Abstract
Objective
While it is now recognized that psychotic experiences (PEs) are associated with an increased risk of later mental disorders, we lack a detailed understanding of the reciprocal time-lagged relationships between first onsets of PEs and mental disorders.
Methods
The WHO World Mental Health (WMH) surveys assessed lifetime prevalence and age-of-onset of PEs and 21 common DSM-IV mental disorders among 31,261 adult respondents from 18 countries.
Results
Temporally primary PEs were significantly associated with subsequent first onset of 8 of the 21 mental disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, adult separation anxiety disorder, bulimia nervosa, alcohol abuse), with ORs (95%CI) ranging from 1.3 (1.2–1.5; major depressive disorder) to 2.0 (1.5–2.6; bipolar disorder). In contrast, 18 of 21 primary mental disorders were significantly associated with subsequent first onset of PEs, with ORs (95% CI) ranging from 1.5 (1.0–2.1; childhood separation anxiety disorder) to 2.8 (1.0–7.8; anorexia nervosa).
Conclusions
While temporally primary PEs are associated with an elevated risk of several subsequent mental disorders, we found that most mental disorder are associated with an elevated risk of subsequent PEs. Further investigation of the underlying factors accounting for these time-order relationships might shed light on the etiology of PEs.
Introduction
A recent study based on data from 18 countries participating in the WHO World Mental Health Surveys (WMH) found that lifetime prevalence of any psychotic experience (PE) among adults was 5.8% (standard error [se] = 0.2) (1). These WMH estimates were broadly consistent with PE prevalence estimates in other population-based studies (2, 3). The relatively high lifetime prevalence of PEs is much higher than the 0.7% mean morbid risk for schizophrenia (4) and raises important questions about the clinical significance of PEs with respect to the risk of psychosis and mental disorders in general.
There is solid evidence from prospective studies that PEs are associated with an increased risk of later psychotic disorders (5–7). In addition, there is some evidence that PEs are associated with increased risk of subsequent non-psychotic mental disorders (8), but this field of research has for the most part examined associations between lifetime PEs and lifetime common mental disorders regardless of temporal priority. For example, there is evidence linking the lifetime prevalence of PE with high prevalence of depression and anxiety disorders (9–12), substance use disorders (13), and behavioural disorders (14). Despite these clues, there is a lack of empirical data on whether temporally primary common mental disorders are associated with an increased risk of subsequent first onset of PEs, and conversely, which mental disorders are predicted by pre-existing PEs.
We had the opportunity to carry out a preliminary exploration of the bidirectional time-lagged associations between PEs and mental disorders using retrospective age-of-onset (AOO) reports within the WMH surveys. The aims of this study were to explore the associations of (a) temporally primary PEs with the subsequent onset of mental disorders and (b) temporally primary mental disorders with the subsequent onset of PEs.
METHOD
Samples
The WMH surveys are a coordinated set of community epidemiological surveys administered in probability samples of the household population in countries throughout the world (www.hcp.med.harvard.edu/WMH) (15). Eighteen of the 29 WMH surveys completed up to now included a Psychosis Module. These 18 countries are distributed across North and South America (Colombia, Mexico, Peru, Sao Paulo in Brazil, USA); Africa (Nigeria); the Middle East (Iraq, Lebanon); Asia (Shenzhen in the People’s Republic of China); the South Pacific (New Zealand); and Europe (Belgium, France, Germany, Italy, the Netherlands, Portugal, Romania, Spain). All 18 surveys were based on multi-stage, clustered area probability household sampling designs (Supplementary Table S1). Individual country sample sizes ranged from 301 in France to 7 263 in New Zealand. The weighted average response rate across all 18 surveys was 72.1%.
In keeping with previous epidemiological studies of PEs (1, 13, 16, 17), we made the a priori decision to exclude individuals who had PEs but who also screened positive for possible schizophrenia/psychosis or manic-depression/mania (i.e. respondents who (a) reported (1) schizophrenia/ psychosis or (2) manic-depression/mania in response to the question “What did the doctor say was causing (this/these) experiences?”; and/or (b) those who ever took any antipsychotic medications for these symptoms). This resulted in the exclusion of 140 respondents (0.4% of all respondents), leaving 31 261 respondents for this study (see Table S1).
Procedures
All surveys were conducted in respondents’ homes by trained lay interviewers. Informed consent was obtained before beginning interviews in all countries. Procedures for obtaining informed consent, ethical approvals and protecting individuals were monitored for compliance by the institutional review boards of the collaborating organisations in each country (18). Standardised interviewer training and quality control procedures were used consistently in the surveys. Full details of these procedures are described elsewhere (18, 19).
All WMH interviews had two parts. Part I, administered to all respondents, contained assessments related to core mental disorders. Part II included additional information relevant to a wide range of survey aims, including assessment of PEs. All Part I respondents who met criteria for any DSM IV mental disorder as well as a probability sample of other respondents were administered Part II. Within the different sites, items related to PEs were either administered to all respondents or a random sample of those administered Part II. Part II respondents were weighted by the inverse of their probability of selection for Part II to adjust for differential sampling. Additional weights were used to adjust for differential probabilities of selection within households, nonresponse, and to match the samples to population socio-demographic distributions. The weighted data are analysed here.
Data collection and Data items
The diagnostic instrument used in the WMH surveys was the WHO Composite International Diagnostic Interview (CIDI) (20), a validated fully-structured diagnostic interview (http://www.hcp.med.harvard.edu/wmhcidi/instruments_download.php) designed to assess the prevalence and correlates of a wide range of mental disorders according to the definitions and criteria of both the DSM-IV and ICD-10 diagnostic systems. WHO translation, back-translation, and harmonisation protocols were used to adapt the CIDI for use in each participating country (21).
Psychotic experiences (PEs)
The CIDI Psychosis Module included questions about 6 PE types – 2 related to hallucinations (HEs: visual hallucinations, auditory hallucinations) and 4 related to delusions (DEs: thought insertion/withdrawal, mind control/passivity, ideas of reference, plot to harm/follow) (Supplementary Material Tables S2a and S2b). The module began by asking respondents if they ever experienced the PEs (e.g., “Have you ever seen something that wasn’t there that other people could not see?”; “Have you ever heard any voices that other people said did not exist?” etc). Positive responses were then probed to determine if the reported PEs ever occurred when the person was ‘not dreaming, not half-asleep, or not under the influence of alcohol or drugs’ (this probe question was included in the main question in European Study of the Epidemiology of Mental Disorders (ESEMeD) countries). Respondents who reported PEs were then asked a further probe question about the age-of-onset (AOO) of PEs (i.e., How old were you the very first time (this/either of these things/any of these things) happened to you?). In the 8.0% of cases where PE AOO was missing, we used imputation to assign predicted values based on a set of predictors that included all the variables in the substantive models (22). Key summary statistics (n, mean, SE, median, IQR) for the observed data (without imputation) and the entire dataset after imputation are shown in Supplementary Material Table S4.
DSM-IV Mental disorders
The WMH survey version of the CIDI assessed lifetime history of 21 mental disorders considered in this paper including mood disorders (major depressive disorder, bipolar disorders); anxiety disorders (panic disorder, generalized anxiety disorder, specific phobia, social phobia, agoraphobia without panic, post-traumatic stress disorder (PTSD), separation anxiety disorder further divided into childhood, and adult separation anxiety disorders); impulse control disorders (intermittent explosive disorder, attention deficit disorder, oppositional defiant disorder, conduct disorder); eating disorder (anorexia nervosa, bulimia nervosa, and binge eating disorder); and substance use disorders (alcohol abuse, alcohol dependence, drug abuse, and drug dependence). The disorders that require childhood onset (e.g., attention deficit disorder, oppositional defiant disorder, conduct disorder, separation anxiety disorder) were included in Part II and are limited to respondents in the age range 18–39/44 due to concerns about recall bias among older respondents (23). All other disorders were assessed for the full sample age range. Clinical reappraisal studies indicate that lifetime diagnoses based on the CIDI have good concordance with diagnoses based on blinded clinical interviews (24).
In keeping with previous research, standardised diagnostic hierarchy rules among the disorders assessed were applied where appropriate (25). When applied, a person is excluded from a diagnosis, even though he/she has sufficient symptoms to meet criteria, because the person may have another disorder that is thought to account for those symptoms. For example, someone who has alcohol dependence cannot be diagnosed with alcohol abuse as dependence is a more severe diagnosis. Therefore, alcohol abuse with hierarchy means it cannot occur exclusively during episodes of alcohol dependence. However, given that the analysis was based on a person-year data array (see below), these diagnoses can change within persons over time. For example, a respondent with an onset of alcohol abuse at age 18 and alcohol dependence at 22 would be coded as having alcohol abuse over the years 18–21 and as having alcohol dependence beginning at age 22.
Statistical Analysis
Discrete-time survival analyses (26) with person-year as the unit of analysis and time-varying measures for prior onset of other mental disorders were used to examine the predictive associations of temporally prior disorders with the subsequent onset of each mental disorder considered in the analysis. A person-year dataset was created such that each year in the life of each respondent (up to and including the age of onset of the outcome disorder or their age at interview, whichever came first) was treated as a separate observational record. We estimated survival models that examined bivariate associations between PEs and only one common mental disorder at a time (with adjustment for age-cohorts, sex, person-year, education, marital and employment status, and country) as well as multivariate models that included information on all temporally primary common mental disorders to predict the outcome disorder. The latter models included measures for both type and number of prior mental disorders. In the case of multivariate models of the associations between temporally primary PEs and later disorders, we controlled for the association of other temporally primary disorders, again including measures for both type and number of these disorders, in order to determine if significant net associations existed between temporally primary PEs and subsequent onsets of the outcome disorders. Measures of number of temporally primary disorders (e.g., dummy predictors for exactly 2, exactly 3, and more than 3 such disorders) provide a coarse characterization of the non-additive associations among the predictor disorders. Previous research has shown that the coefficients associated with these predictors are for the most part negative and significant (27), indicating the presence of “sub-additive” interactions among comorbid disorders. The latter suggests that the predictive associations of having multiple temporally prior disorders increase at a decreasing rate as the number of such disorders increases. A more detailed discussion of the logic of these models is presented elsewhere (27).
All survival coefficients and standard errors were exponentiated to create odds-ratios with 95% confidence intervals. Because the WMH survey data were based on geographically clustered and weighted data, standard errors (SE) were estimated with the Taylor series linearization method (28) using SUDAAN software (29) to adjust for weighting and clustering. Statistical significance was evaluated using F tests or Wald χ2 tests based on design-corrected coefficient variance–covariance matrices. Statistical significance was evaluated consistently using two-tailed .05-level tests.
RESULTS
Lifetime prevalence of DSM-IV mental disorders among respondents with and without psychotic experiences
We first examined lifetime prevalence of mental disorders among respondents with and without PEs regardless of the temporal order of PE and mental disorders (Table 1). Compared to those with no PEs, those with PEs had significantly higher odds of having 20 of the 21 mental disorders examined (based on lifetime prevalence). Odds Ratios [OR] and 95% Confidence Intervals [95%CI] ranged from 1.6 (1.2–2.1) for Drug Abuse to 3.6 (2.6–5.0) for Bulimia nervosa.
Table 1.
Lifetime prevalence of DSM-IV mental disorders among those with and without psychotic experiences across WMH samples.
| Type of mental disorders | Among the total sample (n = 31,261) |
Among those with lifetime psychotic experiences (n = 2,385) |
Among those without lifetime psychotic experiences (n = 28,876) |
Odds ratio between lifetime PE and lifetime mental disorder |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| na | %b | SE | na | %b | SE | na | %b | SE | ORc | (95% C.I) | |
| I. Mood disorders | |||||||||||
| Major depressive disorder with hierarchy | 6824 | 11.8 | 0.2 | 897 | 25.4 | 1.2 | 5927 | 10.9 | 0.2 | 2.3* | (2.0–2.6) |
| Bipolar disorder (broad) | 1212 | 2.3 | 0.1 | 196 | 6.5 | 0.6 | 1016 | 2.1 | 0.1 | 2.8* | (2.2–3.5) |
| II. Anxiety disorders | |||||||||||
| Panic disorder | 1019 | 1.8 | 0.1 | 181 | 4.8 | 0.5 | 838 | 1.6 | 0.1 | 2.7* | (2.2–3.4) |
| Generalized anxiety disorder with hierarchy | 1832 | 3.4 | 0.1 | 276 | 8.0 | 0.6 | 1556 | 3.1 | 0.1 | 2.4* | (2.0–3.0) |
| Social phobia | 2495 | 4.7 | 0.1 | 382 | 12.1 | 0.8 | 2113 | 4.3 | 0.1 | 2.4* | (2.0–2.8) |
| Specific phobia | 4108 | 8.4 | 0.2 | 630 | 20.5 | 1.1 | 3478 | 7.6 | 0.2 | 2.6* | (2.3–3.0) |
| Agoraphobia without panic | 557 | 1.0 | 0.1 | 102 | 3.1 | 0.4 | 455 | 0.9 | 0.1 | 2.8* | (2.0–3.9) |
| Post-traumatic stress disorder | 1811 | 3.7 | 0.1 | 328 | 10.2 | 0.7 | 1483 | 3.3 | 0.1 | 3.0* | (2.5–3.6) |
| Separation anxiety disorder (Child) | 449 | 2.2 | 0.1 | 90 | 5.7 | 0.8 | 359 | 2.0 | 0.1 | 2.3* | (1.6–3.1) |
| Separation anxiety disorder (Adult) | 877 | 3.7 | 0.2 | 186 | 11.1 | 1.0 | 691 | 3.2 | 0.2 | 3.1* | (2.4–4.0) |
| III. Impulse-control disorders | |||||||||||
| Intermittent explosive disorder with hierarchy | 1023 | 3.3 | 0.1 | 153 | 8.4 | 0.7 | 870 | 3.0 | 0.1 | 2.1* | (1.7–2.6) |
| Attention deficit disorder | 368 | 1.5 | 0.1 | 79 | 5.1 | 0.8 | 289 | 1.3 | 0.1 | 2.6* | (1.8–3.9) |
| Oppositional defiant disorder with hierarchy | 311 | 2.2 | 0.2 | 68 | 5.8 | 0.9 | 243 | 1.9 | 0.2 | 2.7* | (1.8–4.1) |
| Conduct disorder | 329 | 2.1 | 0.2 | 64 | 5.4 | 0.8 | 265 | 1.8 | 0.2 | 2.3* | (1.6–3.4) |
| IV. Eating disorders | |||||||||||
| Anorexia nervosa | 69 | 0.4 | 0.1 | 13 | 0.7 | 0.3 | 56 | 0.3 | 0.1 | 1.8 | (0.7–4.4) |
| Binge eating disorder with hierarchy | 563 | 2.1 | 0.1 | 128 | 5.0 | 0.5 | 435 | 1.9 | 0.1 | 2.0* | (1.5–2.6) |
| Bulimia nervosa with hierarchy | 364 | 1.1 | 0.1 | 89 | 3.8 | 0.5 | 275 | 0.9 | 0.1 | 3.6* | (2.6–5.0) |
| V. Substance-use disorders | |||||||||||
| Alcohol abuse with hierarchy | 1908 | 5.1 | 0.2 | 232 | 9.8 | 0.9 | 1676 | 4.8 | 0.2 | 1.7* | (1.4–2.0) |
| Alcohol dependence | 1170 | 2.4 | 0.1 | 205 | 6.3 | 0.6 | 965 | 2.1 | 0.1 | 2.3* | (1.8–2.8) |
| Drug abuse with hierarchy | 644 | 1.8 | 0.1 | 83 | 3.6 | 0.5 | 561 | 1.7 | 0.1 | 1.6* | (1.2–2.1) |
| Drug dependence | 481 | 1.2 | 0.1 | 104 | 3.8 | 0.5 | 377 | 1.0 | 0.1 | 2.7* | (1.9–3.6) |
Unweighted number of respondents in each disorder category;
Estimates were based on weighted data.
Based on a series of logistic regression, each one comparing respondents with psychotic experiences and respondents without psychotic experiences, adjusted for country.
Significant at the .05 level, two-sided test.
Temporal priorities between onset ages of PEs and DSM-IV mental disorders
Table 2 examines the temporal sequence between ages of onset of PEs compared to mental disorders within the subset of respondents having both PEs and particular mental disorders. We show the proportion of respondents with (a) PE onset prior to particular mental disorder onset, (b) PE onset in the same year with the mental disorder, and (c) PE onset after the mental disorder onset, and then test if the proportions with PE onset before or after mental disorders significantly differ (i.e., those with onset in the same year were excluded from this comparison). While the overall findings indicate that most PEs have their onset after mental disorders, this pattern varies between diagnoses. For example, the proportion of respondents with PE onset prior to the onset of bipolar disorders was significantly higher (54%) than the proportion with PE onset after the onset of bipolar disorder (39%) (χ2 = 6.0, p <.02), while there was no difference of PE onset with respect to the onset of depressive disorders (χ2 = 0.1, p=.767). By contrast, for many disorders (e.g. 4 of 8 anxiety disorders, and all of the impulse control disorders), higher proportions of respondents reported PE onsets after the onset of the mental disorders of interest.
Table 2.
Lifetime prevalence of psychotic experiences (PEs) onset before and after the onset of DSM-IV mental disorder.
| Type of mental disorders | Number of respondents with PE and Dx |
Respondents with both lifetime prevalence of mental disorder (Dx) of interest and lifetime PEs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % with PE onset prior to Dx onset |
% with PE onset and Dx onset in the same year |
% with PE onset after Dx onset |
Chi-square goodness of fit test for equal proportiona |
||||||
| n | %b | SE | %b | SE | %b | SE | χ21 | P-value | |
| I. Mood disorders | |||||||||
| Major depressive disorder with hierarchy | 897 | 44.5 | 2.0 | 10.2 | 1.2 | 45.4 | 2.2 | 0.1 | [0.767] |
| Bipolar disorder (broad) | 196 | 53.7 | 5.0 | 7.2 | 2.5 | 39.1 | 5.0 | 6.0** | [0.024] |
| II. Anxiety disorders | |||||||||
| Panic disorder | 181 | 42.2 | 5.3 | 10.9 | 3.9 | 46.9 | 5.3 | 1.0 | [0.343] |
| Generalized anxiety disorder with hierarchy | 276 | 46.1 | 4.2 | 6.8 | 1.9 | 47.1 | 4.3 | 0.0 | [0.839] |
| Social phobia | 382 | 22.6 | 2.8 | 6.7 | 2.2 | 70.7 | 3.3 | 113.0** | [<.001] |
| Specific phobia | 630 | 6.7 | 1.2 | 4.5 | 0.9 | 88.9 | 1.5 | 549.8** | [<.001] |
| Agoraphobia without panic | 102 | 28.4 | 5.8 | 3.7 | 1.9 | 68.0 | 5.9 | 38.2** | [<.001] |
| Post-traumatic stress disorder | 328 | 40.0 | 3.9 | 11.2 | 2.3 | 48.8 | 3.6 | 4.2** | [0.047] |
| Separation anxiety disorder (Child) | 90 | 9.1 | 2.8 | 3.8 | 2.0 | 87.1 | 3.4 | 163.5** | [<.001] |
| Separation anxiety disorder (Adult) | 186 | 46.7 | 5.0 | 7.5 | 1.9 | 45.8 | 5.0 | 0.0 | [0.893] |
| III. Impulse-control disorders | |||||||||
| Intermittent explosive disorder with hierarchy | 153 | 25.4 | 3.3 | 5.3 | 2.3 | 69.4 | 3.9 | 105.0** | [<.001] |
| Attention deficit disorder | 79 | 9.4 | 4.9 | 2.9 | 2.7 | 87.6 | 6.1 | 3145.0** | [<.001] |
| Oppositional defiant disorder with hierarchy | 68 | 17.6 | 5.8 | 1.1 | 1.1 | 81.3 | 5.9 | 401.3** | [<.001] |
| Conduct disorder | 64 | 17.7 | 5.9 | 4.9 | 3.4 | 77.5 | 7.2 | 54.0** | [<.001] |
| IV. Eating disorders | |||||||||
| Anorexia nervosa | 13 | 23.6 | 13.6 | - | - | 76.4 | 13.6 | - | - |
| Binge eating disorder with hierarchy | 128 | 44.7 | 5.9 | 6.9 | 2.9 | 48.3 | 5.9 | 0.2 | [0.656] |
| Bulimia nervosa with hierarchy | 89 | 49.8 | 6.3 | 8.4 | 4.0 | 41.8 | 6.4 | 1.4 | [0.266] |
| V. Substance-use disorders | |||||||||
| Alcohol abuse with hierarchy | 232 | 50.1 | 4.6 | 2.6 | 1.2 | 47.3 | 4.6 | 0.5 | [0.494] |
| Alcohol dependence | 205 | 43.3 | 4.4 | 6.3 | 2.6 | 50.4 | 4.8 | 2.8 | [0.109] |
| Drug abuse with hierarchy | 83 | 55.0 | 6.9 | 3.2 | 2.0 | 41.8 | 6.8 | 36.6** | [0.026] |
| Drug dependence | 104 | 48.2 | 6.3 | 7.6 | 3.8 | 44.2 | 6.2 | 2.5 | [0.192] |
Chi-square goodness of fit tests were performed on a reduced subset of respondents comparing % with PE onset prior to Dx onset versus % with PE onset after Dx onset.
Estimates were based on weighted data.
"-" Goodness of fit test cannot be computed due to small expected counts (<5).
Time-lagged associations between lifetime PEs and subsequent onset of mental disorders
A limitation of the results in Table 2 is that they do not take into consideration differences in the age-of-onset distributions of the different disorders in relation to the age of onset distribution of PEs. This issue is addressed in Table 3 (a more detailed presentation of these analyses can be found in Supplementary material Table S4), where we show the results from bivariate and multivariate models of the associations between PEs and subsequent mental disorders (i.e. the temporal order requires the onset of PE to precede the onset of the mental disorder of interest). In the bivariate analysis, the associations are adjusted for age-cohort, gender, person-year, employment status and country as well as time-varying education and marital status (without mental disorder co-morbidity). In these models, preceding PEs were significantly associated with increased odds for 16 of the 21 mental disorders. It is noteworthy that 3 of the 5 remaining disorders have childhood onsets by definition (AHHD, conduct disorder, childhood separation anxiety disorder). These three disorders also had elevated ORs with temporally prior PEs in the range 1.7–2.5, but none of these elevated ORs was statistically significant due to the rarity of PEs occurring prior to the onsets of these disorders. In the multivariate models, we further adjusted for temporally prior comorbid mental disorders (i.e. adjusting for comorbid mental disorders that had onsets prior to the onset of the mental disorder of interest, but not necessarily prior to the onset of PEs). Those with PEs were significantly more likely to subsequently experience 8 of 21 disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, adult separation anxiety disorder, bulimia nervosa, alcohol abuse), with ORs (95%CI) ranging from 1.3 (1.2–1.5) for major depressive disorder to 2.0 (1.5–2.6) for bipolar disorder. In the multivariate models shown in Supplementary Table S4, the ORs associated with number of temporally prior disorders were for the most part lower than 1.0, indicating a general pattern of sub-additive interactions whereby the incremental association of each additional temporally primary disorder lessens in magnitude as the number of such disorders increases.
Table 3.
Associations between lifetime psychotic experiences (PEs) and the subsequent onset of DSM-IV mental disorders.
| Type of mental disorders | Bivariate modelsa | Multivariate modelsb | ||
|---|---|---|---|---|
| Odds of disorder | Odds of disorder | |||
| OR | (95% C.I) | OR | (95% C.I) | |
| I. Mood disorders | ||||
| Major depressive disorder with hierarchy | 1.6* | (1.4–1.9) | 1.3* | (1.2–1.5) |
| Bipolar disorder (broad) | 2.7* | (2.0–3.5) | 2.0* | (1.5–2.6) |
| II. Anxiety disorders | ||||
| Panic disorder | 2.0* | (1.5–2.8) | 1.3 | (0.9–1.8) |
| Generalized anxiety disorder with hierarchy | 1.9* | (1.5–2.4) | 1.4* | (1.1–1.8) |
| Social phobia | 2.0* | (1.5–2.7) | 1.4* | (1.0–1.8) |
| Specific phobia | 1.0 | (0.7–1.5) | 0.9 | (0.6–1.3) |
| Agoraphobia without panic | 2.0* | (1.2–3.4) | 1.2 | (0.7–2.1) |
| Post-traumatic stress disorder | 2.0* | (1.6–2.6) | 1.3* | (1.1–1.7) |
| Separation anxiety disorder (Child) | 1.7 | (0.9–3.2) | 1.2 | (0.6–2.3) |
| Separation anxiety disorder (Adult) | 2.7* | (1.9–3.6) | 1.6* | (1.2–2.2) |
| III. Impulse-control disorders | ||||
| Intermittent explosive disorder with hierarchy | 1.5* | (1.1–2.1) | 1.2 | (0.9–1.6) |
| Attention deficit disorder | 2.5 | (0.8–7.4) | 1.8 | (0.6–5.6) |
| Oppositional defiant disorder with hierarchy | 2.6* | (1.2–5.7) | 2.1 | (0.9–5.0) |
| Conduct disorder | 1.9 | (0.9–4.1) | 1.2 | (0.5–3.0) |
| IV. Eating disorders | ||||
| Anorexia nervosa | 0.9 | (0.3–2.8) | 0.7 | (0.2–2.0) |
| Binge eating disorder with hierarchy | 1.7* | (1.1–2.5) | 1.0 | (0.7–1.6) |
| Bulimia nervosa with hierarchy | 3.2* | (2.2–4.8) | 1.9* | (1.2–3.1) |
| V. Substance-use disorders | ||||
| Alcohol abuse with hierarchy | 1.7* | (1.3–2.3) | 1.4* | (1.1–1.9) |
| Alcohol dependence | 1.9* | (1.4–2.7) | 1.1 | (0.8–1.7) |
| Drug abuse with hierarchy | 1.9* | (1.2–2.8) | 1.4 | (0.9–2.1) |
| Drug dependence | 2.3* | (1.5–3.4) | 1.4 | (0.9–2.0) |
Significant at the .05 level, 2-sided test.
Lifetime PEs was used as a predictor of mental disorder onset in separate discrete-time survival model controlling for age-cohorts, gender, person-years, country, time-varying education, time-varying marriage and employment status.
Lifetime PEs was used as a predictor of mental disorder onset in separate discrete-time survival model including the controls specified above, other temporally primary mental disorders and number of temporally primary mental disorders (2,3,4 5+ disorders). Full details of these models are shown in Supplementary material Table S4.
Time-lagged associations between lifetime mental disorders and subsequent onset of PEs
Next we examined the associations between preceding mental disorders and the subsequent onset of PEs (Table 4). In bivariate models, 20 of the 21 mental disorders were significantly associated elevated odds of subsequent PEs (drug abuse was the only disorder not associated with later PE onset – note that our study excluded PEs that were experienced under the influence of alcohol or drugs). In multivariate models, we found that 18 of the 21 mental disorders were significantly associated with the later onset of PEs, with ORs (95%CI) ranging from 1.5 (1.0–2.1) for associated with the later onset of PEs, with ORs (95%CI) ranging from 1.5 (1.0–2.1) for childhood separation anxiety disorder to 2.8 (1.0–7.8) for anorexia nervosa. As with the previous models predicting mental disorders after temporally primary PEs (Supplementary Table S4), the ORs (95% CIs) associated with number of temporally primary mental disorders (2, 3, 4, 5+) were for the most part less than 1, confirming the existence of sub-additive interactions among mental disorders in predicting first onset of temporally secondary PEs. The joint associations of all the number-of-disorder coefficients were significant (χ24 = 31.1, p <.001).
Table 4.
Associations between DSM-IV mental disorders and the subsequent onset of psychotic experiences.
| Type of mental disorders | Bivariate modelsa | Multivariate modelb | ||
|---|---|---|---|---|
| OR | (95% C.I) | OR | (95% C.I) | |
| I. Mood disorders | ||||
| Major depressive disorder with hierarchy | 2.5* | (2.1–3.0) | 2.2* | (1.8–2.7) |
| Bipolar disorder (broad) | 2.6* | (1.9–3.7) | 1.9* | (1.3–2.9) |
| II. Anxiety disorders | ||||
| Panic disorder | 2.5* | (1.8–3.4) | 1.6* | (1.2–2.3) |
| Generalized anxiety disorder with hierarchy | 2.4* | (1.8–3.1) | 1.7* | (1.2–2.3) |
| Social phobia | 2.2* | (1.8–2.6) | 1.5* | (1.2–2.0) |
| Specific phobia | 2.6* | (2.2–2.9) | 2.2* | (1.8–2.7) |
| Agoraphobia without panic | 2.5* | (1.8–3.6) | 1.9* | (1.3–2.7) |
| Post-traumatic stress disorder | 2.5* | (1.9–3.2) | 1.8* | (1.3–2.4) |
| Separation anxiety disorder (Child) | 2.1* | (1.5–3.0) | 1.5* | (1.0–2.1) |
| Separation anxiety disorder (Adult) | 2.8* | (2.1–3.9) | 1.9* | (1.3–2.8) |
| III. Impulse-control disorders | ||||
| Intermittent explosive disorder with hierarchy | 2.4* | (1.9–3.0) | 1.9* | (1.5–2.5) |
| Attention deficit disorder | 2.7* | (2.0–3.8) | 1.7* | (1.2–2.4) |
| Oppositional defiant disorder with hierarchy | 3.0* | (2.1–4.3) | 2.0* | (1.3–3.1) |
| Conduct disorder | 2.5* | (1.7–3.7) | 1.4 | (0.9–2.3) |
| IV. Eating disorders | ||||
| Anorexia nervosa | 2.9* | (1.1–8.1) | 2.8* | (1.0–7.8) |
| Binge eating disorder with hierarchy | 2.2* | (1.6–3.2) | 1.8* | (1.2–2.6) |
| Bulimia nervosa with hierarchy | 2.7* | (1.8–4.2) | 1.8* | (1.2–3.0) |
| V. Substance-use disorders | ||||
| Alcohol abuse with hierarchy | 1.8* | (1.4–2.5) | 1.8* | (1.3–2.5) |
| Alcohol dependence | 2.4* | (1.8–3.3) | 2.0* | (1.3–2.9) |
| Drug abuse with hierarchy | 1.5 | (1.0–2.3) | 1.1 | (0.7–1.8) |
| Drug dependence | 2.6* | (1.7–3.9) | 1.3 | (0.8–2.3) |
| Joint effect of all types of disorders, χ221, [p-value] | 130.4** | [<.001] | ||
| Difference between types of disorders, χ220, [p-value] | 27.9 | [0.111] | ||
| VI. Number of disorders | ||||
| 2 disorders | 1.0 | (0.7–1.3) | ||
| 3 disorders | 0.5* | (0.4–0.8) | ||
| 4 disorders | 0.4* | (0.2–0.8) | ||
| 5+ disorders | 0.2* | (0.1–0.4) | ||
| Joint effect of all number of disorders, χ24, [p-value] | 31.1** | [<.001] | ||
Significant at the .05 level, 2-sided test.
Each lifetime mental disorder type was used as a predictor of psychotic experiences onset in separate discrete-time survival model controlling for age-cohorts, gender, person-years, country, time-varying education, time-varying marriage and employment status.
The model was estimated with dummy variables for all temporally primary mental disorders and number of temporally primary mental disorders entered simultaneously including the controls specified above.
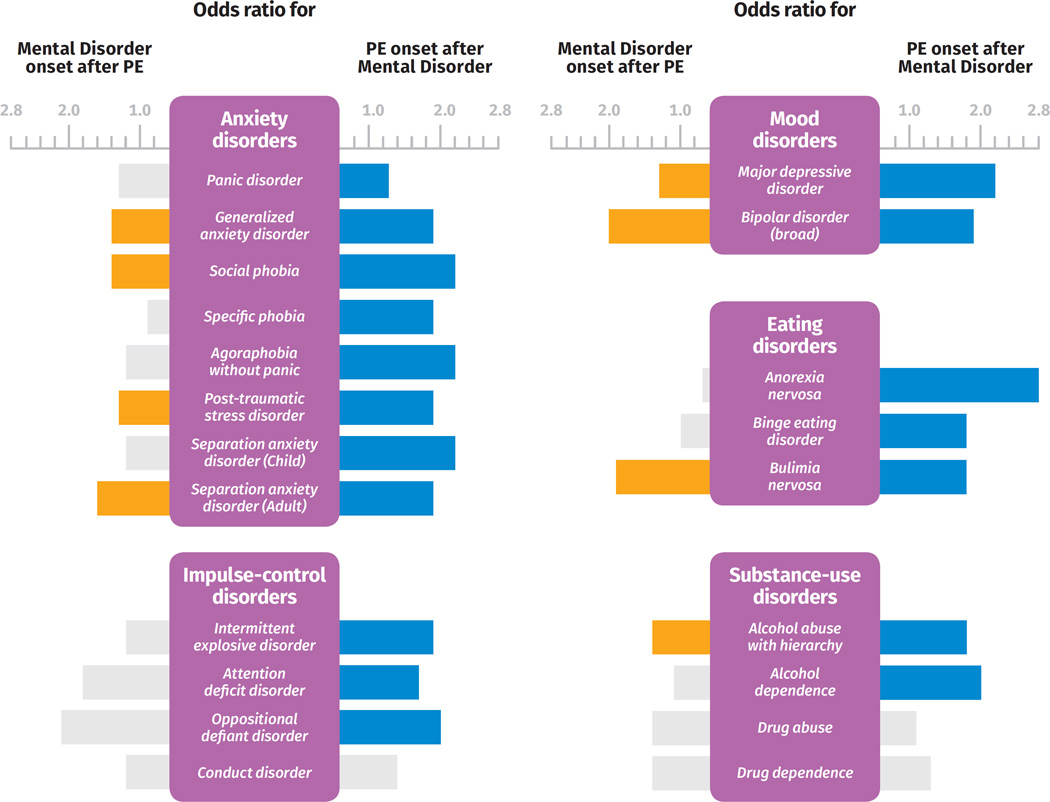
The key findings from Tables 3 and 4 are summarized in the Figure, which allows quick comparisons between; (a) the ORs for the onset of mental disorders after the onset of PEs, with (b) the ORs for the onset of PEs after the onset of mental disorders, when sorted by mental disorder type.
Figure 1.
Summary of the bidirectional association between psychotic experiences and mental disorders
The Odds ratios were extracted from Tables 3 and 4. Bars to the left (in orange if significant) represent the odds ratio of the onset of mental disorders after the onset of Psychotic Experiences. Bars to the right (in blue if significant) represent the Odds Ratio for the onset of mental disorders after the onset of Psychotic Experiences. Odds ratios for nonsignificant associations are shown in light gray.
DISCUSSION
This large, cross-national study demonstrates that individuals with PEs are at increased risk of experiencing a wide range of mental disorders at some stage in their life compared to other people in the population. The mental disorders include mood, anxiety, impulse control, and eating and substance-use disorders. The lack of specificity in these associations even in multivariate models is consistent with the hypothesis that PEs may be non-specific markers of a wide range of mental disorders (30).
Our temporally-ordered analyses have provided new insights into the bidirectional relationship between PEs and mental disorders. We found a strikingly consistent increased risk of PE onset after nearly all of the mental disorders we examined. That is, most mental disorders were associated with increased risk of subsequent PEs even in multivariate models. Of the 21 disorders examined in this study, only three externalizing disorders did not significantly predict subsequent PEs in the multivariate model (i.e. conduct disorder, drug abuse, drug dependence).
We also found that the onset of PEs predicted significantly increased risk of subsequent major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, adult separation anxiety disorder, bulimia nervosa, and alcohol abuse. Thus, in addition to risk of psychotic disorders (8), an increased risk of subsequent mood disorders, some anxiety disorders, eating disorders and substance use disorders are seen after PE onset. These findings call into the question the specificity of the association between PEs and psychotic disorders (31).
Curiously, in the analyses of PEs predicting subsequent onset of mental disorders, PEs predicted none of the four impulse control disorders and only one of the four substance-use disorders (alcohol abuse), while PEs did predict a number of the mood and anxiety disorders. These findings reveal that the temporal relationships between PEs and mental disorders are bidirectional for some disorders. Importantly, this is not due to the different age-of-onset distributions of these disorders, as pervasively significant time-lagged bivariate associations were found across disorders. The differential came only when we estimated multivariate models in which we can see unique incremental associations of a single temporally primary disorder with a temporally secondary disorder net of the additive effects of comorbid disorders. Taking into account the temporal order of the variables of interest, sub-additive associations between comorbid mental disorders and PEs were identified in both analyses (i.e. this feature is also bidirectional).
While the current study has many strengths (e.g. large sample size, range of countries, uniform methodology for data collection), it also has several important limitations. We relied on lay interviewers to administer the questionnaire. We excluded those who were screen-positive for possible psychotic disorders (based on self-report, use of antipsychotic medications to treat PEs). However, we did not have access to valid measures of clinical psychotic disorders, an important consideration for understanding the utility of PEs as predictors of later psychotic disorders. We relied on retrospective reports about age of onset, which might have led to a recall bias despite the use of special age of onset probes in the CIDI that have been shown to improve the accuracy of retrospective age of onset reporting (32). And we estimated models that assumed that predictive associations were additive and independent of age-of-onset, time-since-onset, and, in the models for temporally primary PEs predicting later disorders, interactions of PEs with disorders occurring prior to the onset of the outcome disorders. More complex models that relaxed these simplifying assumptions might well shed light on the asymmetries found here in the time-lagged multivariate associations between PEs and other disorders. Large prospective surveys would be needed to confirm the temporal order between the variables examined in this study. An additional caution is that even in the presence of unequivocal information on temporal ordering of onsets, we cannot infer causality, as common unmeasured causes may influence associations between temporally primary and secondary disorders (see below).
Future directions
Our findings provide a heuristic framework for the generation of new hypotheses related to PEs, which we will explore in subsequent studies. For example, it will be of interest to see what proportion of early versus late onset PEs arise (a) de novo, or (b) following the onset of a mental disorder, as well as the extent to which early-onset PEs and PEs of different types (e.g., DEs vs. HEs) predict the subsequent first onset of different types of mental disorders and do so differentially as a complex function of age-of-onset, time-since-onset, and existence of complex comorbidities. It is also feasible that an underlying diathesis might lead to both an increased risk of PE and an increased risk of mental disorders (regardless of the temporal order of these variables). It is plausible that familial/genetic factors and/or adverse early life exposures (e.g. childhood adversities, cannabis use) could lead to both an increased risk of later mental disorders and an increased risk of PEs (33–35). The comprehensive nature of the WMH survey will allow us to explore these and other related hypotheses in future analyses, albeit within the context of the inherent limitations of cross-sectional data assessed with fully-structured diagnostic interviews.
CONCLUSIONS
Individuals with PEs were significantly more likely to have subsequent first onsets of 8 of 21 common mental disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, separation anxiety disorder (adult), bulimia nervosa, alcohol abuse). Conversely, most temporally primary mental disorders were significantly associated with the subsequent first onset of PEs. Our findings have important implications for the understanding of how PEs fit into the structure of psychopathology and within psychiatric taxonomy. A better understanding of how PEs unfold across the lifespan and interact with mental disorder may provide clues to help guide clinical management.
Supplementary Material
Acknowledgments
(Funding/Support)
Each World Mental Health (WMH) country obtained funding for its own survey. The Sao Paulo Megacity Mental Health Survey is supported by Thematic Project Grant 03/00 204-3 from the State of Sao Paulo Research Foundation; the Shenzhen Mental Health Survey, by the Shenzhen Bureau of Health and the Shenzhen Bureau of Science, Technology, and Information; the Colombian National Study of Mental Health, by the Ministry of Social Protection and the Saldarriaga Concha Foundation; the European Study of the Epidemiology of Mental Disorders project, by contracts QLG5−1999−01042 and 2004123 from the European Commission, the Fondo de Investigacion Sanitaria (the Piedmont Region [Italy]), grant FIS 00/0028 from the Instituto de Salud Carlos III (Spain), grant SAF 2000−158-CE from the Ministerio de Ciencia y Tecnologia (Spain), grants Centro de Investigacion Biomedica en Red CB06/02/0046 and Redes Tematicas de Investigacion Cooperativa en Salud RD06/0011 REM-TAP from the Departament de Salut, Generalitat de Catalunya, Spain, Instituto de Salud Carlos III, other local agencies, and an unrestricted educational grant from GlaxoSmithKline; the Iraq Mental Health Survey (IMHS), by funding from the Japanese and European funds through United Nations Development Group Iraq Trust Fund; the Lebanese National Mental Health Survey, by the Lebanese Ministry of Public Health, the World Health Organization (WHO) (Lebanon), anonymous private donations to the Institute for Development, Research, Advocacy and Applied Care, Lebanon, and unrestricted grants from Janssen Cilag, Eli Lilly and Company, GlaxoSmithKline, Roche, and Novartis; the Mexican National Comorbidity Survey (MNCS), by grant INPRFMDIES 4280 from The National Institute of Psychiatry Ramon de la Fuente, grant CONACyT-G30544-H from the National Council on Science and Technology, and supplemental support from the Pan American Health Organization; the New Zealand Mental Health Survey, by the New Zealand Ministry of Health, Alcohol Advisory Council, and the Health Research Council; the Nigerian Survey of Mental Health and Well-being, by the WHO (Geneva), the WHO (Nigeria), and the Federal Ministry of Health, Abuja; the Peruvian World Mental Health Study, by the National Institute of Health of the Ministry of Health of Peru; and the US National Comorbidity Survey Replication, by grant U01-MH60220 from the National Institute of Mental Health, the National Institute on Drug Abuse, the Substance Abuse and Mental Health Services Administration, grant 044708 from the Robert Wood Johnson
Foundation, and the John W. Alden Trust. The Portuguese Mental Health Study was carried out by the Department of Mental Health, Faculty of Medical Sciences, NOVA University of Lisbon, with the collaboration of the Portuguese Catholic University, and was funded by the Champalimaud Foundation, the Gulbenkian Foundation, the Foundation for Science and Technology (FCT) and the Ministry of Health. The Romania WMH study projects ‘Policies in Mental Health Area’ and ‘National Study regarding Mental Health and Services Use’ were carried out by the National School of Public Health and Health Services Management (former the National Institute for Research and Development in Health, present National School of Public Health Management and Professional Development, Bucharest), with technical support from Metro Media Transylvania, the National Institute of Statistics – National Center for Training in Statistics, Cheyenne Services SRL, Statistics Netherlands and were funded by the Ministry of Public Health (formerly the Ministry of Health) with supplemental support from Eli Lilly Romania SRL.
Additional Contributions
The surveys discussed in this article were administered in conjunction with the WHO WMH Survey Initiative. The WMH staff assisted with instrumentation, fieldwork, and data analysis, and these activities were supported by grant R01MH070884 from the US National Institute of Mental Health, the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, grants R13-MH066849, R01-MH069864, and R01 DA016558 from the US Public Health Service, Fogarty International Research Collaboration award R01-TW006481 from the Fogarty International Center, the Pan American Health Organization, Eli Lilly and Company, Ortho-McNeil Pharmaceutical, Inc, GlaxoSmithKline, and Bristol-Myers Squibb.
Role of the Funder/Sponsor
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
During the past 3 years, Dr Kessler has been a consultant for Hoffman-La Roche, Inc, Johnson & Johnson Wellness and Prevention, and Sanofi and has served on advisory boards for Mensante Corporation, Plus One Health Management, Lake Nona Institute, and US Preventive Medicine.
Evelyn Bromet has funding from CDC/NIOSH (U01OH010712; R. Kotov; PI; U01OH010718, B. Luft, PI; and U01OH010718, A. Gonzalez, PI) and NIA (R01AG049953, S. Clouston, PI).
John McGrath received John Cade Fellowship APP1056929 from the National Health and Medical Research Council.
Footnotes
Disclaimer
The views and opinions expressed in this report are those of the authors and should not be construed to represent the views or policies of any of the sponsoring organizations, agencies, or the WHO.
Conflict of Interest:
All other authors have no conflict of interest.
Disclosures:
No other disclosures were reported.
REFERENCES
- 1.McGrath JJ, Saha S, Al-Hamzawi A, Alonso J, Bromet EJ, Bruffaerts R, Caldas-de-Almeida JM, Chiu WT, de Jonge P, Fayyad J, Florescu S, Gureje O, Haro JM, Hu C, Kovess-Masfety V, Lepine JP, Lim CC, Mora ME, Navarro-Mateu F, Ochoa S, Sampson N, Scott K, Viana MC, Kessler RC. Psychotic Experiences in the General Population: A Cross-National Analysis Based on 31261 Respondents From 18 Countries. JAMA Psychiatry. 2015;72:697–705. doi: 10.1001/jamapsychiatry.2015.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuevo R, Chatterji S, Verdes E, Naidoo N, Arango C, Ayuso-Mateos JL. The Continuum of Psychotic Symptoms in the General Population: A Cross-national Study. Schizophr Bull. 2010;36:475–485. doi: 10.1093/schbul/sbq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- 4.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. Schizophrenia Research. 2006;81:182–183. [Google Scholar]
- 5.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 6.Werbeloff N, Drukker M, Dohrenwend BP, Levav I, Yoffe R, van Os J, Davidson M, Weiser M. Self-reported Attenuated Psychotic Symptoms as Forerunners of Severe Mental Disorders Later in Life. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.1580. [DOI] [PubMed] [Google Scholar]
- 7.Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44:181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- 8.Kaymaz N, Drukker M, Lieb R, Wittchen HU, Werbeloff N, Weiser M, Lataster T, van Os J. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–2253. doi: 10.1017/S0033291711002911. [DOI] [PubMed] [Google Scholar]
- 9.Armando M, Nelson B, Yung AR, Ross M, Birchwood M, Girardi P, Fiori Nastro P. Psychotic-like experiences and correlation with distress and depressive symptoms in a community sample of adolescents and young adults. Schizophr Res. 2010;119:258–265. doi: 10.1016/j.schres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Saha S, Scott J, Varghese D, McGrath J. Anxiety and depressive disorders are associated with delusional-like experiences: a replication study based on a National Survey of Mental Health and Wellbeing. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stochl J, Khandaker GM, Lewis G, Perez J, Goodyer IM, Zammit S, Sullivan S, Croudace TJ, Jones PB. Mood, anxiety and psychotic phenomena measure a common psychopathological factor. Psychol Med. 2014:1–11. doi: 10.1017/S003329171400261X. [DOI] [PubMed] [Google Scholar]
- 12.Varghese D, Scott J, Welham J, Bor W, Najman J, O'Callaghan M, Williams G, McGrath J. Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull. 2011;37:389–393. doi: 10.1093/schbul/sbp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha S, Scott JG, Varghese D, Degenhardt L, Slade T, McGrath JJ. The association between delusional-like experiences, and tobacco, alcohol or cannabis use: a nationwide population-based survey. BMC Psychiatry. 2011;11:202–210. doi: 10.1186/1471-244X-11-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelleher I, Keeley H, Corcoran P, Lynch F, Fitzpatrick C, Devlin N, Molloy C, Roddy S, Clarke MC, Harley M, Arseneault L, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201:26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- 15.Kessler RC, Ustun TB. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York: Cambridge University Press; 2008. [Google Scholar]
- 16.Saha S, Scott JG, Johnston AK, Slade TN, Varghese D, Carter GL, McGrath JJ. The association between delusional-like experiences and suicidal thoughts and behaviour. Schizophr Res. 2011;132:197–202. doi: 10.1016/j.schres.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Scott J, Chant D, Andrews G, Martin G, McGrath J. Association between trauma exposure and delusional experiences in a large community-based sample. Br J Psychiatry. 2007;190:339–343. doi: 10.1192/bjp.bp.106.026708. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Üstün TB. The World Health Organization Composite International Diagnostic Interview. In: Kessler RC, Üstün TB, editors. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York: Cambridge University Press; 2008. pp. 58–90. [Google Scholar]
- 19.Kessler RC, Haro JM, Heeringa SG, Pennell BE, Ustun TB. The World Health Organization World Mental Health Survey Initiative. Epidemiol Psichiatr Soc. 2006;15:161–166. doi: 10.1017/s1121189x00004395. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harkness J, Pennell B, Villar A, Gebler N, Aguilar-Gaxiola S, Bilgen I. Translation procedures and translation assessment in the World Mental Health Survey Initiative. In: Kessler RC, Uston TB, editors. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York: Cambridge University Press; 2008. [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 23.Kessler RC, Angermeyer M, Anthony JC, R DEG, Demyttenaere K, Gasquet I, G DEG, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- 24.Haro JM, Arbabzadeh-Bouchez S, Brugha TS, de Girolamo G, Guyer ME, Jin R, Lepine JP, Mazzi F, Reneses B, Vilagut G, Sampson NA, Kessler RC. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res. 2006;15:167–180. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer JD, Willett JB. It's about time: using discrete-time survival analysis to study duration and the timing of events. Journal of Educational Statistics. 1993;18:155–195. [Google Scholar]
- 27.Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, Stein DJ, Zaslavsky AM, Aguilar-Gaxiola S, Alonso J, Andrade L, Benjet C, de Girolamo G, de Graaf R, Demyttenaere K, Fayyad J, Haro JM, Hu C, Karam A, Lee S, Lepine JP, Matchsinger H, Mihaescu-Pintia C, Posada-Villa J, Sagar R, Ustun TB. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch Gen Psychiatry. 2011;68:90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolter KM. Introduction to Variance Estimation. New York: Springer-Verlag; 1985. [Google Scholar]
- 29.RTI International: SUDAAN: Software for the statistical analysis of correlated data [computer program] Research Triangle Park, North Carolina, USA: RTI International; 1999. [Google Scholar]
- 30.Saha S, Scott JG, Varghese D, McGrath JJ. The association between general psychological distress and delusional-like experiences: a large population-based study. Schizophr Res. 2011;127:246–251. doi: 10.1016/j.schres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher I, Cannon M. Whither the psychosis-neurosis borderline. Schizophr Bull. 2014;40:266–268. doi: 10.1093/schbul/sbt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knäuper B, Cannell CF, Schwarz N, Bruce ML, Kessler RC. Improving accuracy of major depression age-of-onset reports in the US National Comorbidity Survey. International Journal of Methods in Psychiatric Research. 1999;8:39–48. [Google Scholar]
- 33.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood Adversities Increase the Risk of Psychosis: A Meta-analysis of Patient-Control, Prospective- and Cross-sectional Cohort Studies. Schizophr Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, Alati R, Williams GM, Bor W, Najman JM. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67:440–447. doi: 10.1001/archgenpsychiatry.2010.6. [DOI] [PubMed] [Google Scholar]
- 35.van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102:1251–1260. doi: 10.1111/j.1360-0443.2007.01875.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.