Abstract
Cross-section studies suggest that measures of pain sensitivity, derived from quantitative sensory testing (QST), are elevated in persons with chronic pain conditions. However, little is known about whether development of chronic pain is preceded by elevated pain sensitivity or pain sensitivity increases as a result of prolonged experience of pain. Here we used QST to test static (single suprathreshold stimuli) and dynamic (temporal sensory summation) pain processing of thermal stimuli. Muscle pain was induced using high-intensity exercise (DOMS). Multi-level modeling approaches determined the daily covariation among static and dynamic QST measures and pain intensity. Variation in responses to static pain sensitivity was not associated with pain intensity from DOMS while, in contrast, variation in dynamic pain sensitivity was positively associated with variation in pain intensity from DOMS. This finding supports the use of TSS as a marker of the central pain state and potentially as an appropriate measure for treatment monitoring.
Keywords: Pain, Pain sensitivity, Quantitative sensory testing, Delayed onset muscle soreness
1. Introduction
Individuals with chronic musculoskeletal pain conditions demonstrate elevated pain sensitivity in response to experimental pain testing. This finding is consistent across conditions and experimental testing modalities. For example, individuals with fibromyalgia consistently report higher pain intensity than pain free individuals when tested using standard stimuli. This is the case whether static or dynamic measures of pain sensitivity are assessed. Similar findings are reported for individuals with chronic back or neck pain, osteoarthritis, and orofacial pain conditions. The limitation to these findings is that the studies that show these differences between individuals with and without chronic pain are cross-sectional. Consequently what remains unknown is whether individuals with chronic musculoskeletal pain conditions had elevated pain sensitivity prior to the painful episode precipitating the chronic pain condition.
Recent clinical trials of body-based interventions for these chronic musculoskeletal pain conditions show effectiveness in patients with spine pain, carpal tunnel syndrome and other peripheral monoarticular pain conditions such as osteoarthritis of the knee or hand [8,9]. Body-based interventions are a class of complementary and alternative medicine interventions in which movement is caused in joints, muscles or nerves and includes techniques such as joint manipulation and massage. Laboratory and clinical studies indicate hypoalgesia to mechanical [11–13,25], thermal [4,5,14] and electrical [23] stimuli occur in response to these interventions. In our studies of healthy participants and patients with pain we have identified immediate reductions in temporal sensory summation of thermal pain (TSS) after both interventions that target joints [4,5,14] and interventions that target the neurovascular bundle [3]. These are relevant findings as TSS is thought to represent a dynamic behavioral measure of activity dependent modulation of neurons at the dorsal horn observed in animal studies in response to similar stimulation parameters [21]. Additionally, the magnitude of TSS influences the effect of fear on the relationship between pain and disability. The observed reductions in TSS after body-based interventions are therefore hypothesized to indicate changes that would be favorable for clinical outcome. Recent work supporting this theory indicates available plasticity within the nervous system (reductions in TSS after an intervention) to be prognostic of recovery from shoulder pain [24].
Combined, the findings of elevated pain sensitivity in chronic pain conditions and responsiveness of pain sensitivity measures to intervention suggest experimental pain sensitivity to thermal stimuli to be important markers of pain states and useful for treatment monitoring. For both these reasons, the purpose of this analysis was to determine the extent to which measures of thermal pain sensitivity vary with pain intensity over time.
We enrolled fifty-two pain-free participants (36 women; average age 22.4 years; average BMI 24.1). All participants read and signed an informed consent form approved by the University Institutional Review Board. Participants were excluded if they met any of the following criteria: previous participation in a conditioning program specific to trunk extensors in the past 3 months, any current back pain, any chronic medical conditions that may affect pain perception, kidney dysfunction, major psychiatric disorder, history of previous injury including surgery to the lumbar spine, cardiac conditions, osteoporosis, or liver dysfunction, or performance of any intervention for symptoms induced by exercise before the termination of their participation in the protocol.
2. Measures
2.1. Pain intensity
Exercise induced pain intensity was measured with a visual analog scale (VAS) consisting of a 100 mm line anchored at one end with “none” and at the other with “‘worst imaginable” [20]. Participants rated “worst pain in the back or legs today” by placing a mark along the 100 mm line.
2.2. Pain sensitivity
We used thermal quantitative sensory testing to assess pain sensitivity over lumbar innervated areas of the lower extremity using a computer-controlled Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel) through a 900 mm2 Peltier thermode. All participants underwent a practice session prior to the first testing session. During this practice subjects experienced the range of temperatures to which they were to be exposed. Participants practiced using the rating scale to rate the intensity of the first pain experienced in response to each stimulus. In order to standardize the scaling instructions and to clarify the distinction between the sensory intensity and affective dimensions, a standardized instructional set was used for all subjects during every exposure to the thermal stimuli. The scale instructions were repeated for every set of ratings within each session [22].
First, we measured responses to single suprathreshold stimuli. Participants rated single thermal stimuli of 47 °C and 49 °C, presented in a random order. Single stimuli have been described as basal or state measures of pain sensitivity [1] and thought to be predominantly A-delta fiber mediated [19].
Next, we used a train of 10 thermal pulses to the posterior calf of the non-dominant leg to induce TSS. Each stimulus started at a baseline temperature of 39 °C, peaked at 51 °C, then returned to baseline with a rise and decline rate of 10 °C/s (stimulus frequency = 0.33 Hz). Subjects were instructed to attend to the delayed pain sensation felt after each heat pulse (second pain), and verbally rate the intensity of this sensation using a numerical scale from 0 (no pain sensation) to 100 (most intense pain imaginable). TSS was calculated by subtracting the rating of the 1st pulse from that of the 5th pulse. Before the test, participants underwent training to acclimate them to the range of thermal stimuli and rating of ‘second’ pain. TSS represents a dynamic aspect of pain processing [1] and is predominantly c-fiber mediated [19].
2.3. Induction of muscle pain
We induced pain in the low back using our previously reported delayed-onset muscle soreness protocol [6]. Briefly, participants’ isometric trunk extensor torque was measured using a Medx Lumbar Exercise machine. Participants then performed a trunk extension exercise at 80% of his or her maximum torque until fatigue (indicated by a reduction to 50% of the pre-exercise torque). Details of this model and subsequent disability and impairment in motor performance are summarized elsewhere [6].
Daily associations between the ratings of the thermal stimuli and muscle pain intensity over 1-week were tested using multilevel modeling techniques. In these models pain intensity was the dependent variable and we were primarily interested in the fixed effects of the single stimulus and dynamic stimulus (TSS). Fixed effects represent ‘average effects,’ or effects that hold for all members of the group. We also performed random effects tests. A significant random variance term would indicate that the magnitude of the within-person relationship between pain intensity and pain sensitivity may differ substantially across individuals. Assuming that each value for each measure would be correlated with the previous value of that measure, we chose to use an autoregressive covariance structure (AR1). AR1 rho was found to be significantly different from zero and this parameter model was chosen for the analysis.
Peak pain intensity reported by participants ranged from 0 to 68 on the VAS. Pain peaked between 24 and 48 h after exercise and resolved over the subsequent week of testing. The daily ranges of responses to quantitative sensory testing recorded from participants are shown in Table 1, indicating considerable individual variability in pain. Ratings at both 47 and 49 °C varied from 0 to 80 in the pain free baseline testing session.
Table 1.
Diurnal variation in ranges of participant responses to quantitative sensory testing.
| Baseline | Day 1 | Day 2 | Day 4 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain intensity (VAS) | 0 | 0 | 0 | 63 | 0 | 68 | 0 | 36 | 0 | 21 |
| 47 °C | 0 | 90 | 0 | 80 | 0 | 85 | 0 | 95 | 0 | 100 |
| 49 °C | 0 | 95 | 0 | 95 | 0 | 87 | 0 | 100 | 0 | 100 |
| Temporal sensory summation | −15 | 50 | −40 | 90 | −30 | 45 | −30 | 80 | −20 | 45 |
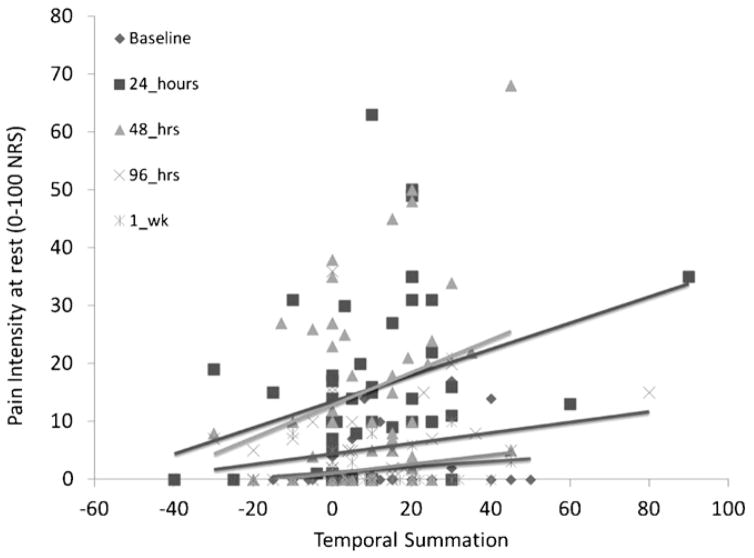
The analysis of the association between responses to 47 °C stimuli and pain intensity indicated no fixed effect of responses to the Bexperimental stimuli (F1,203.4 = 0.015, p = 0.904) nor were effects noted at 49 °C (F1,201.1 = 0.663, p = 0.882). In contrast, TSS was a significant predictor of daily muscle pain intensity (F1,208.9 = 7.253, p = 0.008). The daily association between pain intensity and TSS is graphical displayed in Fig. 1. No significant random effects were identified.
Fig. 1.
Daily variation in temporal sensory summation and muscle pain intensity at rest. Baseline is the first day of testing and the day that muscle pain was induced.
The estimate for the fixed effect of TSS on daily pain intensity was 0.13 (see Table 2).
Table 2.
Fixed effects model with all participants included.
| Source | Estimate | F | Sig. |
|---|---|---|---|
| Intercept | 6.131 | 14.269 | 0.000 |
| 47 °C | 0.006 | 0.015 | 0.904 |
| 49 °C | −0.007 | 0.022 | 0.883 |
| Temporal sensory summation | 0.130 | 7.253 | 0.008 |
For TSS, we noted that 15% of the participants had a negative slope of TSS at the baseline testing; that is, these participants reported less pain during the train of stimuli rather than more. This percentage was consistent across sessions. Consequently we made a post hoc decision to perform an additional analysis to examine the daily variation of this group of participants in a separate model. In that analysis, there were no effects for single stimuli, consistent with the analysis for the entire group of participants. However, the effect for TSS was also non-significant (F1,16.9 = 0.207, p = 0.655) in this model.
3. Discussion
The data from the current analysis in participants with acutely exercise induced low back pain indicate that TSS covaries with pain intensity, at least for those individuals who demonstrate TSS. TSS is considered a dynamic measure of pain processing affected by a variety of factors including the frequency and intensity of the peripheral input as well as the sensitivity of the peripheral receptors and the neurons of the dorsal horn and cortex [1]. Our model uses exercise to induce delayed onset muscle soreness. Based on our work and that of others, muscle damage occurs during exercise driving the changes that generate muscle soreness and pain that mimics low level clinical pain conditions [2,7,18]. One might argue that changes in TSS in our study were related to peripheral sensitivity after muscle damage. Increases in pain sensitivity occur due to activation and sensitization of receptors, such as transient receptor potential vanilloid receptor-1 (TRPV1), through intracellular [10] and extra-cellular [27] kinase signaling pathways or increases in circulating inflammatory cytokines [17].
However, if this were the case, changes in the responses to static stimuli would also be expected. As this was not observed, modulation of spinal cord or cortex responses must have accounted for the changes in TSS. After the inflammation associated with the acute post-injury phase, there is also an increase in brain-derived neurotrophic factor (BDNF)[15] and substance P [16] which both modulate central drive [26]. While we did not directly measure this, it is a logical conclusion supported by our data.
Approximately 15% of our participants did not have TSS. This was expected based on our prior studies of pain free individuals and analyses suggesting pain sensitivity ‘phenotypes’. However, what we had not expected was that individuals who did not experience TSS at baseline showed no statistical covariation in TSS with pain intensity. In prior studies of patients with chronic pain conditions, such as fibromyalgia, there have been no reports of participants who do not have TSS. In fact just the opposite is repeatedly reported; that is, individuals with fibromyalgia have elevated sensitivity and enhanced TSS. This combination of findings is potentially quite significant. We speculate that individuals without TSS may have less likelihood of progressing to chronic pain states. Similarly we speculate that interventions that reduce TSS in those individuals with positive TSS may prevent progression to chronic pain.
The strongest evidence for this latter hypothesis comes from work studying patients undergoing surgical intervention for shoulder pain. In that study, patients who showed reductions in TSS after surgery experienced clinically meaningful improvements in shoulder pain at three months post-intervention. In contrast those patients in whom there was no change in TSS after surgery continued to report pain and interference in daily activity at three months [24].
Other evidence for this speculation comes from intervention studies that show reductions in TSS. Several prior studies by our group indicate changes in TSS after conservative rehabilitation interventions across several musculoskeletal models of pain (e.g. low back pain [5] and carpal tunnel syndrome [3]). Consequently, that interventions affect TSS may indicate that these interventions can have broader effects on other aspects of central sensitization potentially reducing maintenance of musculoskeletal pain conditions.
In summary, the primary finding from this current study was that in general TSS covaries with pain intensity, while responses to single suprathreshold stimuli do not. This finding supports the use of TSS as a marker of the central pain state and potentially as an appropriate measure for treatment monitoring.
HIGHLIGHTS.
Temporal sensory summation (TSS) is elevated in persons with chronic pain conditions.
Little is known about longitudinal changes in TSS during the pain experience.
These findings indicate that TSS varies positively with pain intensity.
Acknowledgments
This project was completed with support by grant #AR054331-01A2 from the National Institutes of Arthritis, Musculoskeletal and Skin Diseases, and by the University of Florida, USA. This project was completed with the assistance of Maggie E. Horn, DPT, MPH.
References
- 1.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. Journal of Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj P, Graven-Nielsen T, Wright A, Davies LAI, Arendt-Nielsen L. Muscle hyperalgesia in postexercise muscle soreness assessed by single and repetitive ultrasound stimuli. Journal of Pain. 2000;1:111–121. [Google Scholar]
- 3.Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. Journal of Orthopaedic and Sports Physical Therapy. 2009;39:709–723. doi: 10.2519/jospt.2009.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskeletal Disorders. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, Jr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Physical Therapy. 2009;89:1292–1303. doi: 10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop MD, Horn ME, George SZ. Exercise-induced pain intensity predicted by pre-exercise fear of pain and pain sensitivity. Clinical Journal of Pain. 2011;27:398–404. doi: 10.1097/AJP.0b013e31820d9bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop MD, Horn ME, Lott DJ, Arpan I, George SZ. Magnitude of spinal muscle damage is not statistically associated with exercise-induced low back pain intensity. The Spine Journal. 2011;11:1135–1142. doi: 10.1016/j.spinee.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, Delitto A. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Annals of Internal Medicine. 2004;141:920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JA, Fritz JM, Childs JD, Kulig K. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: study protocol of a randomized clinical trial [ NCT00257998] BMC Musculoskeletal Disorders. 2006;7:11. doi: 10.1186/1471-2474-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. British Journal of Anaesthesia. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Carnero J, Fernandez-de-las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. Journal of Manipulative and Physiological Therapeutics. 2008;31:675–681. doi: 10.1016/j.jmpt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cleland JA, Rodriguez-Blanco C, Alburquerque-Sendin F. Changes in pressure pain thresholds over C5–C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. Journal of Manipulative and Physiological Therapeutics. 2008;31:332–337. doi: 10.1016/j.jmpt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-de-las-Penas C, Perez-de-Heredia M, Brea-Rivero M, Miangolarra-Page JC. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. Journal of Orthopaedic and Sports Physical Therapy. 2007;37:325–329. doi: 10.2519/jospt.2007.2542. [DOI] [PubMed] [Google Scholar]
- 14.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskeletal Disorders. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi K, Morita Y, Kiyama H, Ono K, Tohyama M. A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry. Brain Research. 1988;464:31–35. doi: 10.1016/0169-328x(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 17.Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Medicine and Science in Sports and Exercise. 1996;28:953–961. doi: 10.1097/00005768-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Medicine and Science in Sports and Exercise. 1995;27:1263–1269. [PubMed] [Google Scholar]
- 19.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. Journal of Investigative Dermatology. 1977;69:167–171. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 20.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 21.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. Journal of Neurophysiology. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 22.Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–216. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 23.Terrett AC, Vernon H. Manipulation and pain tolerance. A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. American Journal of Physical Medicine. 1984;63:217–225. [PubMed] [Google Scholar]
- 24.Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. Journal of Pain. 2012;13:81–89. doi: 10.1016/j.jpain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. doi: 10.1016/S0304-3959(96)03221-6. [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang ZY, Xu H, Clapham D, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. Journal of Neuroscience. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]