Abstract
Objectives
Few routine systems exist to test older, asymptomatic children for HIV. Testing all children in the population has high uptake but is inefficient, while testing only symptomatic children increases efficiency but misses opportunities to optimize outcomes. Testing children of HIV-infected adults in care may efficiently identify previously undiagnosed HIV-infected children before symptomatic disease.
Methods
HIV-infected parents in HIV care in Nairobi, Kenya were systematically asked about their children’s HIV status and testing history. Adults with untested children ≤12 years old were actively referred and offered the choice of pediatric HIV testing at home or clinic. Testing uptake and HIV prevalence were determined, as were bottlenecks in pediatric HIV testing cascade.
Results
Of 10,426 HIV-infected adults interviewed, 8,287 reported having children, of whom 3,477 (42%) had children of unknown HIV status, and 611 (7%) had children ≤12 years of unknown HIV status. Following implementation of active referral, the rate of pediatric HIV testing increased 3.8-fold from 3.5 to 13.6 children tested per month, (RR: 3.8, 95%CI: 2.3–6.1). Of 611 eligible adults, 279 (48%) accepted referral and were screened, and 74 (14%) adults completed testing of 1 or more children. HIV prevalence among 108 tested children was 7.4% and median age was 8 years (IQR: 2–11); one child was symptomatic at testing.
Conclusions
Referring HIV-infected parents in care to have their children tested revealed many untested children and significantly increased the rate of pediatric testing; prevalence of HIV was high. However, despite increases in pediatric testing, most adults did not complete testing of their children.
Keywords: pediatric, HIV testing, older children, targeted testing, index case testing
INTRODUCTION
An estimated 2.1 million children worldwide are HIV-infected, most of whom were born prior to scale up of prevention of mother-to-child HIV transmission (PMTCT) programs [1–3]. While PMTCT programs have resulted in marked declines in new infant HIV infections and include systems for early infant diagnosis, older children are not routinely tested in PMTCT and are often diagnosed when symptomatic. According to a national survey done in 2012, nearly 60% of HIV-infected children in Kenya are undiagnosed [5]. Outcomes of HIV-infected children who are diagnosed and treated when symptomatic are worse than among those who are treated prior to symptomatic disease [6–12].
Routine testing of all children in the general population may be costly and inefficient due to a low overall HIV prevalence. Conversely, provider initiated testing and counseling (PITC), when incompletely implemented tends to preferentially test the sickest children who have advanced disease [13] but when close to universal uptake is achieved has high yield and identifies children prior to severe illness [14]. Testing children of adults attending HIV care may provide an efficient way to diagnose HIV-infected children prior to symptoms and improve outcomes [15, 16], as these children have a higher probability of being HIV-exposed than the general population. Children have lagged behind adults in HIV care and treatment globally, with only 24% on ART, compared to 38% of adults [4]. UNAIDS has called for 90–90–90 targets—to identify 90% of infected individuals, treat and retain 90% [17]. The first step for children to attain these goals is diagnosis. Targeted HIV testing for the children of HIV-infected parents may be a more efficient strategy for case detection than universal screening [15, 16, 18, 19].
In this study we systematically offered testing to the children of HIV-infected parents in an HIV treatment program in Kenya. We determined the number of eligible children, uptake, and yield of pediatric testing under this active, targeted model.
METHODS
Ethics statement
The study was approved by the University of Washington Institutional Review Board (IRB) and the Kenyatta National Hospital (KNH)/University of Nairobi (UoN) Ethics and Research Committee. Oral informed consent was obtained from each adult participant enrolling in the study; written informed consent was then obtained from the subset of those completing pediatric testing. Older children whose parents agreed provided written assent for the HIV test. At the time of this study, children ≥16 years (or emancipated minors > 14) could provide independent consent for HIV testing, while others required parental consent; these older ages were not included in this study. Two external groups from Kenya and the US assisted in determining the appropriate upper age for inclusion in this study; both agreed that the current approach was not appropriate for adolescents age ≥13, recommending further formative work to address adolescents’ distinct needs.
Study design
This prospective cohort study evaluated uptake of a targeted HIV testing intervention for children. The Counseling and Testing for Children at Home (CATCH) pilot study began recruitment at Kenyatta National Hospital (KNH) in November of 2013 and completed enrollment in September of 2014. KNH is a national referral hospital that serves a large catchment area around Nairobi, including suburbs, urban, and peri-urban areas. Clients were recruited and referred from the KNH Voluntary Counseling and Testing Clinic (VCT), PMTCT Clinic, and Comprehensive Care Centre (CCC), a PEPFAR-supported program that provides free HIV management and antiretroviral therapy. Aggregate hospital testing records from the CCC clinic were collected for the period of January 2013 to August 2014 to determine overall testing rates at the site.
Caregivers were eligible if they were HIV-infected and had at least one child 12 years of age or younger of unknown HIV status. Willingness to have a child tested for HIV was not an enrollment criterion and efforts were made to enroll parents who did not want to test children for HIV. Recruitment staff encouraged all eligible adults to speak with study staff, and reassured clients that they would not be pressured to test children. Children were considered to be of unknown HIV status if their biological mother was HIV-infected or if their biological mother’s partner was HIV-infected but her HIV status was unknown AND either of the following criteria were met: a) the child had never been tested, b) the child had tested negative as an infant but had not had confirmatory negative testing after cessation of breastfeeding, or c) the caregiver felt unsure about the child’s status and wished for the child to be tested. Caregivers were able to have any children formally in their care (either biological parent or legal guardian) tested as part of the study.
Recruitment and enrollment
Each HIV-infected clinic attendee was evaluated by existing clinic staff at appointment arrival and registration using a simple log which asked whether the adult had any children of unknown HIV status, children’s ages, and whether the adult had previously been approached about the study. Adults were invited to participate in the study if they had any children of unknown HIV status ≤12 years. No additional health care workers were added to complete the recruitment and referral; this active referral differed from standard of care, in which health care workers determined whether an adult had children of unknown status sporadically. Caregivers were referred to the study regardless of their interest in testing their children. Caregivers who declined referral to the study were not contacted further. Caregivers were screened by study staff and a subset was further excluded due to incompletely assessed eligibility. At enrollment, study staff collected information about caregiver sociodemographic characteristics, adult and child HIV testing and treatment history, and reasons a parent might want to have child testing. Caregivers were given the choice of having home-based, clinic-based, or no HIV testing for their children.
HIV testing and care referral
Following enrollment, caregivers were asked to schedule a home or clinic HIV testing visit. Caregivers were reminded of their appointment to test their children by phone in advance. Caregivers who missed the testing visit were rescheduled by phone a maximum of 2 additional times, after which they were considered to have declined testing. Children were tested for HIV either in their home or at the CATCH-pilot clinic at KNH. Kenyan national HIV testing guidelines were followed [20]; children over 18 months who had ceased breastfeeding were tested by a series of two rapid HIV serologic tests. Children under 18 months or who were recently breastfed were tested first by rapid serologic HIV test and confirmed by HIV DNA PCR test. HIV DNA PCR tests were conducted at the University of Nairobi laboratory as previously described [21].
HIV-infected children and HIV-exposed infants were referred to the HIV care clinic or PMTCT clinic of their caregiver’s choice to begin treatment. Caregivers of HIV-infected children were re-contacted by phone or in-person appointment post-diagnosis to confirm linkage to care, defined as child enrolling at an HIV care clinic and opening a file.
Statistical analysis
An interrupted time series analysis using Prais-Winsten regression (linear regression that models first order autocorrelation) was used to compare the mean number of children tested monthly during the period prior to the introduction of the CATCH-pilot study intervention and during the CATCH-pilot study intervention period. To assess the relative increase in proportion of children tested monthly during the pre- and during-intervention periods, a generalized linear model with a log link, normal family, and robust standard errors was used. In both models, a linear term was included to account for natural temporal trends in testing; an interaction term was considered to assess a change in slope between the pre- and during-intervention time periods. November 2013 was considered a washout period and was not included in analyses.
In estimates of the rates of testing, the denominator of the number of adults in care with children <13 of unknown HIV status was assumed to be constant across the 20 month period. This denominator was estimated by multiplying the number of adults active in care by the proportion of adults who self-reported having children ages 0–12 of unknown HIV status.
In analyses to determine the overall gap in uptake of testing, adjustments were made to the denominator of adults with children of unknown status ages 0–12 to adjust for initial errors in assessments of eligibility during the recruitment and referral process and to account for referred clients not being screened due to staff eligibility. To estimate this corrected denominator, the number of adults referred was multiplied by the proportion of adults screened and then by the proportion of those who were deemed eligible.
All analyses were conducted using Stata 14 IC (StataCorp, College Station, TX). All tests were two-sided with alpha of 0.05.
RESULTS
Higher uptake at CCC than at VCT and PMTCT clinics
The referral model was evaluated in VCT, PMTCT, and CCC (HIV care programs) in a pilot period, during which time 11 adults were screened at VCT with 0 enrolled, 508 women were screened in PMTCT with 10 enrolled, and 632 adults were screened at CCC and 50 were enrolled. Because the number of enrollees from VCT and PMTCT clinics was low, recruitment ended at these sites in February 2014 (PMTCT) and March 2014 (VCT) and the following analyses include only the parents derived from the CCC treatment program, described below.
Burden of untested children high in HIV care clinics, and prevalence of HIV among tested children is high
During the intervention period between December 2013 and September 2014, 10,426 adults were screened at KNH CCC, of whom 8,287 had children, 3,477 (42%) had children of unknown HIV status, and 611 (7%) had children of unknown status ≤12 years. Among these 611, 320 declined referral to CATCH-pilot study, 12 were not screened due to staff unavailability, and 279 were screened. Among those screened, 40 were ineligible, 123 were eligible but not enrolled (of 133 who initially declined enrollment, 10 later enrolled), and 116 enrolled (Figure 1). Among 116 HIV-infected adults enrolled, 74 (64%) tested their children and had 108 children tested, of whom 8 (7.4%) were HIV-infected; median age was 8 years (IQR: 2–11). One child was symptomatic at the time of testing and all children linked to care, at which time all children were WHO stage I and median CD4 count was 666 (IQR: 508–2304) (n=6).
FIGURE 1.
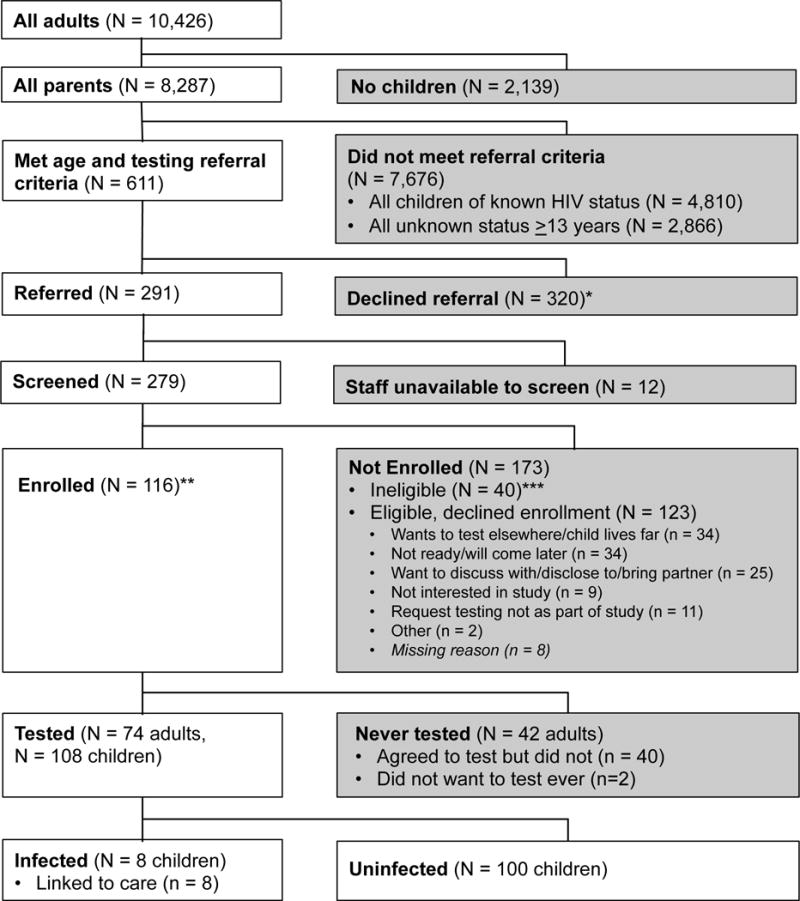
Study flow chart. *Reasons for declining referral were not possible to collect as referral conducted by non-study clinic staff. **Of 116 enrolled, 106 enrolled on same day, while 10 enrolled after booking appointment to return. ***Reasons for ineligibility: Children all ≥13 years (n = 17), no children of unknown HIV status (n = 10), caregiver not infected (n = 5), enrolled in other HIV study (n = 3), missing reason (n = 5).
Systematic assessment and active referral increased testing rates
After controlling for temporal trends and autocorrelation, on average, 10.1 more children were tested per month during the 9 month period at KNH CCC when the team implemented active referral compared to the 10 month period prior to the intervention (13.6 vs 3.5 children per month, p<0.001) (Figure 2), a 3.8-fold increase in the proportion of children tested (RR: 3.8, 95%CI: 2.3–6.1, p<0.001).
FIGURE 2.
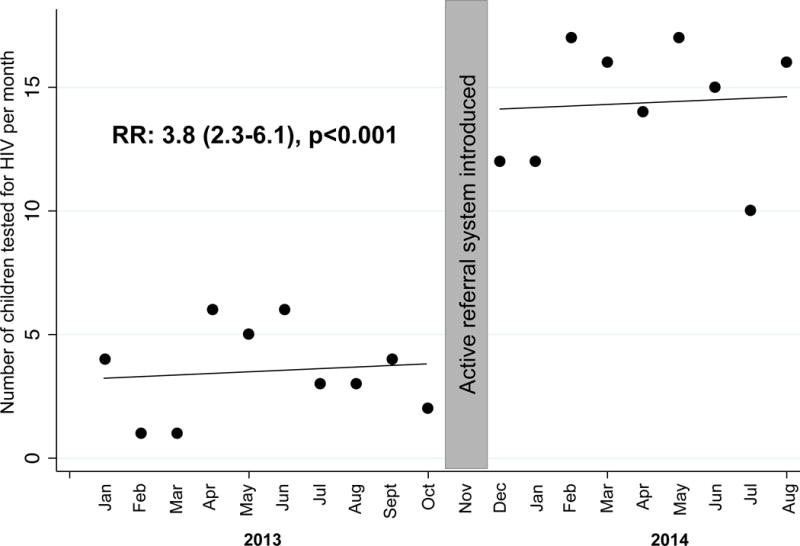
Changes in uptake of pediatric HIV testing before and after introduction of active referral system. Each dot represents the number of children tested each month during the 10 months prior to study initiation, and using an active referral approach during the 9-month study period. November 2013 was considered a washout period.
Absolute uptake of testing remained low
Overall, after adjusting for the possibility of ineligibility among those caregivers who declined referral, 86% (95%CI: 83–89%) of eligible caregivers did not test their children with the study staff during the study period (Figure 3). The most common reasons for being eligible but not enrolled were having children who lived far away or wanting to test children at another location (27%), wanting to return later or being in a hurry (35%), wanting to consult with/disclose to/bring their partner (20%), not being interested in a research study (7%), or wanting testing but outside of a research setting (9%) (Figure 1). The most common reasons for ineligibility among those screened were that the caregiver was HIV-uninfected (14%), all children were too old for study criteria (≥13) (49%), no children were of unknown HIV status (29%), and being enrolled in another HIV study (9%) (Figure 1). Men showed a trend towards being less likely than women to enroll (14% vs 20%, p=0.08). Caregivers who enrolled but did not test did not differ significantly from those who did test in terms of caregiver sex, socio-economic status, partnership status, age, education, or time since HIV diagnosis (data not shown). The number of adults with children of unknown status needed to approach to test one child (NNT) was 5, and to identify one positive child was 66.
FIGURE 3.
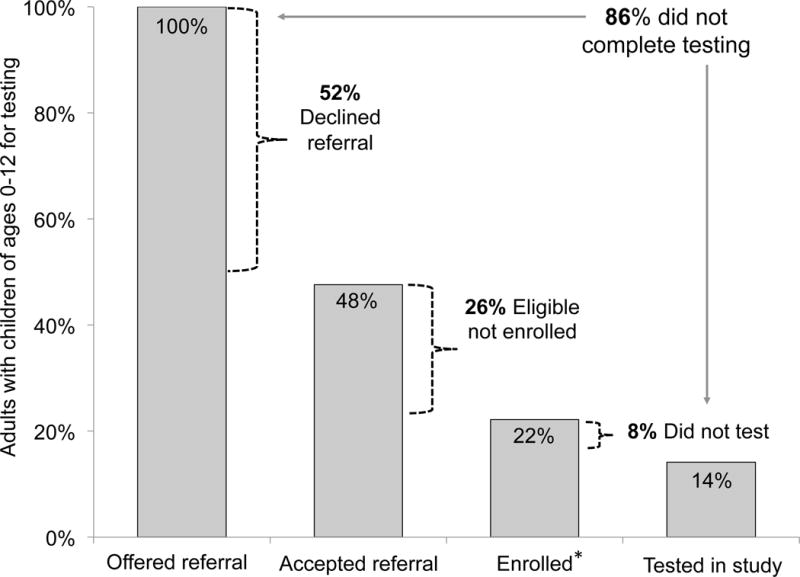
Drop off in pediatric HIV testing cascade. Bar charts show proportion of caregivers completing each stage of study participation from recruitment to testing. * 14% of those screened were ineligible.
Clinic-based testing preferred over home-based testing
Among the 74 parents who tested children, 23 (31%) elected home-based testing and had 46 children tested, while 51 (69%) elected clinic-based testing and had 62 children tested. On average, there were 2.0 children tested per adult in the home-based testing group and 1.2 children tested per adult in the clinic-based testing group (p<0.001). Among 23 parents who completed home-based testing, 15 (65%) reported that they would have accepted clinic-based testing, had home-based testing not been available. Among 51 parents who completed clinic-based testing, 24 (47%) said they would have accepted home-based testing, had clinic-based testing not been available. Had only clinic-based testing been available, 66/74 (89%) parents report that they would have tested their children. However, had only home-based testing been available, 47/74 (64%) report that they would have tested their children.
DISCUSSION
In this study, we found that active referral for testing identified a large number of untested older children of HIV-infected adults in care and significantly increased pediatric HIV testing rates. Pediatric HIV prevalence was 7.4%, higher than in the general population (1%)[5] and higher than would be estimated in early infant diagnosis programs with effective PMTCT (<1–3%). The active referral model did not require additional staffing and could be feasibly implemented at a wide range of facilities with limited additional costs. While home-based testing did allow a small proportion of adults who found clinic-based testing unacceptable to test, the majority of home-based tested caregivers reported that they would have tested at clinic had home-based testing not been available. All HIV-infected children identified in this study linked to care and had generally high CD4 counts. Targeted testing for the children of HIV-infected adults in care is an efficient and easily scalable strategy for case detection prior to symptomatic disease.
As UNAIDS and PEPFAR both prioritize prompt diagnosis and treatment of HIV-infected children to achieve the 90–90–90 targets, innovative models to efficiently diagnose children prior to symptomatic illness are critical. Door-to-door home-based testing of all children is one approach to facilitate HIV diagnosis among children. In prior studies of home-based testing, there is generally high uptake of testing but relatively low HIV prevalence (1%) [5, 15], with higher prevalence in children with risk characteristics such as orphanhood or a suspected or confirmed HIV-infected mother [19, 22, 23]. PITC through opt-out models also has generally high uptake and detects higher HIV prevalence than door-to-door testing [14, 15, 24, 25]; however, children identified through PITC tend to be already ill with HIV, at which point ART confers less benefit compared to their asymptomatic peers [13, 26]. Additionally, PITC—while recommended universally in inpatient and outpatient settings in Kenya—tends to be incompletely implemented and tends to test the sickest children [27]. However, studies with optimized PITC through opt-out models have had higher testing uptake than our study, comparable yield, and also identified children prior to serious illness [14, 28]. Upstream strategies that efficiently identify children prior to symptomatic illness need to be scaled. In Kenya, 55% of children with HIV-infected parents have not been tested, representing an opportunity to scale up targeted testing [5].
We identified several gaps in the pediatric HIV testing and care cascade. Many HIV-infected adults in care (>35%) had children >12 years old who were untested. Our current study was limited to ≤12 year olds because of the lack of carefully formulated adolescent HIV testing approaches that consider issues such as who receives test results, adolescent and parental disclosure, and order of result dissemination when caregivers initiate adolescent testing. This burden of untested adolescents illustrates the critical need for better models and formative research to optimize adolescent HIV testing.
While efficient for case detection, testing children of HIV-infected adults in care had suboptimal uptake in our study. At the VCT site, uptake may have been limited due to adults being newly diagnosed and needing time to process their own diagnosis. In the CCC site, we found that many adults had eligible children, but did not complete testing; this could be due to systems-level or individual-level barriers. Parents reported wishes to test their children elsewhere, consult a partner, or take more time to consider the testing decision as reasons for not testing. In previous studies, HIV-infected caregivers have faced emotional, social, structural, and organizational barriers to testing their children [16, 29–31]. Logistically, bringing children for testing may be inconvenient due to challenges in affording transport, time off of work, or childcare [29]. Parents report fearing emotional suffering for their child if tested—through discrimination and stigma—as well as fears of a child testing positive or dying from HIV, if infected [16, 29, 30]. Parents may not want to test children because a child seems healthy or the parent has uncertainty about the benefits of testing either before symptomatic illness or before sexual debut [29], or because the parent feels guilt or blame regarding having possibly infected the child [16, 30]. Finally, parents reported fearing inadvertent disclosure of their own status as barriers to testing children [16, 29, 31]. Interventions that overcome individual-level barriers—such as addressing logistical challenges; testing before children become symptomatic; allaying parents’ fears about child suffering; addressing parents’ guilt or blame, and providing support to cope with a child’s diagnosis, care, and disclosure—are needed to increase uptake of testing.
On a health systems level, we found that a systematic assessment and referral process quadrupled the number of children being tested monthly. It was outside of the scope of this study to implement other systems-level interventions. We were not able to discern the relative contribution of individual-level vs. systems-level barriers; studies that incorporate systems-level and individual-level interventions could be used to infer the potential differential benefit of interventions.
While our approach increased testing rates over passive referral, only 14% of caregivers tested children in our study. A study in Kenya by Kulzer et al. that used a family-based model to encourage pediatric HIV testing among adults in HIV care programs had 57% uptake, higher than we observed, with high prevalence of infection among tested children (18%). This model used a systems-level family-focused approach that identified all untested family members and noted them in the HIV-infected adult’s medical file, prompting health care workers to ask repeatedly about child testing. The intervention also included a wide range of services to overcome individual-level challenges to testing–assisted disclosure, counseling, support groups, kids play groups, etc.[18]. While uptake was high in this study, costs may have also been high. A similar model that used the systems-level intervention alone (card in the medical record of clients to prompt identification and testing of family members) was tested in Kenya by Mongare et al. Most (88%) of adults in care had a card filled; among all HIV-exposed children identified, uptake of testing was 32% and prevalence of infection was 11%[32]. While not directly comparable, all of these studies suggest that simple, systems-levels interventions may have a sizeable impact on uptake of pediatric HIV testing, but that individual-level interventions may be additionally needed to optimize uptake.
In our study, a systematic assessment and one-time referral had 14% uptake (nearly four times the uptake in the absence of the referral process); in the Mongare et al. study, a systematic assessment and repeat referral model had ~28% uptake (adjusted for incomplete card distribution); in the Kulzer et al. study, a systematic assessment and repeat referral model plus counseling, assisted disclosure, and group activities had 57% uptake. Cost-effectiveness studies are needed to prioritize systems-level and individual-level interventions to maximize uptake with limited resources.
Strengths of our study included systematic collection of recruitment data, which enabled estimation of gaps in uptake that would have been missed had we focused only on the enrolled cohort, and a large sample size. Additionally, our study tested active referral mechanisms using existing healthcare workers, reflecting “real life” effectiveness. Our study is limited in that it was not possible to confirm whether parents tested their children elsewhere, or to determine why parents refused referral. The reasons for not testing given by parents who accepted referral but did not enroll may be different than the reasons that would be given by parents who rejected referral. The precision of our estimate of HIV prevalence was wide. This model is limited to HIV-infected parents engaged in care; in Kenya, 53% of HIV-infected adults are unaware of their status, and 11% of those diagnosed are not engaged in care [5]. Finally, this study was conducted at one urban site and results may not be generalizable.
CONCLUSIONS
Active referral for testing the children of HIV-infected adults in care increased pediatric HIV testing 4-fold and revealed a high prevalence of pediatric HIV. However, most parents with eligible children did not complete pediatric testing. Other approaches are needed to assist parents not testing their children promptly. Systems-level and individual-level interventions may be promising to increase uptake of testing, and merit investigation. Many parents had older untested children outside our age range, suggesting the need for adolescent-focused testing strategies.
Acknowledgments
The authors thank the CATCH-pilot study participants and their families, without whom this research would not be possible. We thank the CATCH-pilot administrative, clinical, and data team for their dedication and support. We thank the Kenyatta National Hospital staff at the PMTCT, VCT, and CCC clinics for their tremendous effort in recruiting study participants. We thank the Kenyan National AIDS and STI Control Programme (NASCOP) for valuable input during study design, conduct, and dissemination. We thank Dr. Bhavna Chohan’s lab for performing HIV PCR tests. We thank Ms. Laurén Gómez and Ms. Hannah Han for data management assistance. We thank members of the Kizazi Working Group (UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh)) and Kenya Research & Training Center (KRTC) for their support during the preparation of this article.
Funding: The Counseling and Testing for Children at Home (CATCH) Study was funded by A83526 (University of Washington Royalty Research Fund, PI Slyker) and by R21 HD079637 (NIH, John-Stewart). ADW was supported by F31 MH099988 (NIH); CMW was supported by UW A83526 (UW RRF); INN and DCW were supported by R01 HD023412 (NIH); KS was supported by K02TW009207; GJS was supported by R01 HD023412 and K24 HD054314 (NIH); JAS was supported by K01 AI087369. This publication was also supported in part by the University of Washington CFAR (P30 AI027757), REDCap UL1TR000423 from NCRR/NIH, and the UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh).
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funding sources were not involved in the analyses or interpretation of data. None of the authors was paid to write this article by a pharmaceutical company or other agency.
Footnotes
Author contributions: ADW, KS, DCW, GJS, JAS conceptualized the article; ADW, GJS, JAS prepared the final draft; ADW, CMW, INN, EMO, KS, IWI, JPH, DCW, GJS, JAS made contributions and revisions. All authors approved the final draft.
Partial data presented previously at 2015 International AIDS Society (IAS).
References
- 1.UNAIDS. 2015 Progress Report on the Global Plan: towards the elimination of new HIV infections among children and keeping their mothers alive. 2015 [Google Scholar]
- 2.Kellerman SE, Ahmed S, Feeley-Summerl T, Jay J, Kim M, Phelps BR, et al. Beyond prevention of mother-to-child transmission: keeping HIV-exposed and HIV-positive children healthy and alive. AIDS. 2013;27(Suppl 2):S225–233. doi: 10.1097/QAD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellerman S, Essajee S. HIV testing for children in resource-limited settings: what are we waiting for? PLoS Med. 2010;7:e1000285. doi: 10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. The Gap Report. 2014 [Google Scholar]
- 5.National AIDS and STI Control Programme MoH K. Kenya AIDS Indicator Survey 2012: Final Report. Nairobi: NASCOP; 2014. [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR, 3rd, et al. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23:1893–1901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Slyker JA, et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J. 2012;31:729–731. doi: 10.1097/INF.0b013e3182587796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner A, Slyker J, Langat A, Inwani I, Adhiambo J, Benki-Nugent S, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:10. doi: 10.1186/s12887-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath CJ, Chung MH, Richardson BA, Benki-Nugent S, Warui D, John-Stewart GC. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–355. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabue MM, Buck WC, Wanless SR, Cox CM, McCollum ED, Caviness AC, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130:e591–599. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyenaar JK, Novosad PM, Ferrer KT, Thahane LK, Mohapi EQ, Schutze GE, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in lesotho. Pediatr Infect Dis J. 2010;29:340–345. doi: 10.1097/INF.0b013e3181bf8ecb. [DOI] [PubMed] [Google Scholar]
- 13.Girardi E, Sabin CA, Monforte AD. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(Suppl 1):S3–8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 14.Ferrand RA, Meghji J, Kidia K, Dauya E, Bandason T, Mujuru H, et al. Implementation and Operational Research: The Effectiveness of Routine Opt-Out HIV Testing for Children in Harare, Zimbabwe. J Acquir Immune Defic Syndr. 2016;71:e24–29. doi: 10.1097/QAI.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govindasamy D, Ferrand RA, Wilmore SM, Ford N, Ahmed S, Afnan-Holmes H, et al. Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18:20182. doi: 10.7448/IAS.18.1.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed S, Kim MH, Sugandhi N, Phelps BR, Sabelli R, Diallo MO, et al. Beyond early infant diagnosis: case finding strategies for identification of HIV-infected infants and children. AIDS. 2013;27(Suppl 2):S235–245. doi: 10.1097/QAD.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: 2014. [Google Scholar]
- 18.Kulzer LJ, Penner JA, Marima R, Oyaro P, Oyanga AO, Shade SB, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 2012;15:8. doi: 10.1186/1758-2652-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vreeman RC, Nyandiko WM, Braitstein P, Were MC, Ayaya SO, Ndege SK, et al. Acceptance of HIV testing for children ages 18 months to 13 years identified through voluntary, home-based HIV counseling and testing in western Kenya. J Acquir Immune Defic Syndr. 2010;55:e3–10. doi: 10.1097/QAI.0b013e3181f0758f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National AIDS and STI Control Programme (NASCOP) MoPHaS. National Guidelines for HIV Testing and Counselling in Kenya. 2nd. Nairobi, Kenya: 2010. [Google Scholar]
- 21.Panteleeff DD, John G, Nduati R, Mbori-Ngacha D, Richardson B, Kreiss J, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS. 2010;24:735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 23.Were WA, Mermin JH, Wamai N, Awor AC, Bechange S, Moss S, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006;43:91–95. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 24.Mutanga JN, Raymond J, Towle MS, Mutembo S, Fubisha RC, Lule F, et al. Institutionalizing provider-initiated HIV testing and counselling for children: an observational case study from Zambia. PLoS One. 2012;7:e29656. doi: 10.1371/journal.pone.0029656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kankasa C, Carter RJ, Briggs N, Bulterys M, Chama E, Cooper ER, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCollum ED, Preidis GA, Golitko CL, Siwande LD, Mwansambo C, Kazembe PN, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sibanda EL, Hatzold K, Mugurungi O, Ncube G, Dupwa B, Siraha P, et al. An assessment of the Zimbabwe ministry of health and child welfare provider initiated HIV testing and counselling programme. BMC Health Serv Res. 2012;12:131. doi: 10.1186/1472-6963-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranzer K, Meghji J, Bandason T, Dauya E, Mungofa S, Busza J, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. doi: 10.1371/journal.pmed.1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rwemisisi J, Wolff B, Coutinho A, Grosskurth H, Whitworth J. ‘What if they ask how I got it?’ Dilemmas of disclosing parental HIV status and testing children for HIV in Uganda. Health Policy Plan. 2008;23:36–42. doi: 10.1093/heapol/czm040. [DOI] [PubMed] [Google Scholar]
- 30.Buzdugan R, Watadzaushe C, Dirawo J, Mundida O, Langhaug L, Willis N, et al. Positive attitudes to pediatric HIV testing: findings from a nationally representative survey from Zimbabwe. PLoS One. 2012;7:e53213. doi: 10.1371/journal.pone.0053213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John-Stewart GC, Wariua G, Beima-Sofie KM, Richardson BA, Farquhar C, Maleche-Obimbo E, et al. Prevalence, perceptions, and correlates of pediatric HIV disclosure in an HIV treatment program in Kenya. AIDS Care. 2012 doi: 10.1080/09540121.2012.749333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mongare J, O F, Ojoo S, Ooko H, Chege M, Wandina D, Redfield R, Client-centered HIV. Client-centered HIV testing and counseling as a strategy for scaling up access to HIV prevention and care services. 7th IAS Conference on HIV Pathogenesis; Kuala Lumpur, Malaysia. 2013. [Google Scholar]


