG, is down-regulated, prompted us to focus on this particular group of lysosomal enzymes and to further investigate their role in TB pathogenesis.
FIG. 1.
Gene array blot analysis of THP-1 human monocytes after infection with M. tuberculosis. Infection of THP-1 monocytes for 24 h with M. tuberculosis strain Erdman (Erd) results in decreased expression of CatG (no. 21) and cystatin C (no. 13), but increased expression of CatB (no. 19), CatD (no. 20), CatL (no. 22), DCC (no. 23), integrin α5 or FNR (no. 36), Integrin β2 (no. 46), MT1-MMP (no. 62), MMP-26 (no. 69), NCAM-1 (no. 74), PECAM-1 (no. 76), and TIMP-3 (no. 93). Blots are representative of triplicate experiments.
TABLE 1.
Identity of genes differentially regulated in THP-1 cells after M. tuberculosis infection
| Position | Gene | GenBank Acc. No. accession no. | Description | Fold changea |
|---|---|---|---|---|
| 8 | CEACAM5 | NM_004363 | Carcinoembrionic antigen-related CAM 5 | +1.33 |
| 13 | CST3 | NM_000099 | Human cystatin C | −1.13 |
| 19 | CTSB | L16510 | Human cathepsin B | +1.76 |
| 20 | CTSD | M11233 | Human cathepsin D | +2.14 |
| 21 | CTSG | NM_001911 | Human cathepsin G | −1.35 |
| 22 | CTSL | X12451 | Human cathepsin L | +1.79 |
| 23 | DCC | NM_005215 | Deleted in colorectal carcinoma | +2.66 |
| 36 | ITGA5 | X06256 | Integrin α5 (fibronectin receptor, α chain) | +1.87 |
| 46 | ITGB2 | Y00057 | Integrin β2 | +1.95 |
| 62 | MT1-MMP | D26512 | Membrane-type matrix metalloproteinase 1 | +2.26 |
| 69 | MMP-26 | AF291664 | Matrix metalloproteinase 26 | +2.32 |
| 74 | NCAM-1 | U63041 | Neural cell adhesion molecule 1 | +1.66 |
| 76 | PECAM-1 | NM_000442 | Platelet/endothelial cell adhesion molecule 1 | +1.94 |
| 93 | TIMP3 | NM_000362 | Tissue inhibitor of metalloproteinase 3 | +1.79 |
Fold change calculated by densitometry scanning of array blots from untreated versus M. tuberculosis-infected cells.
Infection of THP-1 cells or exposure to LPS results in differential gene regulation of cathepsins G, D, and B.
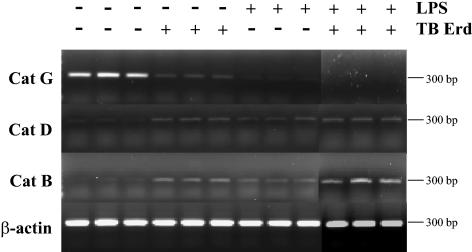
THP-1 monocytic cells become adherent and acquire features of macrophages when infected with M. tuberculosis or exposed to the bacterial endotoxin LPS. We wanted to determine if this induced differentiation and/or activation of THP-1 cells by LPS, similarly to infection with M. tuberculosis bacilli, would result in differential regulation of cathepsin genes. THP-1 cells were infected with M. tuberculosis Erdman bacilli (at MOI of 1 to 5) before and after treatment with LPS (at 10 μg/ml) for 24 h. RNA was isolated from LPS-treated and/or M. tuberculosis bacillus-infected cells and analyzed by RT-PCR for expression of cathepsin genes. The results (Fig. 2) showed that CatG expression was highly abundant in untreated THP-1 cells and that infection with M. tuberculosis bacilli (done in triplicate) resulted in decreased expression of CatG mRNA (approximately fivefold decrease). In cells treated with LPS alone or infected with M. tuberculosis bacilli after LPS treatment, there was complete inhibition of CatG expression. In contrast, expression of CatD and CatB is very low in untreated THP-1 cells, but M. tuberculosis infection or exposure to LPS results in a slight increase in mRNA synthesis.
FIG. 2.
Differential expression of cathepsin G, D, and B genes after M. tuberculosis infection and exposure to LPS. RT-PCR analysis was used to determine differences in the expression of cathepsin genes in THP-1 monocytes after infection with M. tuberculosis Erdman (Erd), exposure to LPS, and combined treatment. As shown, CatG gene expression is decreased, while the expression of CatD and CatB is increased after infection and/or LPS treatment. The β-actin housekeeping gene used as a positive control did not change.
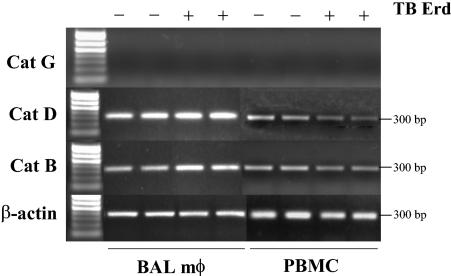
To determine if mature macrophages, in comparison to the human THP-1 macrophage cell line, showed differential gene regulation of cathepsins G, D, and B, we tested macrophages isolated from BAL fluid and blood. BAL macrophages and PBMCs were obtained from a normal donor, cultured (only adherent cells were selected), and infected for 24 h with M. tuberculosis Erdman bacilli, and the expression of cathepsins G, D, and B was determined by RT-PCR. Figure 3 shows that CatG mRNA is absent in mature BAL macrophages and adherent PBMCs and does not change after M. tuberculosis infection. However, cathepsin D and B mRNAs are highly abundant in both cell preparations, and a slight increase in expression is seen in BAL macrophages after M. tuberculosis bacillus infection. These results, showing that CatG mRNA is strongly down-regulated after activation of THP-1 monocytes and is absent in BAL macrophages and PBMCs, prompted us to further investigate the role of this cathepsin during M. tuberculosis pathogenesis.
FIG. 3.
Differential expression of cathepsin G, D, and B genes in BAL macrophages (mφ) and PBMCs. BAL macrophages isolated from human lung and PBMCs were infected with M. tuberculosis Erdman (Erd) bacilli for 24 h, and the expression of CatG, CatD, and CatB was determined by RT-PCR. Both untreated and M. tuberculosis-infected cells show no expression of CatG mRNA. BAL macrophages show a slight increase in CatD and CatB after infection, while PBMCs show a slight decrease. The expression of the β-actin control gene did not change. Results are representative of three individual human samples.
Exposure of THP-1 cells to M. tuberculosis bacilli or LPS results in decreased expression and activity of CatG.
To examine for CatG protein, whole-cell lysates were prepared from THP-1 cells infected with M. tuberculosis Erdman bacilli, before and after treatment with LPS, and analyzed by Western blots. A polyclonal antibody raised against whole CatG isolated from human neutrophils and that recognizes two of the major isoforms (28 to 30 kDa) of CatG was used. As shown in Fig. 4, CatG protein is abundant in untreated THP-1 cells, while infection with M. tuberculosis Erdman bacilli and exposure to LPS results in a dramatic decrease in CatG protein expression. These results confirm our gene array and RT-PCR results showing decreased transcription of CatG after infection or LPS exposure.
FIG. 4.
Infection of THP-1 monocytes and exposure to LPS result in decrease expression of CatG protein. THP-1 cells were infected with M. tuberculosis Erdman (Erd) bacilli for 24 h, exposed to LPS alone, or infected after LPS exposure. Total cell lysates were prepared and analyzed for the presence of CatG by Western blot. As shown, M. tuberculosis infection, exposure to LPS, or combined treatment results in decreased expression of two major protein bands of 28 to 30 kDa corresponding to CatG.
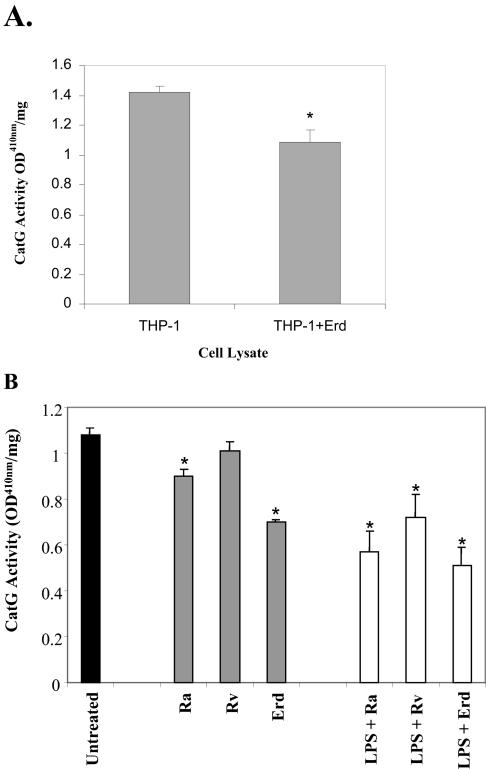
Next, we wanted to determine if M. tuberculosis infection of THP-1 cells would also result in a decrease in CatG enzymatic activity. THP-1 cells were infected with M. tuberculosis Erdman bacilli at an MOI of 1 to 5, and cell lysates were tested for CatG activity toward the colorimetric peptide substrate Suc-Ala-Ala-Pro-Phe-pNA. As shown in Fig. 5A, infection of THP-1 cells resulted in a significant decrease in CatG activity detected in cell lysate samples of 50 μl. In order to be able to conduct these assays under non-biosafety level 3 conditions, we decided to test bacilli that were fixed and killed in 2% paraformaldehyde. THP-1 cells were exposed to formalin-fixed, killed M. tuberculosis bacilli (strains H37Ra, H37Rv, and Erdman), before and after LPS-induced maturation into adherent cells, and CatG activity was determined. Figure 5B shows that exposure of THP-1 cells to nonviable M. tuberculosis strains H37Ra, H37Rv, and Erdman results in decreased CatG activity in comparison to untreated cells. Interestingly, the most significant decrease in CatG activity (35% decrease) was obtained after exposure to the virulent M. tuberculosis strain Erdman. Exposure of cells after LPS activation resulted in further decrease in CatG activity, and as before, the highest decrease in activity (53% decrease) was obtained with strain Erdman. These results demonstrate that M. tuberculosis infection, or exposure to nonviable M. tuberculosis bacilli containing cell surface molecules that are resistant to the cross-linking agent paraformaldehyde, can induce the down-regulation of CatG activity in THP-1 monocytes. In addition, treatment with LPS prior to exposure to M. tuberculosis bacilli results in further decrease in CatG enzymatic activity.
FIG. 5.
CatG activity is decreased in THP-1 monocytes after M. tuberculosis infection or exposure to killed M. tuberculosis bacilli or endotoxin LPS. (A) THP-1 monocytic cells were infected with M. tuberculosis Erdman (Erd) or (B) exposed to formalin-fixed, killed M. tuberculosis bacillus strains H37Ra (Ra), H37Rv (Rv), and Erdman, before and after exposure to endotoxin LPS. Cell lysates were tested for CatG activity towards the colorimetric substrate peptide Suc-Ala-Ala-Pro-Phe-pNA. As shown, exposure to live or dead M. tuberculosis bacilli and LPS results in a significant decrease in CatG activity. OD, optical density. *, P < 0.05 (Student's t test) in comparison to the untreated control.
LPS-induced down-regulation of CatG in M. tuberculosis-infected THP-1 monocytes results in increased survival of bacilli.
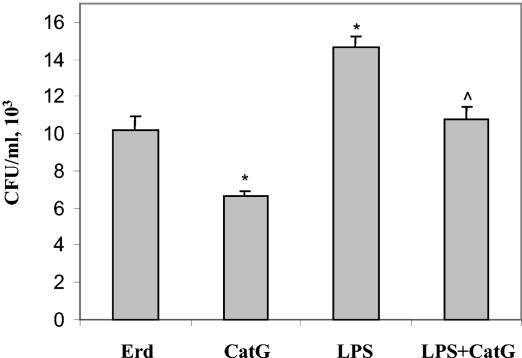
We showed that infection of THP-1 monocytes with M. tuberculosis bacilli results in decreased transcription and translation of CatG. Therefore, we wanted to determine if the down-regulation of CatG is advantageous to intracellular replication of M. tuberculosis bacilli in macrophages. THP-1 cells were treated with purified CatG (1 h), LPS (for 24 h), or both LPS and CatG. After treatment, cells were infected with M. tuberculosis Erdman bacilli at an MOI of 1 to 5 for 24 h, the cell pellets were lysed in 0.1% Triton X-100, and dilutions were plated to determine bacillary counts in CFU per milliliter. Figure 6 shows that treatment of cells with CatG (5 μg/ml) for 1 h prior to infection results in a significant decrease (∼40%) in CFU and therefore a decrease in survival of bacilli in cultures. This decrease could be attributed to the extracellular killing of bacilli by CatG. Infection of THP-1 cells after LPS induction (which downregulates CatG expression) results in a significant increase in bacillary counts after 24 h (∼30%) in comparison to the level in untreated cells. However, treatment with CatG after LPS exposure only partially reverses the effects of LPS but does not result in decreased survival in comparison to that of the untreated control. This suggests that the addition of exogenous CatG cannot completely overcome the effects of LPS down-regulation of intracellular levels of CatG. Taken together, these results suggest that the down-regulation of CatG expression in THP-1 monocytes increases the viability of M. tuberculosis bacilli and therefore is advantageous to intracellular survival.
FIG. 6.
LPS-induced down-regulation of CatG results in increased survival of bacilli in THP-1 cells. THP-1 cells were treated with purified CatG (1 h), LPS (24 h), or with both LPS and CatG. After treatment, cells were infected with M. tuberculosis Erdman (Erd) bacilli for 24 h, the cell pellets were lysed in 0.1% Triton X-100, and dilutions were plated to determine bacillary counts in CFU per milliliter. Values represent the mean and standard deviation from triplicate experiments. *, P < 0.05 (Student's t test) in comparison to untreated control (Erd). ^, P < 0.05 in comparison to LPS treatment alone.
CatG peptides show tuberculocidal activity in vitro.
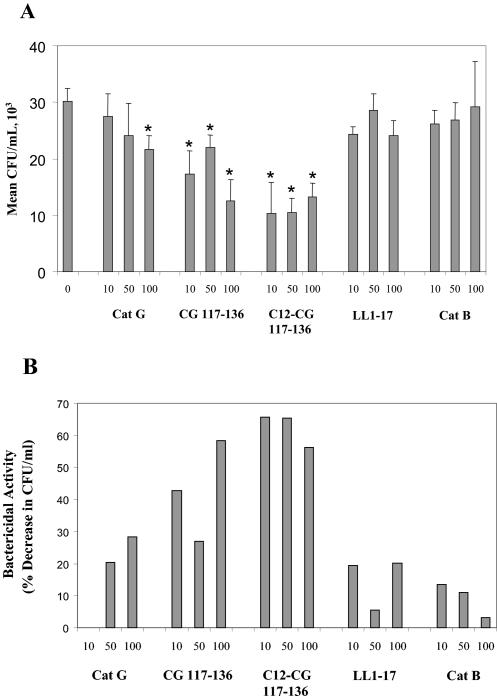
It has been shown that synthetic peptides of CatG, CG117-136, and the fatty acid-modified version C12-CG 117-136, have broad bactericidal activity against gram-negative and -positive bacteria (31). We tested peptides CG117-136 and C12-CG117-136 and purified neutrophil full-length CatG for bactericidal activity in cultures of M. tuberculosis Erdman bacilli. Also, we tested full-length CatB and control peptide LL1-17. M. tuberculosis Erdman cultures (1 × 106 to 2 × 106/ml) were incubated for 24 h at 37°C with the above agents at 10, 50, and 100 μg/ml, and aliquots were plated for colony counts. Figure 7A shows the average number of CFU per milliliter from triplicate experiments obtained after incubation with increasing concentrations of full-length CatG, CatG peptides CG117-136 and C12-CG117-136, full-length CatB, and control peptide LL1-17. Figure 7B shows bactericidal activity, expressed as percent decrease in CFU per milliliter in relation to untreated bacilli. As shown, full-length CatG added to bacilli at 10 μg/ml results in an approximately 10% decrease in viability, while at 50 to 100 μg/ml resulted in a 20 to 30% decrease in viability. Both synthetic peptides resulted in significantly higher bactericidal activity in comparison to full-length CatG. Peptide CG117-136 at 10 and 50 μg/ml showed 42 and 27% reductions, respectively, while a 58% decrease in viability was obtained with 100 μg/ml. The FA-modified peptide C12-CG117-136 showed the highest bactericidal activity, since treatment with 10 μg/ml resulted in more than 60% decrease in CFU per milliliter. However, increasing doses of 50 to 100 μg/ml also resulted in about 50 to 60% decrease in viability, suggesting that saturation levels of activity are reached at 10 μg/ml. On a molar-to-molar basis comparison, 100 μg (3.47 μM) of CatG resulted in a 30% decrease in CFU per milliliter, while 10 μg (4.6 μM) of CG117-136 resulted in a 43% decrease and 10 μg of C12-CG117-136 (4.3 μM) resulted in a more than 60% decrease in CFU per milliliter. No decrease in viability of M. tuberculosis bacilli was obtained with control peptide LL-1-17 and full-length CatB at similar concentrations. Therefore, these results demonstrate that CatG has antimicrobial activity against M. tuberculosis bacilli and that similarly to its activity against other pathogenic bacteria, it is due to the cationic peptide domain CG117-136. Studies are under way to further characterize the mechanisms by which these peptides are bactericidal to M. tuberculosis bacilli.
FIG. 7.
CatG and synthetic cationic peptides show tuberculocidal activity. Full-length CatG, CatG synthetic peptides CG117-136, C12-CG 117-136, control peptide LL1-17, and full-length CatB were tested (in triplicate) at 10, 50, and 100 μg/ml for bactericidal activity against M. tuberculosis bacilli. M. tuberculosis Erdman cultures (1 × 106 to 2 × 106/ml) were incubated for 24 h at 37°C with the above agents, and aliquots were plated for CFU determinations. (A) Average CFU per milliliter from triplicate experiments obtained after incubation; (B) bactericidal activity, expressed as percent decrease in CFU per milliliter in relation to untreated bacilli. *, P < 0.05 (Student's t test) in comparison to untreated control.
DISCUSSION
One major limitation in the development of more effective drug treatments to control the spread of TB is a poor understanding of the host cellular and molecular mechanisms involved in TB pathogenesis, in particular, granuloma formation and the sequelae of tissue-damaging responses that lead to caseous necrosis, liquefaction, and cavity formation. Work in our laboratory focuses on defining the host tissue remodeling responses during pulmonary TB, the processes involved in ECM expression, deposition, and degradation. We believe that the major pathological consequences of pulmonary M. tuberculosis infection are caused by the dysregulated expression of tissue remodeling genes, most of which are still unknown. Therefore, the main objective of our work is to identify host genes differentially regulated in human macrophages early on during M. tuberculosis infection by the use of gene array analysis and to determine their function and relevance to TB pathogenesis.
Here, we demonstrate by gene array analyses that infection of THP-1 monocytes with M. tuberculosis Erdman bacilli for 24 h results in differential expression of 14 genes from a collection of 96 genes, including those coding for 43 CAMs, 15 ECMs, 32 proteases, and 6 protease inhibitors. Among these, we found that levels of six CAMs (CEACAM5, DCC, integrin α5, integrin β2, NCAM-1, and PECAM-1) were increased 1- to 2.5-fold, as determined by densitometry scanning. This result was expected, since it is known that CAMs are important in M. tuberculosis-host cell interactions, in processes such as receptor binding, macrophage activation, phagocytosis, and cell trafficking, and in many other processes during TB pathogenesis (11). Most interesting was the finding of differential expression of several proteases (cathepsins B, G, D, and L; MT1-MMP; and MMP-26), and protease inhibitors (cystatin C and TIMP-3), early during infection (24 h) of cells. In a previous study (23), gene array analysis of THP-1 monocytes infected with M. tuberculosis bacilli for 6 to 12 h also revealed the up-regulation of numerous genes involved in inflammatory processes, such as CAMs, integrins, chemokines, cytokines, and curiously the matrix metalloproteinase MMP-9. We recently showed that infection of THP-1 monocytes or exposure to M. tuberculosis mannosylated glycans results in tremendous secretion of bioactive MMP-9, but only a slight increase in mRNA was detected by RT-PCR (25, 26). In the present study, no increase in MMP-9 mRNA expression was detected by gene array, while high levels of MMP-9 activity were present in the media (data not shown), suggesting that M. tuberculosis infection of THP-1 cells results mostly in the release and activation of MMP-9 stored in secretory granules. Therefore, although gene array is a powerful method for the discovery of differentially regulated genes, parallel analyses of protein expression and activity are always necessary.
Our gene array results showing differential expression of cathepsin genes prompted us to focus in this family of lysosomal proteases. By RT-PCR analysis, we showed that M. tuberculosis infection of THP-1 cells or exposure to LPS endotoxin induced the expression of catB and -D genes, while complete inhibition in the expression of catG was observed. Cathepsins are a large family of lysosomal proteases that are classified according to their active site and substrate specificity (22). They are present in most tissues, synthesized as inactive zymogens, targeted from the endosomal compartments to lysosomes via the mannose 6-phosphate receptor pathway, and activated by proteolytic cleave of an N-terminal propeptide (40). Cathepsins not only function in intralysosomal protein degradation, but they participate in tissue remodeling by degrading extracellular matrix proteins when secreted in a microenvironment that allows their activity (40). Cathepsins B and L are cysteine proteases, while CatD and CatG are aspartyl and serine proteases, respectively. Very little information exists on the role of cathepsins during M. tuberculosis infection. One study by Dannenberg's group demonstrated high levels of CatD present in mature epithelioid macrophages surrounding the caseous and liquefied areas near the wall of pulmonary cavities in M. tuberculosis-infected rabbits (7). Cathepsins B and D are weakly acidic lysosomal proteases with a pH optima of 5. Therefore, it is possible that during infection, the secretion of these cathepsins in the acidified caseous centers of granulomas contributes to tissue liquefaction and cavitation. The finding that catB and -D gene expression is induced by M. tuberculosis infection or LPS activation after 24 h suggests that these cathepsins also play a role during the early tissue remodeling events involved in granuloma formation.
We were intrigued by the finding of a strong down-regulation of catG gene expression after M. tuberculosis infection and exposure to LPS. By Western blot analysis, we demonstrated that catG protein expression was also decreased (approximately fivefold) after 24 h, coinciding with the down-regulation of catG mRNA. In addition, catG activity assays revealed a significant decrease in enzyme activity after infection or exposure of THP-1 cells to formalin-fixed killed M. tuberculosis bacilli and LPS. These studies demonstrate that in THP-1 monocytes, infection with live bacilli and exposure to components of the cell wall in M. tuberculosis bacilli or to LPS endotoxin can result in transcriptional silencing of the catG gene. CatG is a major serine proteinase present in the azurophilic granules of neutrophils, and monocytes (29, 30) that is synthesized during the promyelocytic and promonocytic stages of maturation, respectively (1, 37). Previous studies showed that CatG is highly abundant in the human leukemic monocyte cell line U937 (30, 38) and that treatment with phorbol ester tetradecanoyl phorbol acetate results in transcriptional down-regulation (14, 17, 38). THP-1 monocytes, similarly to U937 cells, are at a stage of differentiation where CatG is abundant. When these cells are exposed to potent antigens, such as live or dead M. tuberculosis bacilli or bacterial LPS, they become adherent, acquire morphological features of mature macrophages, and loose the expression of CatG. However, M. tuberculosis infection or LPS exposure of THP-1 cells also results in increased expression of catB and -D genes. We showed by RT-PCR that cell preparations rich in mature macrophages, such as human BAL and PBMCs, do not express CatG, but express high basal levels of CatB and -D. We also showed that M. tuberculosis infection of BAL macrophages results in further induction in the expression of these cathepsins. Therefore, our studies demonstrate that M. tuberculosis or LPS-induced differentiation and/or activation of THP-1 monocytes results in a “cathepsin switch” that is transcriptionally regulated. Current studies in our laboratory are intended to identify the transcriptional mechanisms involved in this “cathepsin switch” that occurs in monocytes and their relevance to TB pathogenesis.
We wanted to determine if the lack of expression of CatG in THP-1 monocytes is advantageous to M. tuberculosis infection. CatG is not only involved in phagolysosomal degradation and proteolysis of extracellular matrix components during inflammatory reactions (6, 36), but it is known to have potent nonoxidative antibacterial activity that is independent of its serine protease activity (34). CatG is a highly cationic arginine-rich protein that exerts its bactericidal activity through a mechanism involving membrane depolarization (32). We found that THP-1 cells treated with LPS for 24 h and infected with M. tuberculosis bacilli had higher bacillary counts (30% increase) than untreated controls. Although this increase in bacillary load can be due to multiple factors, such as an overall activation of macrophages with increased phagocytosis, we believe that the down-regulation of CatG plays a major role. We found that addition of CatG to THP-1 cells prior to infection results in a significant decrease in bacillary counts (40% decrease), while treatment of cells after LPS induction does not result in a significant decrease in the viability of bacilli. Our interpretation of these results is that, in untreated THP-1 cells, the exogenously added CatG is bactericidal to bacilli present extracellularly, resulting in decreased infection and bacillary counts. These cells also have high levels of endogenous CatG that can be bactericidal to internalized bacilli before enzyme synthesis is completely abolished within 24 h of infection. However, in LPS-induced cells, the addition of CatG only partially decreased bacillary counts in comparison to LPS treatment alone, suggesting that addition of exogenous enzyme cannot completely overcome the LPS-induced down-regulation of intracellular levels of CatG.
It has been demonstrated that CatG contains cationic peptide domains that by themselves can exert antibacterial activity against gram-negative and -positive bacteria (2, 35). The 20-amino-acid synthetic peptide CG117-136, which contains an N-terminal hydrophobic and C-terminal cationic domain, showed the highest bactericidal activity against P. aeruginosa and S. aureus (35). The bactericidal activity of this peptide is dependent on its high arginine content (31) and covalent attachment of a C8-12 saturated fatty acid to the N or C terminus enhances its activity against multidrug-resistant strains of bacteria (18). We tested peptides CG117-136 and C12-CG117-136 against M. tuberculosis Erdman bacilli in broth cultures for 24 h and found that both peptides resulted in significantly higher bactericidal activity in comparison to full-length CatG. At the lowest concentration used (10 μg/ml), peptides CG117-136 and C12-CG117-136 were five- and sevenfold more active than full-length CatG, respectively. The lipid-modified peptide C12-CG117-136 was the most active, showing more than 60% decrease in CFU per milliliter at the lowest concentrations (10 to 50 μg/ml). Also, when compared on a molar basis, CatG peptides were more bactericidal than full-length cathepsin G. These results are in agreement with previous studies showing that the bactericidal activity of CatG is independent of its proteolytic activity and that cationic peptides are responsible for its broad-spectrum activity (31, 33, 35). Although the antimicrobial mechanism of action of CatG is not completely understood, it has been proposed that it acts by depolarizing the cytoplasmic membrane (20, 21) by a mechanism similar to the antimicrobial peptides defensins (16) and magainins (39). It is believed that the bactericidal activity of cationic peptides is due initially to the interaction of arginine amino groups with negatively charged groups in microbial surfaces, such as the unsubstituted phosphates of LPS in gram-negative bacteria or the teichoic acids of gram-positive bacteria (31). However, studies with peptide CG117-136 suggest that the guanidinium side chain of arginine also plays an important role in ionic and hydrogen bonding interactions (31). In P. aeruginosa, treatment with these peptides results in a rapid loss of amino acid transport capabilities and macromolecular synthesis, activities that are consistent with damage to the cytoplasmic membrane (31, 35). In S. aureus, resistance to peptide CG117-136 is associated with deletion of the major cold shock gene cspA, suggesting that cspA or genes under its regulation are important for susceptibility to CatG peptides (15).
It is not known how CatG or its cationic peptides kill M. tuberculosis bacilli and how significantly relevant is this observation to TB pathogenesis. In the present study, we demonstrate that the down-regulation of CatG in THP-1 monocytes coincides with increases in bacillary counts and that addition of CatG or peptides to M. tuberculosis broth cultures results in decreased viability. We can speculate that during the early stages of infection, monocytes are able to mount a significant CatG-dependent killing response against invading M. tuberculosis bacilli. CatG from lysosomes is emptied into phagosomes where the full-length protein or its proteolytic peptides are able to kill bacilli. But, this response is short lived due to the dramatic down-regulation of CatG that is concomitant with the M. tuberculosis-induced maturation of these cells into macrophages. In that sense, the down-regulation of CatG in macrophages is advantageous to M. tuberculosis and may represent an important mechanism by which the organism is able to evade the host innate immune defense mechanisms. In addition, the “cathepsin switch” in macrophages, resulting in the increased expression of acidic cathepsins, such as CatB and -D, may be also advantageous to M. tuberculosis, since these proteases could participate in tissue destruction and the formation of pulmonary cavities, events that are necessary for the transmission and perpetuation of tuberculosis infection. Further studies are under way to determine the transcriptional regulatory mechanisms involved in this “cathepsin switch” and its implications to TB pathogenesis.
Acknowledgments
We thank J. Pohl of the Microchemical Facilty of Emory University for providing the antimicrobial peptides used in this study.
This work was supported by National Institute of Allergy and Infectious Diseases grants 1RO1 AI-37937 (J. Roman) and RO1 AI-43316 (W. Shafer) and a Veterans Affairs Merit Review Award (to C. A. Rivera-Marrero). W. Shafer is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
Editor: A. D. O'Brien
REFERENCES
- 1.Bainton, D. F., J. L. Ullyot, and M. G. Farquhar. 1971. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134:907-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore, N., J. Travis, V.C. Onunka, J. Pohl, and W. M. Shafer. 1990. Identification of the primary antimicrobial domains in human neutrophil catG. J. Biol. Chem. 265:13584-13588. [PubMed] [Google Scholar]
- 3.Barret, A. J., and G. Cathepsin. 1981. Methods Enzymol. 80:561-565. [DOI] [PubMed] [Google Scholar]
- 4.Bloom, B. R., and C. J. L. Murray. 1992. Commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Capodici, C., and R. A. Berg. 1989. Cathepsin G degrades denatured collagen. Inflammation 13:137-145. [DOI] [PubMed] [Google Scholar]
- 7.Converse, P. J., A. M. Dannenberg, Jr., J. E. Estep, K. Sugisaki, Y. Abe, B. H. Schofield, and L. M. Pitt. 1996. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect. Immun. 64:4776-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannenberg, A. M. 1984. Chemical and enzymatic host factors in resistance to tuberculosis, p. 721-760. In G. P. Kubica and L. G. Wayne (ed.), The mycobacteria. Marcel Dekker, Inc., New York, N.Y.
- 9.Dannenberg, A. M., and G. A. W. Rook. 1994. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication, p. 459-483. In B. R. Blood (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 10.Denholm, E. M., and F. M. Wolber. 1991. A simple method for the purification of human peripheral blood monocytes. A substitute for Sepracell-MN. J. Immunol. Methods 144:247-251. [DOI] [PubMed] [Google Scholar]
- 11.DesJardin, L. E., T. M. Kaufman, B. Potts, B. Kutzbach, H. Yi, and L. S. Schlesinger. 2002. Mycobacterium tuberculosis-infected human macrophages exhibit enhanced cellular adhesion with increased expression of LFA-1 and ICAM-1 and reduced expression and/or function of complement receptors, FcγRII and the mannose receptor. Microbiology 148:3161-3171. [DOI] [PubMed] [Google Scholar]
- 12.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W.H.O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 13.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, R. D., N. L. Connolly, D. Burnett, E. J. Campbell, R. M. Senior, and T. J. Ley. 1990. Developmental regulation of the human cathepsin G gene in myelomonocytic cells. J. Biol. Chem. 265:1524-1530. [PubMed] [Google Scholar]
- 15.Katzif, S., D. Danavall, S. Bowers, J. T. Balthazar, and W. M. Shafer. 2003. The major cold shock gene, cspA, is involved in the susceptibility of Staphylococcus aureus to an antimicrobial peptide of human cathepsin G. Infect. Immun. 71:4304-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehrer, R. I., A. Burton, K. Daher, S. S. L. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of defensins with Escherichia coli: mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley, T. J., N. L. Connolly, S. Katamine, M. S. Cheah, R. M. Senior, and K. C. Robbins. 1989. Tissue-specific expression and developmental regulation of the human fgr proto-oncogene. Mol. Cell. Biol. 9:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak, P., J. Pohl, A. Dubin, M. S. Reed, S. E. Bowers, M. T. Fallon, and W. M. Shafer. 2003. The increased bactericidal activity of a fatty acid-modified synthetic antimicrobial peptide of human cathepsin G correlates with its enhanced capacity to interact with model membranes. Int. J. Antimicrob. 21:13-19. [DOI] [PubMed] [Google Scholar]
- 19.Munger, J. S., and H. A. Chapman, Jr. 1996. Tissue destruction by proteases, p. 353-361. In W. N. Rom and S. M. Gray (ed.), Tuberculosis. Little, Brown and Company, Boston, Mass.
- 20.Odeberg, H., and I. Olsson. 1975. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect. Immun. 14:1269-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odeberg, H., and I. Olsson. 1976. Antibacterial activity of cationic proteins from human granulocytes. J. Clin. Investig. 56:1118-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto, H.-H., and T. Schirmeister. 1997. Cysteine proteases and their inhibitors. Chem. Rev. 97:133-171. [DOI] [PubMed] [Google Scholar]
- 23.Ragno, S., M. Romano, S. Howell, D. J. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley, L. W. 1996. Phagocytosis of M. tuberculosis, p. 281-289. In W. N. Room and S. Gray (ed.), Tuberculosis. Little, Brown and Company, Boston, Mass.
- 25.Rivera-Marrero, C. A., W. Schuyler, S. Roser, J. Ritzenthaler, and J. Roman. 2002. Mycobacterium tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. Am. J. Physiol. 282:546-555. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Marrero, C. A., W. Schuyler, S. Roser, and J. Roman. 2000. Induction of MMP-9 mediated gelatinolytic activity in human monocytic cells by cell wall components of Mycobacterium tuberculosis. Microb. Pathog. 29:231-244. [DOI] [PubMed] [Google Scholar]
- 27.Salvesen, G., D. Farley, J. Shulman, A. Pryzbla, C. Reiley, and J. Travis. 1987. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry 26:2289-2293. [DOI] [PubMed] [Google Scholar]
- 28.Schwebach, J. R., W. R., Jacobs, Jr., and A. Casadevall. 2001. Sterilization of Mycobacterium tuberculosis Erdman samples by antimicrobial fixation in a biosafety level 3 laboratory. J. Clin. Microbiol. 39:769-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senior, R. M., E. J. Campbell, J. A. Landis, F. R. Cox, C. Kuhn, and H. S. Koren. 1982. Elastase of U-937 monocyte like cells. Comparisons with elastases derived form human monocytes and neutrophils and murine macrophagelike cells. J. Clin. Investig. 69:384-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senior, R. M., and E. J. Campbell. 1984. Cathepsin G in human mononuclear phagocytes: comparisons between monocytes and U937 monocyte-like cells. J. Immunol. 132:2547-2551. [PubMed] [Google Scholar]
- 31.Shafer, W. M., F. Hubalek, M. Huang, and J. Pohl. 1996. Bactericidal activity of a synthetic peptide (CG117-136) of human lysosomal cathepsin G is dependent on arginine content. Infect. Immun. 64:4842-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer, W. M., S. Katzif, S. Bowers, M. Fallon, M. Hubalek, M. S. Reed, P. Veprek, and J. Pohl. 2002. Tailoring an antibacterial peptide of human lysosomal cathepsin G to enhance its broad-spectrum action against antibiotic-resistant bacterial pathogens. Curr. Pharm. Des. 8:695-702. [DOI] [PubMed] [Google Scholar]
- 33.Shafer, W. M., V. C. Onunka, and L. E. Martin. 1986. Antigonococcal activity of human neutrophil cathepsin G. Infect. Immun. 54:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafer, W. M., J. Pohl, V. C. Onunka, N. Bangalore, and J. Travis. 1991. Human lysosomal catG and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J. Biol. Chem. 266:112-116. [PubMed] [Google Scholar]
- 35.Shafer, W. M., M. E. Shepherd, B. Boltin, L. Wells, and J. Pohl. 1993. Synthetic peptides of human lysosomal cathepsin G with potent antipseudomonal activity. Infect. Immun. 61:1900-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro, S. D., E. J. Campbell, R. M. Senior, and H. G. Welgus. 1991. Proteinases secreted by human mononuclear phagocytes. J. Rheumatol. Suppl. 27:95-98. [PubMed] [Google Scholar]
- 37.van der Meer, J. W., J. S. van de Gevel, R. H. Beelen, D. Fluitsma, E. C. Hoefsmit, and R. van Furth. 1981. Culture of human bone marrow in the Teflon culture bag: identification of the human monoblast. J. Reticuloendothel. Soc. 32:355-369. [PubMed] [Google Scholar]
- 38.Welgus, H. G., N. L. Connolly, and R. M. Senior. 1986. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J. Clin. Investig. 77:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerhoff, H. V., D. Juretic, R. W. Hendler, and M. Zasloff. 1989. Magainins and the disruption of membrane-linked free-energy transduction. Proc. Natl. Acad. Sci. USA 86:6597-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolters, P. J., and H. A. Chapman. 2000. Importance of lysosomal cysteine proteases in lung disease. Respir. Res. 1:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]